Robotic Complete ALPPS (rALPPS)—First German Experiences
Abstract
Simple Summary
Abstract
1. Background
2. Material and Methods
2.1. Patients
2.2. Selection Criteria for rALPPS
2.3. Technique of rALPPS
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Perioperative Parameters
3.3. Comparison with the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkhatov, L.; Aghayan, D.L.; Scuderi, V.; Cipriani, F.; Fretland, A.; Kazaryan, A.M.; Ratti, F.; Armstrong, T.; Belli, A.; Dagher, I.; et al. Long-term oncological outcomes after laparoscopic parenchyma-sparing redo liver resections for patients with metastatic colorectal cancer: A European multi-center study. Surg. Endosc. 2022, 36, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Heij, L.R.; Amygdalos, I.; Heise, D.; Bruners, P.; Ulmer, T.F.; Neumann, U.P.; Lang, S.A. The role of re-resection in recurrent hepatocellular carcinoma. Langenbeck’s Arch. Surg. 2022, 407, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.T.; Lee, J.; Jo, H.H.; Kim, H.G.; Han, J. The effect and therapeutic compliance of adjuvant therapy in patients with cholangiocarcinoma after R0 resection: A retrospective study. Yeungnam Univ. J. Med. 2023, 40, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-D.; Xiao, L. Associating liver partition and portal vein ligation for staged hepatectomy in the treatment of colorectal cancer liver metastases. World J. Gastrointest. Surg. 2021, 13, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.B.L.; Colasanti, M.; Aldrighetti, L.; Guglielmi, A.; Cillo, U.; Mazzaferro, V.; Valle, R.D.; De Carlis, L.; Gruttadauria, S.; Di Benedetto, F.; et al. Is minimally invasive liver surgery a reasonable option in recurrent HCC? A snapshot from the I Go MILS registry. Updat. Surg. 2022, 74, 87–96. [Google Scholar] [CrossRef]
- Knitter, S.; Andreou, A.; Kradolfer, D.; Beierle, A.S.; Pesthy, S.; Eichelberg, A.-C.; Kästner, A.; Feldbrügge, L.; Krenzien, F.; Schulz, M.; et al. Minimal-Invasive Versus Open Hepatectomy for Colorectal Liver Metastases: Bicentric Analysis of Postoperative Outcomes and Long-Term Survival Using Propensity Score Matching Analysis. J. Clin. Med. 2020, 9, 4027. [Google Scholar] [CrossRef]
- Andreou, A.; Struecker, B.; Raschzok, N.; Krenzien, F.; Haber, P.; Wabitsch, S.; Waldbaur, C.; Touet, E.-M.; Eichelberg, A.-C.; Atanasov, G.; et al. Minimal-invasive versus open hepatectomy for hepatocellular carcinoma: Comparison of postoperative outcomes and long-term survivals using propensity score matching analysis. Surg. Oncol. 2018, 27, 751–758. [Google Scholar] [CrossRef]
- Kabir, T.; Tan, Z.Z.; Syn, N.L.; Wu, E.; Lin, J.D.; Zhao, J.J.; Tan, A.Y.H.; Hui, Y.; Kam, J.H.; Goh, B.K.P. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: Meta-analysis. Br. J. Surg. 2021, 109, 21–29. [Google Scholar] [CrossRef]
- Choi, G.H.; Chong, J.U.; Han, D.H.; Choi, J.S.; Lee, W.J. Robotic hepatectomy: The Korean experience and perspective. HepatoBiliary Surg. Nutr. 2017, 6, 230–238. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Xu, S.; Hu, M.-G.; Zhao, Z.-M.; Wang, Z.-H.; Zhao, G.-D.; Li, C.-G.; Tan, X.-L.; Liu, R. Short- and long-term outcomes after robotic and open liver resection for elderly patients with hepatocellular carcinoma: A propensity score-matched study. Surg. Endosc. 2022, 36, 8132–8143. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Oberlin, O.; Sergent, G.; Lebuffe, G.; Gambiez, L.; Ernst, O.; Pruvot, F.-R. Remnant Liver Volume to Body Weight Ratio ≥0.5%: A New Cut-Off to Estimate Postoperative Risks after Extended Resection in Noncirrhotic Liver. J. Am. Coll. Surg. 2007, 204, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Markar, S.; Partelli, S.; Fotheringham, T.; Low, D.; Imber, C.; Malago, M.; Kocher, H.M. Portal Vein Embolization and Ligation for Extended Hepatectomy. Indian J. Surg. Oncol. 2014, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right Portal Vein Ligation Combined with In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Perrakis, A.; Rahimli, M.; Gumbs, A.A.; Negrini, V.; Andric, M.; Stockheim, J.; Wex, C.; Lorenz, E.; Arend, J.; Franz, M.; et al. Three-Device (3D) Technique for Liver Parenchyma Dissection in Robotic Liver Surgery. J. Clin. Med. 2021, 10, 5265. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wakabayashi, G.; Kim, H.-J.; Choi, G.-H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.J.; Wong, M.J.; Choi, G.H.; Wu, Y.M.; Lai, P.B.; Goh, B.K.P. Systematic review and meta-analysis of robotic versus open hepatectomy. ANZ J. Surg. 2019, 89, 165–170. [Google Scholar] [CrossRef]
- Montalti, R.; Scuderi, V.; Patriti, A.; Vivarelli, M.; Troisi, R.I. Robotic versus laparoscopic resections of posterosuperior segments of the liver: A propensity score-matched comparison. Surg. Endosc. 2016, 30, 1004–1013. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Sbrana, F.; Coratti, A.; Bianco, F.M.; Addeo, P.; Buchs, N.C.; Ayloo, S.M.; Benedetti, E. Totally Robotic Right Hepatectomy. Arch. Surg. 2011, 146, 844–850. [Google Scholar] [CrossRef]
- Truant, S.; Scatton, O.; Dokmak, S.; Regimbeau, J.-M.; Lucidi, V.; Laurent, A.; Gauzolino, R.; Benitez, C.C.; Pequignot, A.; Donckier, V.; et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): Impact of the inter-stages course on morbi-mortality and implications for management. Eur. J. Surg. Oncol. 2015, 41, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.-J.; et al. Prediction of Postoperative Outcome After Hepatectomy With a New Bedside Test for Maximal Liver Function Capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef]
- Franz, M.; Arend, J.; Wolff, S.; Perrakis, A.; Rahimli, M.; Negrini, V.-R.; Stockheim, J.; Lorenz, E.; Croner, R. Tumor visualization and fluorescence angiography with indocyanine green (ICG) in laparoscopic and robotic hepatobiliary surgery—Valuation of early adopters from Germany. Innov. Surg. Sci. 2021, 6, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Croner, R.; Perrakis, A.; Grützmann, R.; Hohenberger, W.; Brunner, M. Roboterassistierte Leberchirurgie. Tumordiagn. Ther. 2016, 37, 259–264. [Google Scholar] [CrossRef]
- Croner, R.; Arend, J.; Franz, M.; Rahimli, M.; Negrini, V.R.; Stockheim, J.; Lorenz, E.; Andric, M.; Perrakis, A. Roboterassistierte Hemihepatektomie rechts. Zentralbl. Chir. 2021, 146, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Solomonov, E.; Tzadok, I.; Stemmer, S.; Biswas, S. Case Report: Robotic ALPPS Procedure for Hepatocellular Carcinoma in the Right Lobe of the Liver. Front. Surg. 2021, 8, 655683. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Quijano, Y.; Ielpo, B.; Fabra, I. First ALPPS procedure using a total robotic approach. Surg. Oncol. 2016, 25, 457. [Google Scholar] [CrossRef]
- Quijano, Y.; Vicente, E.; Ielpo, B.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ferri, V.; Plaza, C.; Lindemann, J.L.; et al. Hepatobilio-pancreatic robotic surgery: Initial experience from a single center institute. J. Robot. Surg. 2017, 11, 355–365. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Magistri, P. First Case of Full Robotic ALPPS for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 865. [Google Scholar] [CrossRef]
- Machado, M.A.C.; Surjan, R.C.; Makdissi, F. Robotic ALPPS. Ann. Surg. Oncol. 2019, 27, 1174–1179. [Google Scholar] [CrossRef]
- Fernandes, E.d.S.M.; de Barros, F.; Magistri, P.; Di Sandro, S.; de Carvalho, P.R.; da Silva, F.R.; Andrade, R.O.; Pimentel, L.S.; Girão, C.L.; de Mello, F.P.; et al. Total robotic ALPPS approach for hepatocellular carcinoma in cirrhotic liver. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2238. [Google Scholar] [CrossRef]
- Hu, M.-G.; Wang, J.; Yin, Z.-Z.; Liu, R. First two-stage robotic ALPPS in HCC patients with hepatic vein invasion: A step-by-step procedure from a clinical case. World J. Surg. Oncol. 2021, 19, 1–6. [Google Scholar] [CrossRef]
- Robles, R.; Parrilla, P.; López-Conesa, A.; Brusadin, R.; de la Peña, J.; Fuster, M.; García-López, J.; Hernández, E. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br. J. Surg. 2014, 101, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Petrowsky, H.; Györi, G.; de Oliveira, M.; Lesurtel, M.; Clavien, P.-A. Is Partial-ALPPS Safer Than ALPPS? A Single-center Experience. Ann. Surg. 2015, 261, e90–e92. [Google Scholar] [CrossRef] [PubMed]
- De Santibañes, E.; Alvarez, F.A.; Ardiles, V.; Pekolj, J.; de Santibañes, M. Inverting the ALPPS paradigm by minimizing first stage impact: The Mini-ALPPS technique. Langenbeck’s Arch. Surg. 2016, 401, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kantas, A.; Ittrich, H.; Koops, A.; Achilles, E.G.; Fischer, L.; Nashan, B. Avoid “All-Touch” by Hybrid ALPPS to Achieve Oncological Efficacy. Ann. Surg. 2016, 263, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Napoli, N.; Kauffmann, E.F.; Presti, G.L.; Moglia, A. Laparoscopic Microwave Liver Ablation and Portal Vein Ligation: An Alternative Approach to the Conventional ALPPS Procedure in Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gall, T.M.H.B.; Sodergren, M.H.P.; Frampton, A.E.M.; Fan, R.; Spalding, D.R.M.; Habib, N.A.C.; Pai, M.M.; Jackson, J.E.F.; Tait, P.F.; Jiao, L.R.M. Radio-frequency-assisted Liver Partition with Portal Vein Ligation (RALPP) for Liver Regeneration. Ann. Surg. 2015, 261, e45–e46. [Google Scholar] [CrossRef]
- Edmondson, M.J.; Sodergren, M.H.; Pucher, P.H.; Darzi, A.; Li, J.; Petrowsky, H.; Campos, R.R.; Serrablo, A.; Jiao, L.R. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery 2016, 159, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.A.; Makdissi, F.F.; Surjan, R.C.; Basseres, T.; Schadde, E. Transition from open to laparoscopic ALPPS for patients with very small FLR: The initial experience. HPB 2017, 19, 59–66. [Google Scholar] [CrossRef]
- Michal, K.; Sau, M.; Tamara, G.M.H.; Long, J.R. A better route to ALPPS: Minimally invasive vs open ALPPS. Surg. Endosc. 2020, 34, 2379–2389. [Google Scholar] [CrossRef]
- Imura, S.; Shimada, M.; Utsunomiya, T.; Morine, Y.; Wakabayashi, G.; Kaneko, H. Current status of laparoscopic liver surgery in Japan: Results of a multicenter Japanese experience. Surg. Today 2014, 44, 1214–1219. [Google Scholar] [CrossRef]
- Pekolj, J.; Alvarez, F.A.; Biagiola, D.; Villegas, L.; Ardiles, V.; de Santibañes, E. Totally Laparoscopic Mini-ALPPS Using a Novel Approach of Laparoscopic-Assisted Transmesenteric Portal Vein Embolization. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; El Amrani, M.; Baillet, C.; Ploquin, A.; Lecolle, K.; Ernst, O.; Hebbar, M.; Huglo, D.; Pruvot, F.-R. Laparoscopic Partial ALPPS: Much Better Than ALPPS! Ann. Hepatol. 2019, 18, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, K.-F.; Schmidt, M.W.; Proctor, T.; Pohl, M.; Wennberg, E.; Karadza, E.; Romero, P.; Kenngott, H.G.; Müller-Stich, B.P.; Nickel, F. Skills in minimally invasive and open surgery show limited transferability to robotic surgery: Results from a prospective study. Surg. Endosc. 2018, 32, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, Q.; Xu, Y.; Wang, W. Comparative clinical outcomes of robot-assisted liver resection versus laparoscopic liver resection: A meta-analysis. PLoS ONE 2020, 15, e0240593. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Fuks, D.; Lee, K.-F.; Zhao, J.J.; Choi, G.H.; Sucandy, I.; Chiow, A.K.H.; Marino, M.V.; Gastaca, M.; Wang, X.; et al. Propensity Score–Matched Analysis Comparing Robotic and Laparoscopic Right and Extended Right Hepatectomy. JAMA Surg. 2022, 157, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.N.; Lok, H.T.; Fung, A.K.Y.; Fong, A.K.W.; Cheung, Y.S.; Wong, J.; Lee, K.F.; Lai, P.B.S. Robotic versus laparoscopic hepatectomy: Application of the difficulty scoring system. Surg. Endosc. 2020, 34, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.; Manas, D.; Jiao, L.R.; Abu Hilal, M.; White, S.A. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand. J. Surg. 2021, 110, 290–300. [Google Scholar] [CrossRef]
- Coletta, D.; Sandri, G.B.L.; Giuliani, G.; Guerra, F. Robot-assisted versus conventional laparoscopic major hepatectomies: Systematic review with meta-analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2218. [Google Scholar] [CrossRef]
- D’Hondt, M.; Devooght, A.; Willems, E.; Wicherts, D.; De Meyere, C.; Parmentier, I.; Provoost, A.; Pottel, H.; Verslype, C. Transition from laparoscopic to robotic liver surgery: Clinical outcomes, learning curve effect, and cost-effectiveness. J. Robot. Surg. 2022, 17, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Baili, E.; Tsilimigras, D.; Filippou, D.; Ioannidis, A.; Bakopoulos, A.; Machairas, N.; Papalampros, A.; Petrou, A.; Schizas, D.; Moris, D. Associating liver partition and portal vein ligation for staged hepatectomy in patients with primary liver malignancies: A systematic review of the literature. JBUON 2019, 24, 1371–1381. [Google Scholar] [PubMed]
- Feldbrügge, L.; Galindo, S.A.O.; Frisch, O.; Benzing, C.; Krenzien, F.; Riddermann, A.; Kästner, A.; Nevermann, N.F.; Malinka, T.; Schöning, W.; et al. Safety and feasibility of robotic liver resection after previous abdominal surgeries. Surg. Endosc. 2022, 36, 2842–2849. [Google Scholar] [CrossRef]
- Heinrich, S.; Lang, H. Evidenz in der minimal-invasiven onkologischen Chirurgie der Leber. Chirurg 2021, 92, 316–325. [Google Scholar] [CrossRef]
- Ozair, A.; Collings, A.; Adams, A.M.; Dirks, R.; Kushner, B.S.; Sucandy, I.; Morrell, D.; Abou-Setta, A.M.; Vreeland, T.; Whiteside, J.; et al. Minimally invasive versus open hepatectomy for the resection of colorectal liver metastases: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 7915–7937. [Google Scholar] [CrossRef]
- Guerra, F.; Guadagni, S.; Pesi, B.; Furbetta, N.; Di Franco, G.; Palmeri, M.; Annecchiarico, M.; Eugeni, E.; Coratti, A.; Patriti, A.; et al. Outcomes of robotic liver resections for colorectal liver metastases. A multi-institutional analysis of minimally invasive ultrasound-guided robotic surgery. Surg. Oncol. 2019, 28, 14–18. [Google Scholar] [CrossRef]
- Lim, C.; Salloum, C.; Tudisco, A.; Ricci, C.; Osseis, M.; Napoli, N.; Lahat, E.; Boggi, U.; Azoulay, D. Short- and Long-term Outcomes after Robotic and Laparoscopic Liver Resection for Malignancies: A Propensity Score-Matched Study. Mol. Med. 2019, 43, 1594–1603. [Google Scholar] [CrossRef] [PubMed]

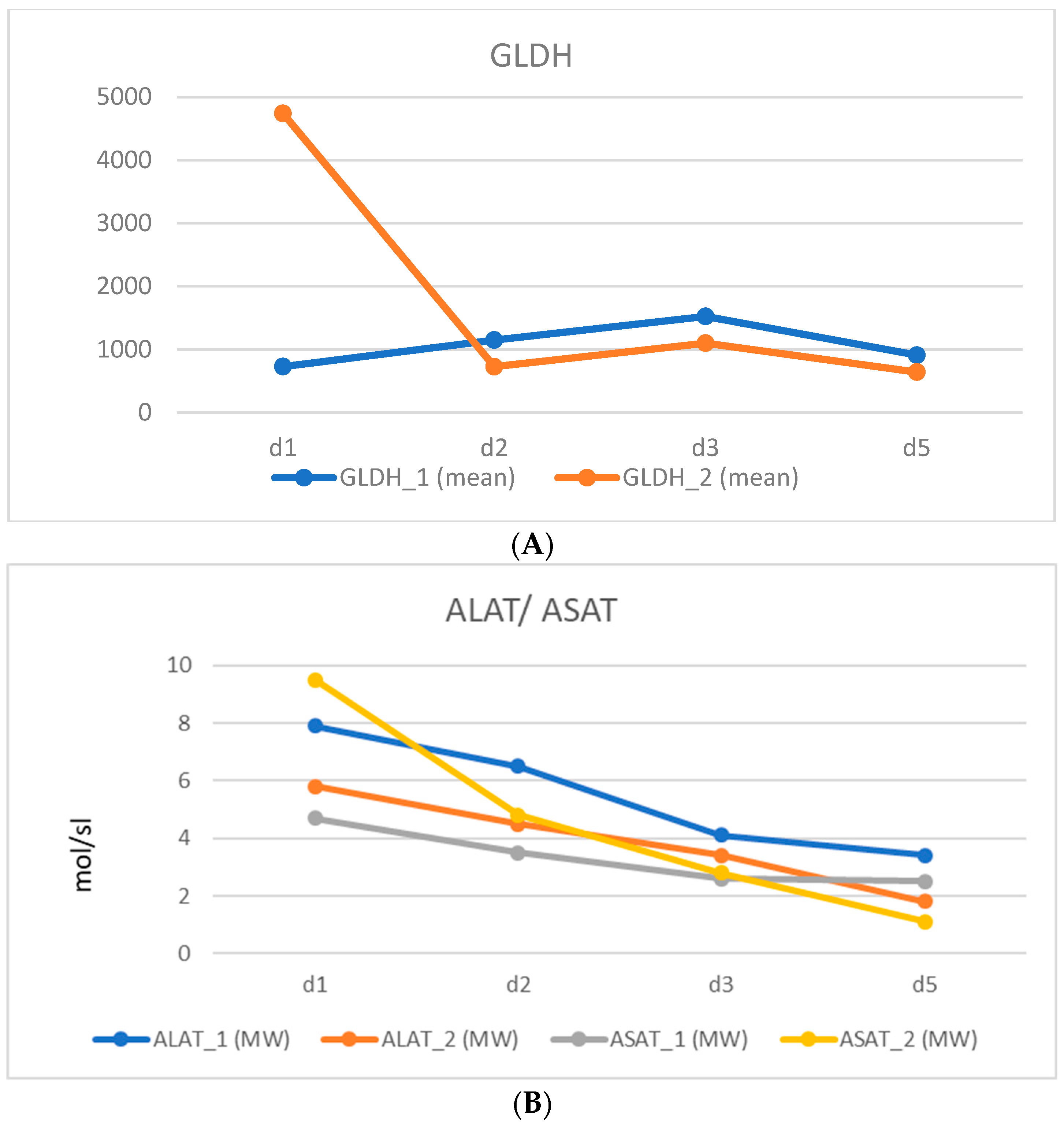
| Total, n | 5 |
| Age; years, mean (SD) | 50.0 (8.%) |
| Gender; female: n (%) | 4 (80) |
| BMI; kg/m² (SD) | 22.7 (3.6) |
| ASA classification (%) | 2 (100) |
| LiMAx; µg/kg/h; mean (SD) | 485.3 (83.5) |
| Neoadjuvant CTX; n (%) | 4 (80) |
| Prior abdominal surgery; n (%) | 3 (60.0) |
| Tumor | |
| 4 (80.0) |
| 1 (20.0) |
| 45.4 (27.9) |
| 4.2 (1.9) |
| 9.5 (17.0) |
| 780.2 (180.2) |
| FLV; mean, mL (SD) | |
| 1. Operation | 380.9 (138.1) |
| 2. Operation | 529.8 (163.0) |
| Hypertrophy; mean %, (SD) | 41.9 (13.2) |
| FLV-BWR | |
| 1. Operation | 0.677 (0.196) |
| 2. Operation | 0.947 (0.223) |
| FLV-BWR Increase; mean %, (SD) | 42.0 (13.1) |
| Time from 1. to 2. operation; d (SD) | 17.8 (5.4) |
| OR time; min (SD) | |
| 1. Operation | 341.2 (145.2) |
| 2. Operation | 440.6 (158.4) |
| Blood loss; mL (SD) | |
| 1. Operation | 140 (54.8) |
| 2. Operation | 660.0 (313.1) |
| Intraoperative transfusions | 1 Packed Red Blood Cells |
| Overall hospital stay; days (SD) | 27.2 (7.7) |
| 90-day morbidity | |
| Clavien–Dindo > 3a (%) | 10 |
| 90-day mortality (%) | 0 |
| Author | Year | Country | Study Design | Diagnose | Cases (n) | Approach |
|---|---|---|---|---|---|---|
| Solomonov et al. [26] | 2013 | Israel | CR | HCC | 1 | rALPPS |
| Vincente et al. [27] | 2016 | Spain | CR | CRLM | 1 | rALPPS |
| Quijano et al. [28] | 2017 | Spain | CR | n.a. | 1 | rALPPS |
| Di Benedetto et al. [29] | 2020 | Italy | CR | HCC | 1 | rALPPS |
| Di Benedetto et al. [30] | 2020 | Italy | CR | ICC | 1 | rALPPS |
| Machado et al. [31] | 2020 | Brazil | CR | CRLM | 1 | rALPPS |
| Fernandes et al. [32] | 2021 | Brazil | SC | HCC | 3 | rALPPS |
| Hu et al. [33] | 2021 | China | CR | HCC | 1 | rALPPS |
| Arend et al. | 2023 | Germany | SC | CRLM/ICC | 5 | rALPPS |
| Author | Operation Time (min)/Mean | Intraoperative Blood Loss (mL)/Mean | Increase FLR (%)/Mean | Time OP 1.–2. (d)/Mean | Hospital Stay (d)/Mean | ||
|---|---|---|---|---|---|---|---|
| OP 1 | OP 2 | OP 1 | OP 2 | ||||
| Solomonov et al. [26] | 410 | 180 | 500 | 500 | 30 | 14 | 10 |
| Vincente et al. [27] | n.a. | n.a. | n.a. | n.a. | 43 | 13 | n.a. |
| Quijano et al. [28] | 540 | 180 | n.a. | n.a. | n.a. | n.a. | 64 |
| Di Benedetto et al. [29] | 495 | 280 | 480 | 200 | 91.6 | 10 | 13 |
| Di Benedetto et al. [30] | 470 | 380 | 150 | 300 | 112 | 14 | 14 |
| Machado et al. [31] | 293 | 245 | 420 | 270 | 46 | 21 | 8 |
| Fernandes et al. [32] | 446.7 | 276.7 | n.d. | 200 | 62.7 | 21 | 18 |
| Hu et al. [33] | 195 | 217 | 250 | 500 | 85 | 12 | 18 |
| Arend et al. | 341.2 | 440.6 | 140 * | 660 * | 39.9 | 17.8 | 27.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arend, J.; Franz, M.; Rose, A.; March, C.; Rahimli, M.; Perrakis, A.; Lorenz, E.; Croner, R. Robotic Complete ALPPS (rALPPS)—First German Experiences. Cancers 2024, 16, 1070. https://doi.org/10.3390/cancers16051070
Arend J, Franz M, Rose A, March C, Rahimli M, Perrakis A, Lorenz E, Croner R. Robotic Complete ALPPS (rALPPS)—First German Experiences. Cancers. 2024; 16(5):1070. https://doi.org/10.3390/cancers16051070
Chicago/Turabian StyleArend, Jörg, Mareike Franz, Alexander Rose, Christine March, Mirhasan Rahimli, Aristotelis Perrakis, Eric Lorenz, and Roland Croner. 2024. "Robotic Complete ALPPS (rALPPS)—First German Experiences" Cancers 16, no. 5: 1070. https://doi.org/10.3390/cancers16051070
APA StyleArend, J., Franz, M., Rose, A., March, C., Rahimli, M., Perrakis, A., Lorenz, E., & Croner, R. (2024). Robotic Complete ALPPS (rALPPS)—First German Experiences. Cancers, 16(5), 1070. https://doi.org/10.3390/cancers16051070








