Ixazomib, Lenalidomide, and Dexamethasone (IRD) Treatment with Cytogenetic Risk-Based Maintenance in Transplant-Eligible Myeloma: A Phase 2 Multicenter Study by the Nordic Myeloma Study Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Treatment
2.2. Methods
2.3. Endpoints
2.4. Statistical Analysis
2.5. Ethics
3. Results
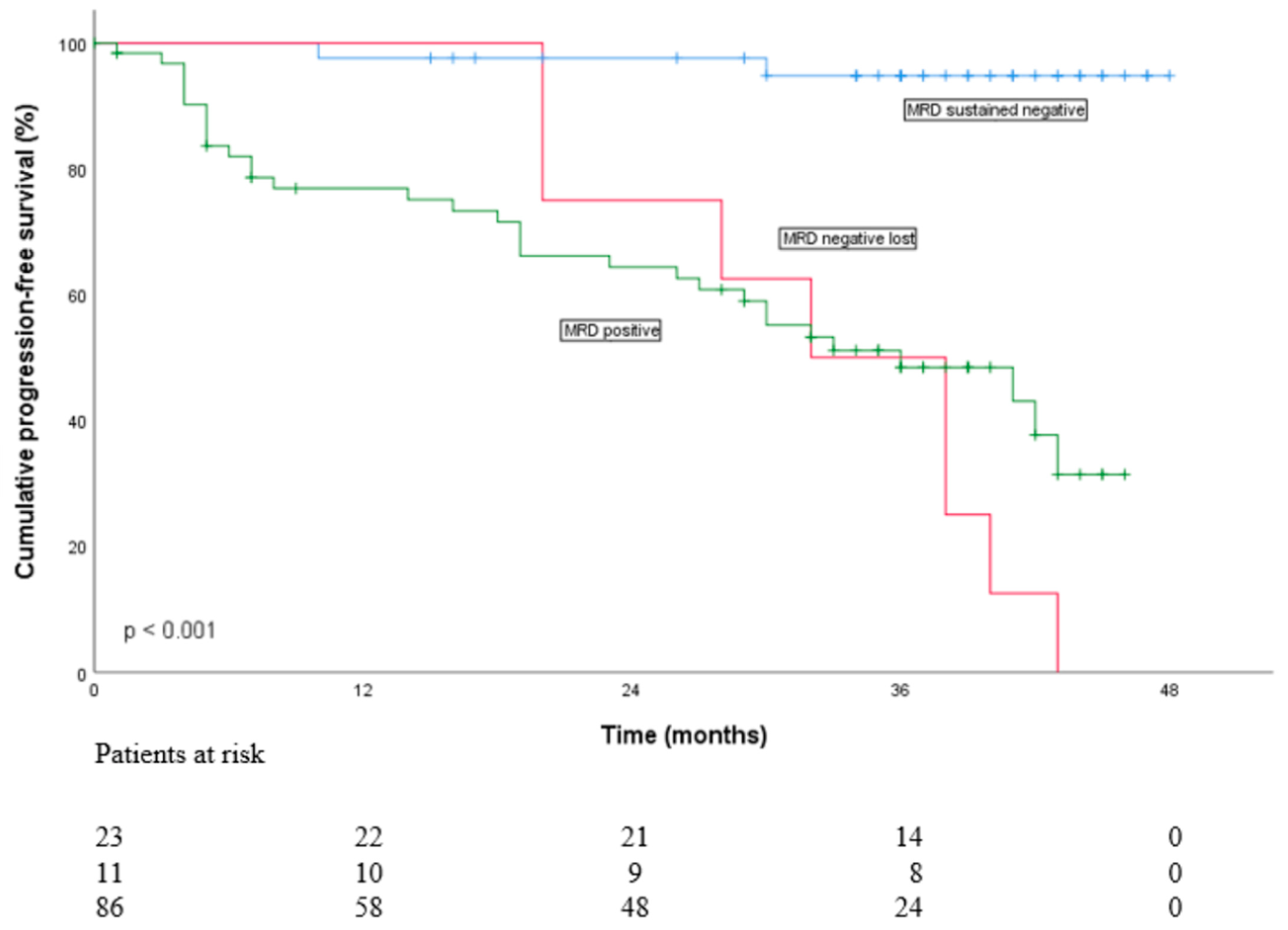
3.1. Flow-MRD Status
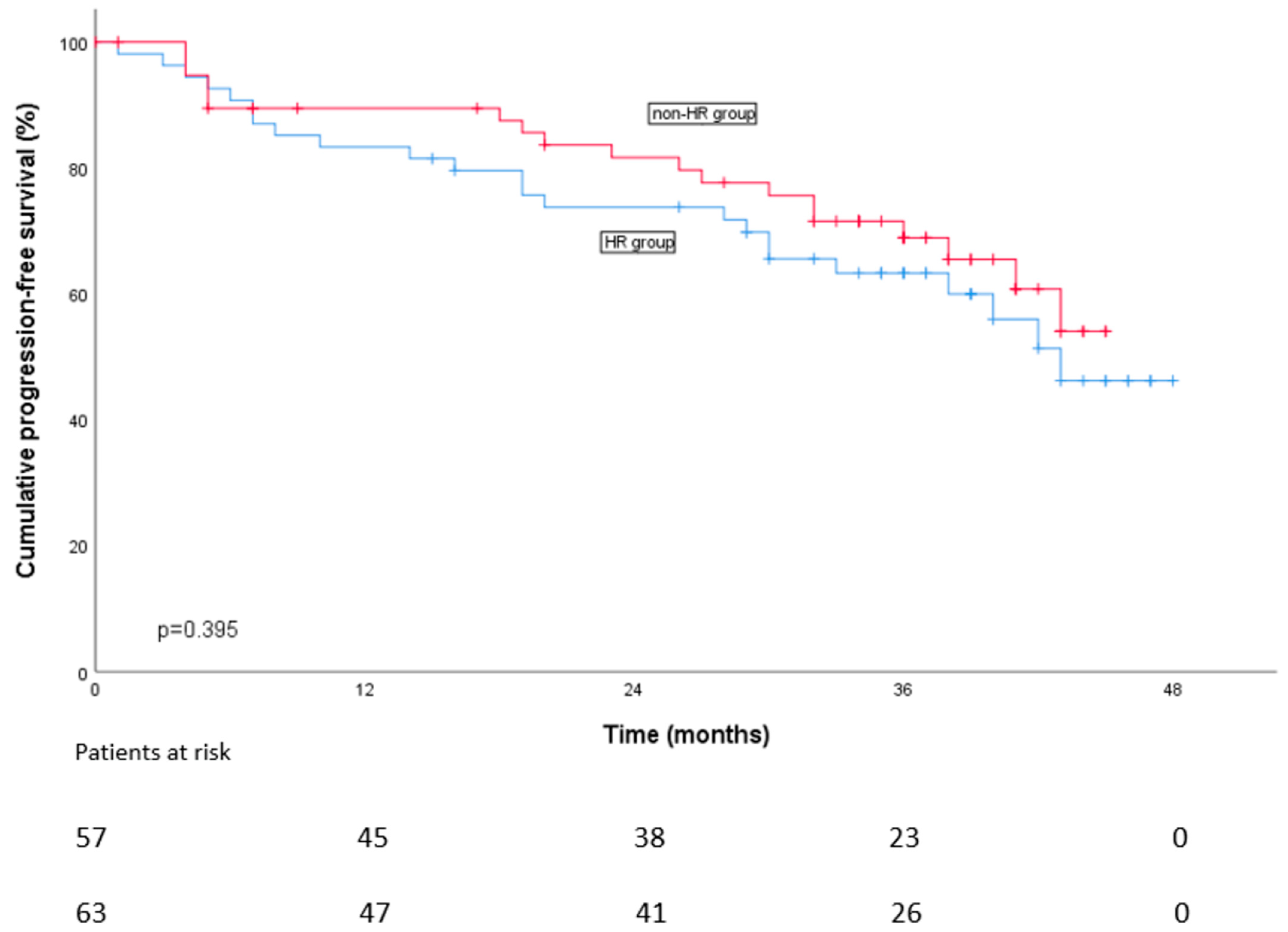
3.2. Progression-Free Survival
3.3. Overall Survival
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirley, M. Ixazomib: First Global Approval. Drugs 2016, 76, 405–411. [Google Scholar] [CrossRef]
- Ninlaro | European Medicines Agency. Available online: https://european-union.europa.eu/index_en (accessed on 7 December 2016).
- Touzeau, C.; Perrot, A.; Roussel, M.; Karlin, L.; Benboubker, L.; Jacquet, C.; Mohty, M.; Facon, T.; Manier, S.; Chretien, M.L.; et al. All-oral triplet combination of ixazomib, lenalidomide, and dexamethasone in newly diagnosed transplant-eligible multiple myeloma patients: Final results of the phase II IFM 2013-06 study. Haematologica 2022, 107, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Rosinol, L.; Oriol, A.; Tamayo, R.; Blanchard, M.; Jarque, I.; Bargay, J.; Hernández, M.-T.; Moraleda, J.; Carrillo-Cruz, E.; Sureda, A.; et al. Ixazomib Plus Lenalidomide/Dexamethasone (IRD) Versus Lenalidomide /Dexamethasone (Rd) Maintenance after Autologous Stem Cell Transplant in Patients with Newly Diagnosed Multiple Myeloma: Results of the Spanish GEM2014MAIN Trial. Blood 2021, 138 (Suppl. S1), 466. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef]
- Slade, M.; Martin, T.G.; Nathwani, N.; Fiala, M.A.; Rettig, M.P.; Gao, F.; Deol, A.; Buadi, F.K.; Kaufman, J.L.; Hofmeister, C.C.; et al. Ixazomib, lenalidomide and dexamethasone consolidation with randomized ixazomib or lenalidomide maintenance after autologous transplant in newly diagnosed multiple myeloma. Leukemia 2022, 36, 2917–2921. [Google Scholar] [CrossRef]
- Paiva, B.; Manrique, I.; Dimopoulos, M.A.; Gay, F.; Min, C.K.; Zweegman, S.; Špička, I.; Teipel, R.; Mateos, M.V.; Giuliani, N.; et al. MRD dynamics during maintenance for improved prognostication of 1280 myeloma patients in TOURMALINE-MM3 and -MM4 trials. Blood 2023, 141, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, R.; Lundan, T.; Kairisto, V.; Pelliniemi, T.T.; Putkonen, M.; Anttila, P.; Huotari, V.; Mäntymaa, P.; Siitonen, S.; Uotila, L.; et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood Cancer J. 2014, 4, e250. [Google Scholar] [CrossRef]
- Medina, A.; Puig, N.; Flores-Montero, J.; Jimenez, C.; Sarasquete, M.E.; Garcia-Alvarez, M.; Prieto-Conde, I.; Chillon, C.; Alcoceba, M.; Gutierrez, N.C.; et al. Comparison of next-generation sequencing (NGS) and next-generation flow (NGF) for minimal residual disease (MRD) assessment in multiple myeloma. Blood Cancer J. 2020, 10, 108. [Google Scholar] [CrossRef]
- Diamond, B.T.; Rustad, E.; Maclachlan, K.; Thoren, K.; Ho, C.; Roshal, M.; Ulaner, G.A.; Landgren, C.O. Defining the undetectable: The current landscape of minimal residual disease assessment in multiple myeloma and goals for future clarity. Blood Rev. 2021, 46, 100732. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Xu, J.; Lin, Z.; Huang, J.; Wang, F.; Yang, Y.; Cui, Y.; Luo, H.; Gao, Y.; Zhai, X.; et al. Minimal residual disease in multiple myeloma: Current status. Biomark. Res. 2021, 9, 75. [Google Scholar] [CrossRef]
- Langerhorst, P.; Noori, S.; Zajec, M.; De Rijke, Y.B.; Gloerich, J.; van Gool, A.J.; Caillon, H.; Joosten, I.; Luider, T.M.; Corre, J.; et al. Multiple Myeloma Minimal Residual Disease Detection: Targeted Mass Spectrometry in Blood vs Next-Generation Sequencing in Bone Marrow. Clin. Chem. 2021, 67, 1689–1698. [Google Scholar] [CrossRef]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- San-Miguel, J.; Avet-Loiseau, H.; Paiva, B.; Kumar, S.; Dimopoulos, M.A.; Facon, T.; Mateos, M.V.; Touzeau, C.; Jakubowiak, A.; Usmani, S.Z.; et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 2022, 139, 492–501. [Google Scholar] [CrossRef]
- Cavo, M.; San-Miguel, J.; Usmani, S.Z.; Weisel, K.; Dimopoulos, M.A.; Avet-Loiseau, H.; Paiva, B.; Bahlis, N.J.; Plesner, T.; Hungria, V.; et al. Prognostic value of minimal residual disease negativity in myeloma: Combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood 2022, 139, 835–844. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.J.; Vidriales, M.B.; García-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Costa, L.J.; Derman, B.A.; Bal, S.; Sidana, S.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia 2021, 35, 18–30. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Rack, K.A.; van den Berg, E.; Haferlach, C.; Beverloo, H.B.; Costa, D.; Espinet, B.; Foot, N.; Jeffries, S.; Martin, K.; O’Connor, S.; et al. European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia 2019, 33, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutiérrez, N.C.; Liebisch, P.; O’Connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. European Myeloma Network. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef]
- Stetler-Stevenson, M.; Paiva, B.; Stoolman, L.; Lin, P.; Jorgensen, J.L.; Orfao, A.; Van Dongen, J.; Rawstron, A.C. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytom. B. Clin. Cytom. 2016, 90, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.; Anttila, P.; Säily, M.; Lundan, T.; Heiskanen, J.; Siitonen, T.; Kakko, S.; Putkonen, M.; Ollikainen, H.; Terävä, V.; et al. RVD induction and autologous stem cell transplantation followed by lenalidomide maintenance in newly diagnosed multiple myeloma: A phase 2 study of the Finnish Myeloma Group. Ann. Hematol. 2019, 98, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Lauwers-Cances, V.; Wuilleme, S.; Belhadj, K.; Manier, S.; Garderet, L.; Escoffre-Barbe, M.; Mariette, C.; Benboubker, L.; Caillot, D.; et al. Up-front carfilzomib, lenalidomide, and dexamethasone with transplant for patients with multiple myeloma: The IFM KRd final results. Blood 2021, 138, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Kumar, S.; Fraser, R.; Estrada-Merly, N.; Giralt, S.; Agrawal, V.; Anderson, L.D., Jr.; Aljurf, M.; Banerjee, R.; Bashey, A.; et al. Impact of Induction Therapy with VRD versus VCD on Outcomes in Patients with Multiple Myeloma in Partial Response or Better Undergoing Upfront Autologous Stem Cell Transplantation. Transpl. Cell 2022, 28, 83.e1–83.e9. [Google Scholar] [CrossRef]
- Kumar, S.; Flinn, I.; Richardson, P.G.; Hari, P.; Callander, N.; Noga, S.J.; Stewart, A.K.; Turturro, F.; Rifkin, R.; Wolf, J.; et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012, 119, 4375–4382. [Google Scholar] [CrossRef]
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Jevremovic, D.; Buad, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; et al. Impact of Post-Transplant Response and Minimal Residual Disease on Survival in Myeloma with High-Risk Cytogenetics. Biol. Blood Marrow Transpl. 2017, 23, 598–605. [Google Scholar] [CrossRef]
- Uttervall, K.; Borg Bruchfeld, J.; Gran, C.; Wålinder, G.; Månsson, R.; Lund, J.; Gahrton, G.; Alici, E.; Nahi, H. Upfront bortezomib, lenalidomide, and dexamethasone compared to bortezomib, cyclophosphamide, and dexamethasone in multiple myeloma. Eur. J. Haematol. 2019, 103, 247–254. [Google Scholar] [CrossRef]
- Menon, T.; Kataria, S.; Adhikari, R.; Khan, H.; Khalid, M.Z.; Saeeduddin, M.O.; Taj, S.; Rehman, U.; Tekin, A.; Singh, R. Efficacy of Daratumumab-Based Regimens Compared to Standard of Care in Transplant-Eligible Multiple Myeloma: A Meta-Analysis. Cureus 2021, 13, e15098. [Google Scholar] [CrossRef]
- Groen, K.; Stege, C.A.M.; Nasserinejad, K.; de Heer, K.; van Kampen, R.J.W.; Leys, R.B.L.; Thielen, N.; Westerman, M.; Wu, K.L.; Ludwig, I.; et al. Ixazomib, daratumumab and low-dose dexamethasone in intermediate-fit patients with newly diagnosed multiple myeloma: An open-label phase 2 trial. EClinicalMedicine 2023, 63, 102167. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Hofmeister, C.C.; Rosenbaum, C.A.; Htut, M.; Vesole, D.H.; Berdeja, J.G.; Liedtke, M.; Chari, A.; Smith, S.D.; Lebovic, D.; et al. Twice-weekly ixazomib in combination with lenalidomide-dexamethasone in patients with newly diagnosed multiple myeloma. Br. J. Haematol. 2018, 182, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Rosiñol, L.; Oriol, A.; Ríos, R.; Blanchard, M.J.; Jarque, I.; Bargay, J.; Hernández, M.T.; Cabañas, V.; Carrillo-Cruz, E.; Sureda, A.; et al. Lenalidomide and dexamethasone maintenance with or without ixazomib, tailored by residual disease status in myeloma. Blood 2023, 142, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.F.; D’Souza, A.; Kassim, A.A.; Costa, L.J.; Innis-Shelton, R.D.; Zhang, M.J.; Huang, J.; Abidi, M.; Aiello, J.; Akpek, G.; et al. Maintenance versus Induction Therapy Choice on Outcomes after Autologous Transplantation for Multiple Myeloma. Biol. Blood Marrow Transpl. 2017, 23, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.; Davies, F.; Pawlyn, C.; Cairns, D.; Striha, A.; Collett, C.; Waterhouse, A.; Jones, J.; Kishore, B.; Garg, M.; et al. Lenalidomide Is a Highly Effective Maintenance Therapy in Myeloma Patients of All Ages; Results of the Phase III Myeloma XI Study. Blood 2016, 128, 1143. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208462s012s013lbl.pdf (accessed on 1 April 2022).
- Patel, K.K.; Shah, J.J.; Feng, L.; Lee, H.C.; Manasanch, E.M.; Olsem, J.; Morphey, A.; Huo, X.J.; Thomas, S.K.; Bashir, Q.; et al. Safety and Efficacy of Combination Maintenance Therapy with Ixazomib and Lenalidomide in Patients with Posttransplant Myeloma. Clin. Cancer Res. 2022, 28, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Moreau, P.; Hari, P.; Mateos, M.V.; Ludwig, H.; Shustik, C.; Masszi, T.; Spencer, A.; Hájek, R.; Romeril, K.; et al. Management of adverse events associated with ixazomib plus lenalidomide/dexamethasone in relapsed/refractory multiple myeloma. Br. J. Haematol. 2017, 178, 571–582. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Gregory, W.M.; de Tute, R.M.; Davies, F.E.; Bell, S.E.; Drayson, M.T.; Cook, G.; Jackson, G.H.; Morgan, G.J.; Child, J.A.; et al. Minimal residual disease in myeloma by flow cytometry: Independent prediction of survival benefit per log reduction. Blood 2015, 125, 1932–1935. [Google Scholar] [CrossRef]
- Paiva, B.; Puig, N.; Cedena, M.T.; Rosiñol, L.; Cordón, L.; Vidriales, M.B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. Measurable Residual Disease by Next-Generation Flow Cytometry in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 784–792. [Google Scholar] [CrossRef]

| Variable | HR Group n = 57 (%) | NHR Group n = 63 (%) | Significance p |
|---|---|---|---|
| Age | 63 (41–70) | 59 (40–70) | 0.089 |
| Gender | 0.228 | ||
| Female | 27 (47) | 23 (37) | |
| Male | 30 (53) | 40 (63) | |
| ECOG | 0.103 | ||
| 0 | 33 (58) | 34 (54) | |
| 1 | 17 (30) | 27 (43) | |
| 2 | 7 (12) | 2 (3) | |
| Heavy chain type † | 0.432 | ||
| IgG | 32 (56) | 43 (68) | |
| IgA | 14 (25) | 12 (19) | |
| IgD | 1(2) | ||
| Light chain type | 0.019 | ||
| Kappa | 30 (53) | 47 (75) | |
| Lambda | 27 (47) | 16 (25) | |
| IMWG risk group * | <0.001 | ||
| low | 1 (2) | 18 (27) | |
| standard | 42 (74) | 39 (62) | |
| high | 14 (24) | 2 (3) | |
| R-ISS | <0.001 | ||
| 1 | 9 (16) | 31 (49) | |
| 2 | 42 (74) | 26 (42) | |
| 3 | 6 (10) | 2 (3) | |
| FISH findings ‡ | |||
| del 13q/-13 | 16 (28) | 7 (11) | |
| del 17p | 10 (18) | 3 (5) | |
| +1q | 42 (74) | ||
| t(4;14) | 14 (25) | ||
| t(11;14) | 10 (18) | 11 (18) | |
| t(14;16) | 4 (7) | ||
| t(6;14) | 1 (2) | ||
| t(14;20) | 3 (5) | ||
| Treatment indication | 0.055 | ||
| CRAB criteria positive | 51 (89) | 62 (98) | |
| Biomarker-based | 6 (11) | 1 (2) |
| Post-Induction | Post-ASCT | After Consolidation | 1 Year on Maintenance | 2 Years on Maintenance | |
|---|---|---|---|---|---|
| n = 120 (%) | n = 120 (%) | n = 120 (%) | n= 120 (%) | n = 120 (%) | |
| sCR | 2(2) | 6 (5) | 11 (9) | 18 (15) | 22 (18) |
| CR | 6 (5) | 18 (15) | 21 (18) | 28 (23) | 20 (17) |
| VGPR | 34 (28) | 34 (28) | 38 (32) | 24 (20) | 17 (14) |
| PR | 59 (49) | 38 (32) | 21 (18) | 11 (9) | 7 (6) |
| SD | 7 (6) | ||||
| PD | 9 (8) | 3 (3) | 4 (3) | 7 (6) | 12 (10) |
| Out other cause † | 3 (3) | 2 (2) | 3 (3) | 1 (1) | |
| Cumulative out | 19 (16) | 24 (20) | 29 (24) | 40 (33) | |
| Death | 1 (1) | 1 (1) |
| AE | Both Groups n = 120 | HR Group n = 57 | NHR Group n = 63 | |||||
|---|---|---|---|---|---|---|---|---|
| Grade ≥ 3 during Ind n (%) | Grade ≥ 3 during Cons n (%) | Grade 1 during Maint n (%) | Grade2 during Maint n (%) | Grade 3 during Maint n (%) | Grade 1 during Maint n (%) | Grade 2 during Maint n (%) | Grade 3 during Maint n (%) | |
| HEMATOLOGIC | ||||||||
| Anemia | 2 (2) | 3 (5) | 1 (2) | 2 (3) | 1 (2) | |||
| Neutropenia | 10 (8) | 9 (8) | 1 (2) | 15 (26) | 20 (32) | |||
| Thrombopenia | 2 (1) | 1 (2) | 4 (7) | 4 (7) | 1 (2) | |||
| NON-HEMATOLOGIC | ||||||||
| Fever | 10 (8) | 2 (2) | 2 (4) | 2 (3) | ||||
| Pneumonia | 5 (4) | 4 (7) | 6 (10) | |||||
| Septicemia | 3 (3) | |||||||
| Upper respiratory tract infection | 3 (3) | 3 (5) | 5 (9) | 1 (2) | 4 (6) | 4 (6) | 1 (2) | |
| Covid19 | 1 (2) | 1 (2) | 1 (2) | 1 (2) | 2 (3) | |||
| Gastrointestinal disorders | 1 (1) | 1 (1) | 3 (5) | 3 (5) | 1 (2) | 1 (2) | 5 (8) | |
| Nausea/fatigue | 2 (2) | 5 (9) | 1 (2) | 1 (2) | 1 (2) | |||
| Elevated ALT | 3 (3) | 3 (5) | 2 (4) | 2 (4) | 4 (6) | 1 (2) | ||
| Acute renal failure | 3 (3) | 1 (1) | ||||||
| Skin reactions | 8 (7) | 1 (1) | 2 (4) | 2 (4) | 2 (3) | |||
| Deep venous thrombosis | 1 (2) | 3 (5) | ||||||
| Pulmonary embolism | 1 (1) | 1 (1) | 1 (2) | |||||
| Arterial thrombosis eye | 1 (2) | |||||||
| Peripheral neuropathy sensory | 2 (2) | 1 (1) | 4 (7) | 3 (5) | 1 (2) | 1 (2) | 1 (2) | 2 (3) |
| Peripheral neuropathy motor | 1 (1) | 2 (4) | 2 (4) | 1 (2) | ||||
| Cardiac/arrythmia | 1 (1) | 1 (2) | ||||||
| Muscle pain | 1 (1) | 1 (2) | 2 (4) | 1 (2) | 1 (2) | 1 (2) | ||
| SPMs | 2 (4) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partanen, A.; Waage, A.; Peceliunas, V.; Schjesvold, F.; Anttila, P.; Säily, M.; Uttervall, K.; Putkonen, M.; Carlson, K.; Haukas, E.; et al. Ixazomib, Lenalidomide, and Dexamethasone (IRD) Treatment with Cytogenetic Risk-Based Maintenance in Transplant-Eligible Myeloma: A Phase 2 Multicenter Study by the Nordic Myeloma Study Group. Cancers 2024, 16, 1024. https://doi.org/10.3390/cancers16051024
Partanen A, Waage A, Peceliunas V, Schjesvold F, Anttila P, Säily M, Uttervall K, Putkonen M, Carlson K, Haukas E, et al. Ixazomib, Lenalidomide, and Dexamethasone (IRD) Treatment with Cytogenetic Risk-Based Maintenance in Transplant-Eligible Myeloma: A Phase 2 Multicenter Study by the Nordic Myeloma Study Group. Cancers. 2024; 16(5):1024. https://doi.org/10.3390/cancers16051024
Chicago/Turabian StylePartanen, Anu, Anders Waage, Valdas Peceliunas, Fredrik Schjesvold, Pekka Anttila, Marjaana Säily, Katarina Uttervall, Mervi Putkonen, Kristina Carlson, Einar Haukas, and et al. 2024. "Ixazomib, Lenalidomide, and Dexamethasone (IRD) Treatment with Cytogenetic Risk-Based Maintenance in Transplant-Eligible Myeloma: A Phase 2 Multicenter Study by the Nordic Myeloma Study Group" Cancers 16, no. 5: 1024. https://doi.org/10.3390/cancers16051024
APA StylePartanen, A., Waage, A., Peceliunas, V., Schjesvold, F., Anttila, P., Säily, M., Uttervall, K., Putkonen, M., Carlson, K., Haukas, E., Sankelo, M., Szatkowski, D., Hansson, M., Marttila, A., Svensson, R., Axelsson, P., Lauri, B., Mikkola, M., Karlsson, C., ... Silvennoinen, R. (2024). Ixazomib, Lenalidomide, and Dexamethasone (IRD) Treatment with Cytogenetic Risk-Based Maintenance in Transplant-Eligible Myeloma: A Phase 2 Multicenter Study by the Nordic Myeloma Study Group. Cancers, 16(5), 1024. https://doi.org/10.3390/cancers16051024








