Palliative Quad Shot Radiation Therapy with or without Concurrent Immune Checkpoint Inhibition for Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Design
2.2. Radiation Simulation and Treatment Planning
2.3. Systemic Therapy
2.4. Follow-Up and Assessments
2.5. Statistical Analysis
3. Results
3.1. Patterns of Treatment Failure
3.2. Clinical Factors Affecting Control and Survival
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Airoldi, M.; Allais, A.; Aluffi Valletti, P.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40 (Suppl. S1), S1–S86. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.A.; Harris, J.; Wheeler, R.H.; Machtay, M.; Schultz, C.; Spanos, W.; Rotman, M.; Meredith, R.; Ang, K.-K. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008, 30, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Harris, J.; Horwitz, E.M.; Nicolaou, N.; Kies, M.; Curran, W.; Wong, S.; Ang, K. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group Protocol 9911. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4800–4805. [Google Scholar] [CrossRef] [PubMed]
- Lartigau, E.F.; Tresch, E.; Thariat, J.; Graff, P.; Coche-Dequeant, B.; Benezery, K.; Schiappacasse, L.; Degardin, M.; Bondiau, P.-Y.; Peiffert, D.; et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 109, 281–285. [Google Scholar] [CrossRef]
- Chen, A.M.; Vaughan, A.; Narayan, S.; Vijayakumar, S. Palliative radiation therapy for head and neck cancer: Toward an optimal fractionation scheme. Head Neck 2008, 30, 1586–1591. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Rosser, B.; Burmeister, B.H.; Jones, M.; Hickey, B.; Baumann, K.; Gogna, K.; Pullar, A.; Poulsen, M.; Holt, T. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment--”Hypo Trial”. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2007, 85, 456–462. [Google Scholar] [CrossRef]
- Agarwal, J.P.; Nemade, B.; Murthy, V.; Ghosh-Laskar, S.; Budrukkar, A.; Gupta, T.; D’Cruz, A.; Pai, P.; Chaturvedi, P.; Dinshaw, K. Hypofractionated, palliative radiotherapy for advanced head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2008, 89, 51–56. [Google Scholar] [CrossRef]
- Al-mamgani, A.; Tans, L.; Van Rooij, P.H.E.; Noever, I.; Baatenburg de Jong, R.J.; Levendag, P.C. Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. Stockh. Swed. 2009, 48, 562–570. [Google Scholar] [CrossRef]
- Vargo, J.A.; Ferris, R.L.; Ohr, J.; Clump, D.A.; Davis, K.S.; Duvvuri, U.; Kim, S.; Johnson, J.T.; Bauman, J.E.; Gibson, M.K.; et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 480–488. [Google Scholar] [CrossRef]
- Comet, B.; Kramar, A.; Faivre-Pierret, M.; Dewas, S.; Coche-Dequeant, B.; Degardin, M.; Lefebvre, J.-L.; Lacornerie, T.; Lartigau, E.F. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-and-neck cancer: A feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 203–209. [Google Scholar] [CrossRef]
- Gogineni, E.; Zhang, I.; Rana, Z.; Marrero, M.; Gill, G.; Sharma, A.; Riegel, A.C.; Teckie, S.; Ghaly, M. Quality of Life Outcomes Following Organ-Sparing SBRT in Previously Irradiated Recurrent Head and Neck Cancer. Front. Oncol. 2019, 9, 836. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, T.S.; Bhatt, N.H.; Shaughnessy, J.N.; Perez, C.; Redman, R.; Silverman, C.; Bumpous, J.; Potts, K.; Dunlap, N.E. Cyclical hypofractionated radiotherapy technique for palliative treatment of locally advanced head and neck cancer: Institutional experience and review of palliative regimens. J. Community Support. Oncol. 2016, 14, 29–36. [Google Scholar] [CrossRef]
- Spanos, W.; Guse, C.; Perez, C.; Grigsby, P.; Doggett, R.L.; Poulter, C. Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: Preliminary report of RTOG 8502. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Spanos, W.J.; Clery, M.; Perez, C.A.; Grigsby, P.W.; Doggett, R.L.; Poulter, C.A.; Steinfeld, A.D. Late effect of multiple daily fraction palliation schedule for advanced pelvic malignancies (RTOG 8502). Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lok, B.H.; Jiang, G.; Gutiontov, S.; Lanning, R.M.; Sridhara, S.; Sherman, E.J.; Tsai, C.J.; McBride, S.M.; Riaz, N.; Lee, N.Y. Palliative head and neck radiotherapy with the RTOG 8502 regimen for incurable primary or metastatic cancers. Oral Oncol. 2015, 51, 957–962. [Google Scholar] [CrossRef]
- Gamez, M.E.; Agarwal, M.; Hu, K.S.; Lukens, J.N.; Harrison, L.B. Hypofractionated Palliative Radiotherapy with Concurrent Radiosensitizing Chemotherapy for Advanced Head and Neck Cancer Using the “QUAD-SHOT Regimen”. Anticancer Res. 2017, 37, 685–691. [Google Scholar] [CrossRef]
- Paris, K.J.; Spanos, W.J.; Lindberg, R.D.; Jose, B.; Albrink, F. Phase I–II study of multiple daily fractions for palliation of advanced head and neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 657–660. [Google Scholar] [CrossRef]
- Toya, R.; Saito, T.; Yamaguchi, K.; Matsuyama, T.; Watakabe, T.; Matsumoto, T.; Yoshida, R.; Hirosue, A.; Murakami, D.; Orita, Y.; et al. Hypofractionated palliative volumetric modulated arc radiotherapy with the Radiation Oncology Study Group 8502 “QUAD shot” regimen for incurable head and neck cancer. Radiat. Oncol. Lond. Engl. 2020, 15, 123. [Google Scholar] [CrossRef]
- Fan, D.; Kang, J.J.; Fan, M.; Wang, H.; Lee, A.; Yu, Y.; Chen, L.; Jillian Tsai, C.; McBride, S.M.; Riaz, N.; et al. Last-line local treatment with the Quad Shot regimen for previously irradiated head and neck cancers. Oral Oncol. 2020, 104, 104641. [Google Scholar] [CrossRef]
- Corry, J.; Peters, L.J.; Costa, I.D.; Milner, A.D.; Fawns, H.; Rischin, D.; Porceddu, S. The ’QUAD SHOT’—A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2005, 77, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet Lond. Engl. 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef]
- Mell, L.K.; Torres-Saavedra, P.; Wong, S.; Chang, S.; Kish, J.A.; Minn, A.J.; Jordan, R.; Liu, T.; Truong, M.T.; Winquist, E.; et al. Radiotherapy with Durvalumab vs. Cetuximab in Patients with Locoregionally Advanced Head and Neck Cancer and a Contraindication to Cisplatin: Phase II Results of NRG-HN004. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 1058. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.-H.; Lin, J.-C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Tao, Y.; Burtness, B.; Tahara, M.; Rischin, D.; Alves, G.V.; Lima, I.P.F.; Hughes, B.G.M.; Pointreau, Y.; Aksoy, S.; et al. LBA5 Primary results of the phase III KEYNOTE-412 study: Pembrolizumab (pembro) with chemoradiation therapy (CRT) vs placebo plus CRT for locally advanced (LA) head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2022, 33, S1399. [Google Scholar] [CrossRef]
- Hughes, R.T.; Gebeyehu, R.R.; Kalada, J.M.; Lycan, T.W.; Frizzell, B.A.; Kinney, R.D.; D’Agostino, R.B.; Bunch, P.M.; Triozzi, P.; Zhang, W.; et al. Quad-shot-immunotherapy: Quad-shot radiotherapy with pembrolizumab for advanced/recurrent head and neck cancer. Future Oncol. Lond. Engl. 2023, 19, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- University of Oklahoma. A Single-arm Phase II Trial of Palliative “QUAD SHOT” Radiotherapy Combined with Pembrolizumab in Patients with Recurrent Head & Neck Cancer. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT04373642 (accessed on 31 December 2022).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lorenz, J.; Fain, R.; Robbins, J.R. Utilization of the ‘QUAD SHOT’ for Palliating Malignancies of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e445. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsantis, I.; Psyrri, A. The promise of immunotherapy in head and neck squamous cell carcinoma: Combinatorial immunotherapy approaches. ESMO Open 2016, 1, e000122. [Google Scholar] [CrossRef]
- Quad-Shot Radiation Therapy in Combination with Pembrolizumab for the Treatment of Locally Advanced, Locally Recurrent, or Metastatic Head and Neck Cancer—NCI. Published 23 June 2016. Available online: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2020-07332 (accessed on 30 October 2023).


| Characteristic | All Patients (n = 70) n (%) | QS + ICI (n = 40) n (%) | QS Alone (n = 30) n (%) | p-Value |
|---|---|---|---|---|
| Age (y): median (IQR) | 65.5 (57.9–77.8) | 63.3 (57.3–70.8) | 67.2 (59.9–80.7) | 0.410 |
| Race | 0.219 | |||
| White | 60 (85.7) | 36 (90.0) | 24 (80.0) | |
| African American | 8 (11.4) | 4 (10.0) | 4 (13.3) | |
| Others | 2 (2.9) | 0 | 2 (6.7) | |
| Sex | 0.366 | |||
| Male | 56 (80.0) | 30 (75.0) | 26 (86.7) | |
| Female | 14 (20.0) | 10 (25.0) | 4 (13.3) | |
| Primary site | 0.371 | |||
| Oropharynx | 23 (32.9) | 12 (30.0) | 11 (36.7) | |
| Oral cavity | 17 (24.3) | 8 (20.0) | 9 (30.0) | |
| Larynx | 11 (15.7) | 8 (20.0) | 3 (10.0) | |
| Cutaneous | 7 (10.0) | 4 (10.0) | 3 (10.0) | |
| Hypopharynx | 4 (5.7) | 1 (2.5) | 3 (10.0) | |
| Paranasal sinus | 3 (4.3) | 3 (7.5) | 0 | |
| Thyroid | 3 (4.3) | 2 (5.0) | 1 (3.3) | |
| Major salivary glands | 2 (2.9) | 2 (5.0) | 0 | |
| P16-positive oropharynx | 11 (55.0) | 7 (58.3) | 4 (50.0) | 0.462 |
| PD-L1 CPS ≥ 1% | 15/21 (71.4) | 10/13 (76.9) | 5/8 (62.5) | 0.683 |
| T-stage | 0.164 | |||
| T0/Tx | 6 (8.6) | 3 (7.5) | 3 (10.0) | |
| T1–2 | 9 (12.9) | 4 (10.0) | 5 (16.7) | |
| T3–4 | 55 (78.5) | 33 (82.5) | 22 (73.3) | |
| N-stage | 0.019 * | |||
| N0–1 | 24 (34.3) | 8 (20.0) | 16 (53.3) | |
| N2–3 | 46 (65.7) | 32 (80.0) | 14 (46.7) | |
| M-stage | 0.415 | |||
| M1 | 18 (25.7) | 12 (30.0) | 6 (20.0) | |
| Smoking history | 0.384 | |||
| Current | 15 (21.4) | 8 (20.0) | 7 (23.3) | |
| Former | 41 (58.6) | 26 (65.0) | 15 (50.0) | |
| None | 14 (20.0) | 6 (15.0) | 8 (26.7) | |
| Smoking pack years: median (IQR) | 30 (18–50) | 25 (18–50) | 44 (18–50) | 0.487 |
| ECOG performance status | 0.316 | |||
| 0 | 10 (14.3) | 6 (15.0) | 4 (13.3) | |
| 1 | 35 (50.0) | 22 (55.0) | 13 (43.3) | |
| 2 | 19 (27.1) | 12 (30.0) | 7 (23.3) | |
| 3 | 6 (8.6) | 0 | 6 (20.0) | |
| Surgery for primary site | 24 (34.3) | 17 (42.5) | 7 (23.3) | 0.077 |
| Prior systemic therapy | 42 (60.0) | 25 (62.5) | 17 (56.7) | 0.402 |
| ICI | 13 (31.0) | 5 (20.0) | 8 (47.1) | |
| Chemotherapy alone | 19 (45.2) | 14 (56.0) | 5 (29.4) | |
| Cetuximab alone | 1 (2.4) | 0 | 1 (5.9) | |
| Chemotherapy + Cetuximab | 9 (21.4) | 6 (24.0) | 3 (17.6) | |
| Prior radiation therapy | 35 (50.0) | 22 (55.0) | 13 (43.3) | 0.235 |
| Concurrent non-ICI systemic therapy | 19 (27.1) | 4 (10.0) | 15 (50.0) | <0.001 * |
| Chemotherapy alone | 9 (12.9) | 3 (7.5) | 6 (40.0) | |
| Cetuximab alone | 4 (5.7) | 0 | 4 (26.7) | |
| Chemotherapy + Cetuximab | 6 (8.6) | 1 (2.5) | 5 (33.3) | |
| Number of QS cycles | 0.507 | |||
| 3 | 52 (74.3) | 30 (75.0) | 22 (73.3) | |
| 4 | 17 (24.3) | 10 (25.0) | 7 (23.3) | |
| 5 | 1 (1.4) | 0 | 1 (3.3) |
| Outcome | All Patients (n = 70) n (%) | QS + ICI (n = 40) n (%) | QS Alone (n = 30) n (%) | p-Value |
|---|---|---|---|---|
| Objective response | 0.487 | |||
| CR | 16 (22.8%) | 8 (20.0%) | 8 (26.7%) | |
| PR | 34 (48.6%) | 20 (50.0%) | 14 (46.7%) | |
| SD | 13 (18.6%) | 8 (20.0%) | 5 (16.7%) | |
| PD | 7 (10.0%) | 4 (10.0%) | 3 (10.0%) | |
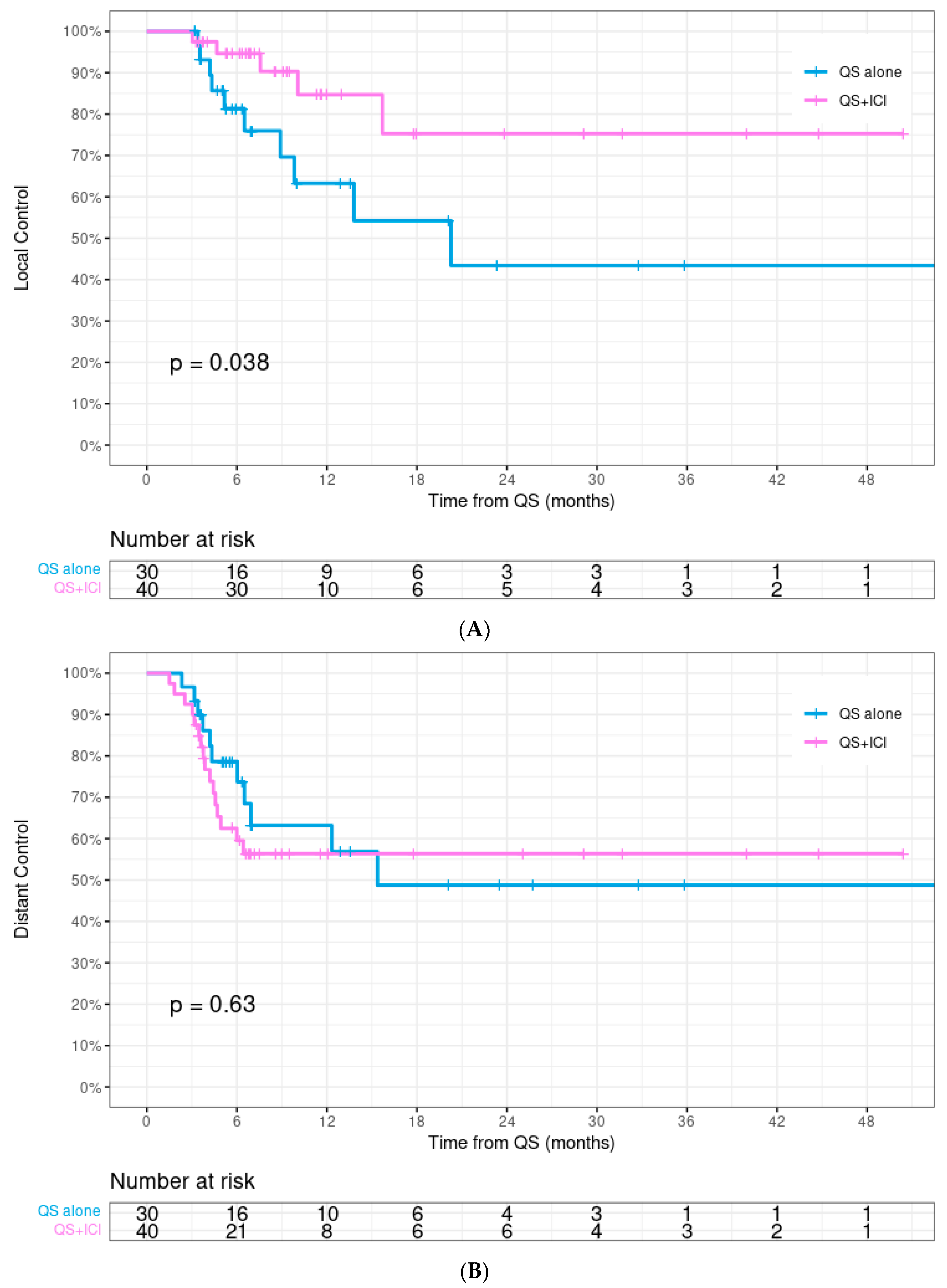
| Local control | 0.038 * | |||
| 12-month | 75.5% | 84.7% | 63.3% | |
| 24-month | 60.1% | 75.3% | 43.4% | |
| Distant control | 0.629 | |||
| 12-month | 59.4% | 56.4% | 63.2% | |
| 24-month | 51.9% | 56.4% | 48.8% | |
| Overall survival | 0.850 | |||
| 12-month | 35.8% | 30.0% | 43.6% | |
| 24-month | 23.2% | 21.8% | 20.3% | |
| Median (95% CI) | 9.4 m (6.5–12.2) | 9.0 m (6.7–11.4) | 10.0 m (5.5–14.5) |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.014 (0.003–319.381) | 0.996 | Not included | |
| Race | 0.594 (0.166–2.125) | 0.423 | Not included | |
| Sex | 1.418 (0.451–4.460) | 0.550 | Not included | |
| Primary site | 0.911 (0.773–1.074) | 0.267 | Not included | |
| T-stage | 0.618 (0.171–2.236) | 0.464 | Not included | |
| N-stage | 3.059 (0.683–13.699) | 0.144 | 1.729 (0.905–3.305) | 0.097 |
| M-stage | 0.334 (0.074–1.499) | 0.152 | 0.397 (0.086–1.838) | 0.237 |
| Smoking | 1.899 (0.523–6.897) | 0.330 | Not included | |
| ECOG PS ≥ 2 | 1.051 (0.325–3.403) | 0.934 | Not included | |
| Surgery | 1.130 (0.385–3.312) | 0.824 | Not included | |
| Prior ST | 10.073 (1.323–76.722) | 0.026 * | 7.035 (0.917–54.001) | 0.061 |
| Conc QS + ICI | 0.337 (0.115–0.989) | 0.048 * | 0.238 (0.073–0.778) | 0.018 * |
| Prior RT | 1.100 (0.398–3.040) | 0.854 | Not included | |
| Adjuvant ICI | 0.978 (0.332–2.877) | 0.968 | Not included | |
| No. of QS cycles | 9507.71 (0.000–5.2 × 10151) | 0.958 | Not included | |
| Toxicity | Grade 1 n (%) | Grade 2 n (%) | Grade 3 n (%) | Any Grade n (%) |
|---|---|---|---|---|
| Acute | ||||
| Dermatitis | 18 (25.7) | 5 (7.1) | 1 (1.4) | 24 (34.3) |
| Mucositis | 10 (14.3) | 8 (11.4) | 1 (1.4) | 19 (27.1) |
| Dysphagia | 6 (8.6) | 12 (17.1) | 5 (7.1) | 23 (32.9) |
| Dysgeusia | 10 (14.3) | 4 (5.7) | 1 (1.4) | 15 (21.4) |
| Nausea/Vomiting | 1 (1.4) | 0 | 0 | 1 (1.4) |
| Pain | 1 (1.4) | 2 (2.9) | 1 (1.4) | 4 (5.7) |
| Late | ||||
| Xerostomia | 25 (35.7) | 11 (15.7) | 1 (1.4) | 37 (52.8) |
| Radionecrosis | 0 | 0 | 6 (8.6) | 6 (8.6) |
| Lymphedema/Fibrosis | 1 (1.4) | 2 (2.9) | 0 | 3 (4.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, R.; Gogineni, E.; Tocaj, G.; Ma, S.J.; Bonomi, M.; Bhateja, P.; Konieczkowski, D.J.; Baliga, S.; Mitchell, D.L.; Jhawar, S.R.; et al. Palliative Quad Shot Radiation Therapy with or without Concurrent Immune Checkpoint Inhibition for Head and Neck Cancer. Cancers 2024, 16, 1049. https://doi.org/10.3390/cancers16051049
Upadhyay R, Gogineni E, Tocaj G, Ma SJ, Bonomi M, Bhateja P, Konieczkowski DJ, Baliga S, Mitchell DL, Jhawar SR, et al. Palliative Quad Shot Radiation Therapy with or without Concurrent Immune Checkpoint Inhibition for Head and Neck Cancer. Cancers. 2024; 16(5):1049. https://doi.org/10.3390/cancers16051049
Chicago/Turabian StyleUpadhyay, Rituraj, Emile Gogineni, Glenis Tocaj, Sung J. Ma, Marcelo Bonomi, Priyanka Bhateja, David J. Konieczkowski, Sujith Baliga, Darrion L. Mitchell, Sachin R. Jhawar, and et al. 2024. "Palliative Quad Shot Radiation Therapy with or without Concurrent Immune Checkpoint Inhibition for Head and Neck Cancer" Cancers 16, no. 5: 1049. https://doi.org/10.3390/cancers16051049
APA StyleUpadhyay, R., Gogineni, E., Tocaj, G., Ma, S. J., Bonomi, M., Bhateja, P., Konieczkowski, D. J., Baliga, S., Mitchell, D. L., Jhawar, S. R., Zhu, S., Grecula, J. C., Dibs, K., Gamez, M. E., & Blakaj, D. M. (2024). Palliative Quad Shot Radiation Therapy with or without Concurrent Immune Checkpoint Inhibition for Head and Neck Cancer. Cancers, 16(5), 1049. https://doi.org/10.3390/cancers16051049






