FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
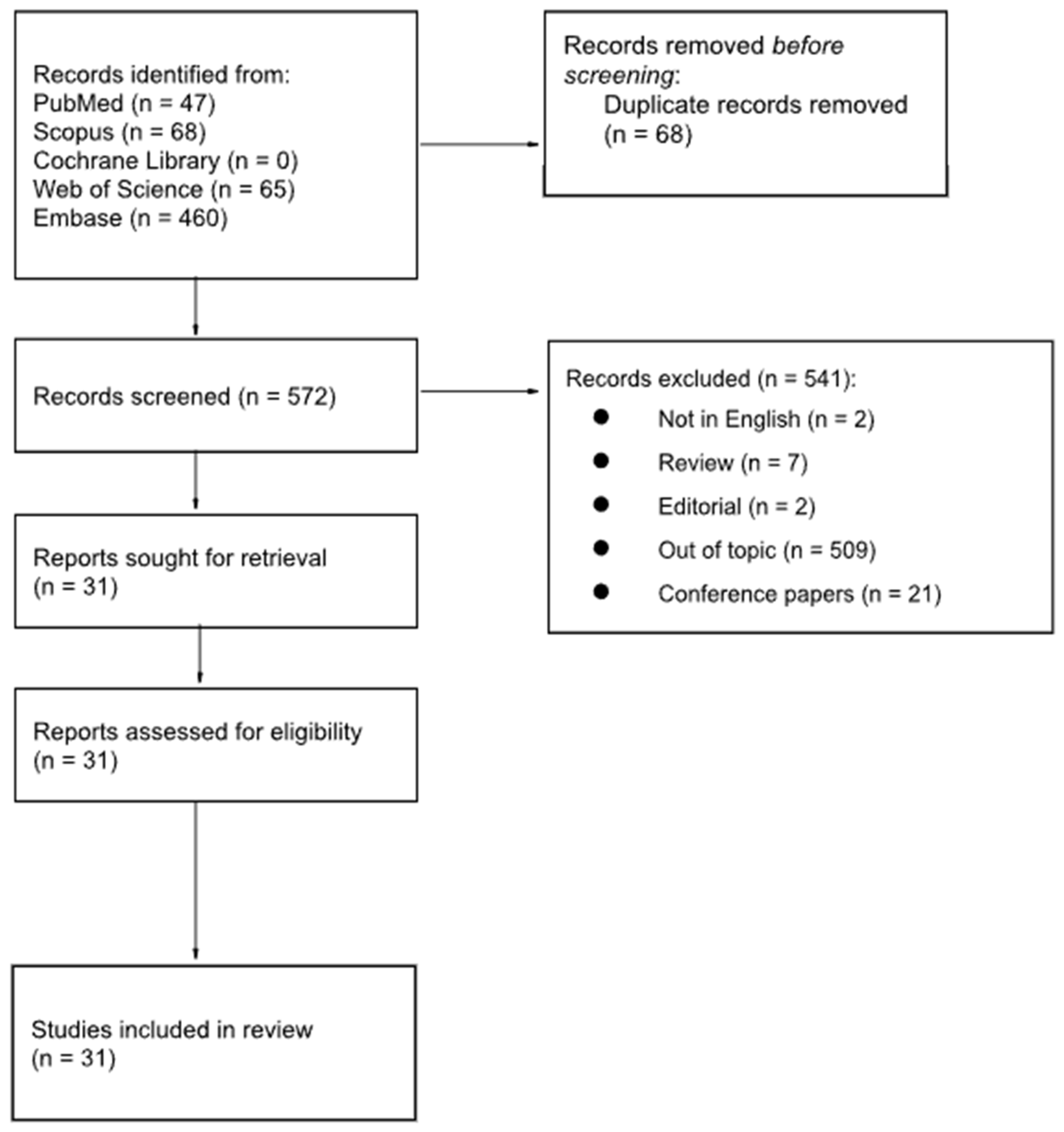
2. Materials and Methods
3. Results
3.1. Thyroid Cancer
| Authors, Ref. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was There a Clear Question for the Study to Address? | Was There a Comparison with an Appropriate Reference Standard? | Did All Patients Get the Diagnostic Test and Reference Standard? | Could the Results of the Test Have Been Influenced by the Results of the Reference Standard? | Is the Disease Status of the Tested Population Clearly Described? | Were the Methods for Performing the Test Described in Sufficient Detail? | What Are the Results? | How Sure Are We about the Results? Consequences and Cost of Alternatives Performed? | Can the Results be Applied to Your Patients/the Population of Interest? | Can the Test be Applied to Your Patient or Population of Interest? | Were All Outcomes Important to the Individual or Population Considered? | What Would be the Impact of Using This Test on Your Patients/Population? | |
| Fu et al. [34] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Sensitivity and specificity | Data are clear | ☺ | ☺ | ☺ | More experience is needed |
| Mu et al. [35] | ☺ | ☹ | ? | ? | ? | ☺ | Detection rate | Not clear | ? | ? | ? | No information about the standard of reference |
| Sayiner et al. [36] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Detection rate | Not clear | ☺ | ☺ | ☺ | Limited data in small population |
| Chen et al. [37] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Descriptive analysis | Not clear | ? | ? | ? | Limited data |
| Ballal et al. [38] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Detection rate and semiquantitative data | Not clear | ☺ | ☺ | ? | The effect on the management is unclear |
| Ballal et al. [52] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Detection rate and semiquantitative data | Not clear | ? | ? | ? | Limited data |
3.2. Theranostics
3.3. Pitfalls
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular Cell-Derived Thyroid Cancer. Nat. Rev. Dis. Primers 2015, 1, 15077. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, R.; Brunaud, L.; Kanauchi, H.; Wong, M.; Fung, M.; Ginzinger, D.; Duh, Q.-Y.; Clark, O.H. Increasing the Effectiveness of Radioactive Iodine Therapy in the Treatment of Thyroid Cancer Using Trichostatin A, a Histone Deacetylase Inhibitor. Surgery 2002, 132, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Haugen, B.R.; Schlumberger, M. Progress in Molecular-Based Management of Differentiated Thyroid Cancer. Lancet 2013, 381, 1058–1069. [Google Scholar] [CrossRef]
- Worden, F. Treatment Strategies for Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Ther. Adv. Med. Oncol. 2014, 6, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Nixon, I.J.; Whitcher, M.M.; Palmer, F.L.; Tuttle, R.M.; Shaha, A.R.; Shah, J.P.; Patel, S.G.; Ganly, I. The Impact of Distant Metastases at Presentation on Prognosis in Patients with Differentiated Carcinoma of the Thyroid Gland. Thyroid 2012, 22, 884–889. [Google Scholar] [CrossRef]
- Shinohara, S.; Kikuchi, M.; Suehiro, A.; Kishimoto, I.; Harada, H.; Hino, M.; Ishihara, T. Characteristics and Prognosis of Patients with Thyroglobulin-Positive and Radioactive Iodine Whole-Body Scan-Negative Differentiated Thyroid Carcinoma. JPN J. Clin. Oncol. 2015, 45, 427–432. [Google Scholar] [CrossRef][Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Manohar, P.M.; Beesley, L.J.; Bellile, E.L.; Worden, F.P.; Avram, A.M. Prognostic Value of FDG-PET/CT Metabolic Parameters in Metastatic Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Yildirim Poyraz, N.; Polat, S.B.; Turkolmez, S.; Ersoy, R.; Cakir, B. Diagnostic Value of 18F-FDG PET/CT in Patients with TENIS Syndrome: Correlation with Thyroglobulin Levels. Ann. Nucl. Med. 2014, 28, 241–247. [Google Scholar] [CrossRef]
- Ozkan, E.; Aras, G.; Kucuk, N.O. Correlation of 18F-FDG PET/CT Findings With Histopathological Results in Differentiated Thyroid Cancer Patients Who Have Increased Thyroglobulin or Antithyroglobulin Antibody Levels and Negative 131I Whole-Body Scan Results. Clin. Nucl. Med. 2013, 38, 326–331. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal. Similarities between Tumor Stroma Generation and Wound Healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Fozzatti, L.; Cheng, S. Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol. Metab. 2020, 35, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68 Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Lasek, W. Cancer Immunoediting Hypothesis: History, Clinical Implications and Controversies. Cent. Eur. J. Immunol. 2022, 47, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, L.; Airò Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Salè, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-Associated Fibroblasts: An Emerging Target of Anti-Cancer Immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Zarrad, F.; Nader, M.; Kersting, D.; Weber, M.; Kazek, S.; Sraieb, M.; Hamacher, R.; et al. Pitfalls and Common Findings in 68Ga-FAPI PET: A Pictorial Analysis. J. Nucl. Med. 2022, 63, 890–896. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding Fibroblast Activation Protein (FAP): Substrates, Activities, Expression and Targeting for Cancer Therapy. Proteomics Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Yazbeck, R.; Jaenisch, S.E.; Abbott, C.A. Potential Disease Biomarkers: Dipeptidyl Peptidase 4 and Fibroblast Activation Protein. Protoplasma 2018, 255, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Tillmanns, J.; Hoffmann, D.; Habbaba, Y.; Schmitto, J.D.; Sedding, D.; Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Fibroblast Activation Protein Alpha Expression Identifies Activated Fibroblasts after Myocardial Infarction. J. Mol. Cell Cardiol. 2015, 87, 194–203. [Google Scholar] [CrossRef]
- Backhaus, P.; Gierse, F.; Burg, M.C.; Büther, F.; Asmus, I.; Dorten, P.; Cufe, J.; Roll, W.; Neri, D.; Cazzamalli, S.; et al. Translational Imaging of the Fibroblast Activation Protein (FAP) Using the New Ligand [68Ga]Ga-OncoFAP-DOTAGA. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Weiner, L.M. The Role of Fibroblast Activation Protein in Health and Malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bennett, S.; Del Mar, C. Evidence-Based Practice across the Health Professions; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Moore, S.A.; Cumming, S.P.; Balletta, G.; Ramage, K.; Eisenmann, J.C.; Baxter-Jones, A.D.; Sherar, L.B. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Fu, H.; Wu, J.; Huang, J.; Sun, L.; Wu, H.; Guo, W.; Qiu, S.; Chen, H. 68 Ga Fibroblast Activation Protein Inhibitor PET/CT in the Detection of Metastatic Thyroid Cancer: Comparison with 18 F-FDG PET/CT. Radiology 2022, 304, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Huang, X.; Jiang, Z.; Li, M.; Jia, L.; Lv, Z.; Fu, W.; Mao, J. [18F]FAPI-42 PET/CT in Differentiated Thyroid Cancer: Diagnostic Performance, Uptake Values, and Comparison with 2-[18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, Z.A.; Elboğa, U.; Sahin, E.; Ozturk, S.; Cayirli, Y.B.; Celen, Y.Z.; Cakici, D. Comparison of 68Ga-FAPI-04 and 18F-FDG PET/CT for Diagnosis of Metastatic Lesions in Patients with Recurrent Papillary Thyroid Carcinoma. Hell J. Nucl. Med. 2023, 26, 41–46. [Google Scholar] [PubMed]
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. 68Ga-DOTA-FAPI-04 PET/CT Imaging in Radioiodine-Refractory Differentiated Thyroid Cancer (RR-DTC) Patients. Ann. Nucl. Med. 2022, 36, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Roesch, F.; Satapathy, S.; Moon, E.S.; Martin, M.; Wakade, N.; Sheokand, P.; Tripathi, M.; Chandekar, K.R.; et al. Head-to-Head Comparison of [68Ga]Ga-DOTA.SA.FAPi with [18F]F-FDG PET/CT in Radioiodine-Resistant Follicular-Cell Derived Thyroid Cancers. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Fu, J.; Huang, J.; Pang, Y.; Chen, H. 68Ga-FAPI PET/CT Versus 18F-FDG PET/CT for Detecting Metastatic Lesions in a Case of Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2021, 46, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ou, L.; Zhang, C. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in Metastases of Papillary Thyroid Carcinoma. Endocrine 2021, 73, 767–768. [Google Scholar] [CrossRef]
- Aghaee, A.; Barashki, S.; Jafarian, A.H.; Zakavi, S.R.; Kamali, H.; Norouzbeigi, N.; Aryana, K. Detection of Osseous Metastasis by [68Ga]Ga-FAPI PET/CT in a Thyroid Cancer Patient with Elevated Thyroglobulin and Negative Radioiodine Scintigraphy. Iran. J. Nucl. Med. 2023, 31, 109–111. [Google Scholar] [CrossRef]
- Fu, H.; Fu, J.; Huang, J.; Su, X.; Chen, H. 68Ga-FAPI PET/CT in Thyroid Cancer With Thyroglobulin Elevation and Negative Iodine Scintigraphy. Clin. Nucl. Med. 2021, 46, 427–430. [Google Scholar] [CrossRef]
- Tatar, G.; Alçın, G.; Erol Fenercioğlu, Ö.; Şahin, R.; Çermik, T.F. Findings of I-131 SPECT/CT, 18F-FDG, and 68Ga-FAPI-04 PET/CT Imaging in a Patient Treated with Radioiodine Therapy for Metastatic Papillary Thyroid Carcinoma. Mol. Imaging Radionucl. Ther. 2023, 32, 57–61. [Google Scholar] [CrossRef]
- Nourbakhsh, S.; Salehi, Y.; Farzanehfar, S.; Ghaletaki, R.; Bakhshi Kashi, M.; Abbasi, M. FAPI PET/CT Provides Higher Uptake and Better Target to Back Ground in Recurrent and Metastatic Tumors of Patients with Iodine Refractory Papillary Thyroid Cancer Compared with FDG PET CT. Nuklearmedizin-NuclearMedicine 2024. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. Pleural Metastasis of Papillary Thyroid Cancer Depicted by 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2022, 47, 467–468. [Google Scholar] [CrossRef]
- Santangelo, G.; Pellino, G.; De Falco, N.; Colella, G.; D’Amato, S.; Maglione, M.G.; De Luca, R.; Canonico, S.; De Falco, M. Prevalence, Diagnosis and Management of Ectopic Thyroid Glands. Int. J. Surg. 2016, 28, S1–S6. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, L.; Fei, M.; Liu, J.; Wang, Z. 68Ga-FAPI–Avid Submental Ectopic Papillary Thyroid Carcinoma and Lateral Neck Lymphadenopathy With Low 18F-FDG Uptake. Clin. Nucl. Med. 2023, 48, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Kushchayev, S.V.; Kushchayeva, Y.S.; Tella, S.H.; Glushko, T.; Pacak, K.; Teytelboym, O.M. Medullary Thyroid Carcinoma: An Update on Imaging. J. Thyroid Res. 2019, 2019, 1893047. [Google Scholar] [CrossRef] [PubMed]
- Pajak, C.; Cadili, L.; Nabata, K.; Wiseman, S.M. 68Ga-DOTATATE-PET Shows Promise for Diagnosis of Recurrent or Persistent Medullary Thyroid Cancer: A Systematic Review. Am. J. Surg. 2022, 224, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibraheem, A.; Alyasjeen, S.F.; Abdlkadir, A.S.; Sheikha, A.A. [68Ga]Ga-DOTA-FAPI-04 PET/CT Depicts Metastases from Medullary Thyroid Cancer That [68Ga]Ga-DOTATOC PET/CT Missed. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 4112–4113. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, S.; Işık, E.G.; Sanli, Y. Liver Metastases from Medullary Thyroid Carcinoma Detected on 68Ga-FAPI-04 PET/CT. Endocrine 2021, 74, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Roesch, F.; Raju, S.; Satapathy, S.; Sheokand, P.; Moon, E.S.; Martin, M.; Awarwal, S.; Tripathi, M.; et al. Head-to-Head Comparison of [68Ga]Ga-DOTA.SA.FAPi and [68Ga]Ga-DOTANOC Positron Emission Tomography/Computed Tomography Imaging for the Follow-Up Surveillance of Patients with Medullary Thyroid Cancer. Thyroid 2023, 33, 974–982. [Google Scholar] [CrossRef]
- Yang, X.; Gong, W.; Chen, Y. 68Ga-FAPI PET/CT Imaging in a Patient with Primary Thyroid Lymphoma. Endocrine 2021, 73, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Ora, M.; Soni, N.; Nazar, A.H.; Dixit, M.; Singh, R.; Puri, S.; Graham, M.M.; Gambhir, S. Fibroblast Activation Protein Inhibitor–Based Radionuclide Therapies: Current Status and Future Directions. J. Nucl. Med. 2023, 64, 1001–1008. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2021, 32, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Huang, J.; Sun, L.; Wu, H.; Chen, H. FAP-Targeted Radionuclide Therapy of Advanced Radioiodine-Refractory Differentiated Thyroid Cancer With Multiple Cycles of 177Lu-FAPI-46. Clin. Nucl. Med. 2022, 47, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; De Loose, J.; Verhulst, E.; De Meester, I.; Van Der Veken, P.; Roesch, F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers 2023, 15, 1889. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Rösch, F.; ArunRaj, S.T.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Bal, C. First-in-Human Experience With 177Lu-DOTAGA.(SA.FAPi)2 Therapy in an Uncommon Case of Aggressive Medullary Thyroid Carcinoma Clinically Mimicking as Anaplastic Thyroid Cancer. Clin. Nucl. Med. 2022, 47, e444–e445. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Zhao, T.; Wang, H.; Chen, Y.; Xu, W.; Pang, Y.; Guo, W.; Sun, L.; Wu, H.; et al. Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study. Clin. Cancer Res. 2023, 29, 4740–4750. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Götz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Disentangling Inflammatory from Fibrotic Disease Activity by Fibroblast Activation Protein Imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491. [Google Scholar] [CrossRef]
- Hotta, M.; Sonni, I.; Benz, M.R.; Gafita, A.; Bahri, S.; Shuch, B.M.; Yu, R.; Liu, S.T.; Czernin, J.; Calais, J. 68Ga-FAPI-46 and 18F-FDG PET/CT in a Patient with Immune-Related Thyroiditis Induced by Immune Checkpoint Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3736–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, J.; Chen, Y. 68Ga-FAPI PET/CT Imaging in a Patient with Thyroiditis. Endocrine 2021, 73, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, L.; Lei, L.; Wang, L.; Chen, Y. Clinical Significance of Diffusely Increased Uptake of 68Ga-FAPI in Thyroid Gland. Front. Med. 2021, 8, 782231. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wu, J.; Wu, J.; Mou, C.; Zhang, C. Follicular Thyroid Adenoma Showing Avid Uptake on 68Ga-DOTA-FAPI-04 PET/CT. Clin. Nucl. Med. 2021, 46, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Can, C.; Gündoğan, C.; Güzel, Y.; Kaplan, İ.; Kömek, H. 68Ga-FAPI Uptake of Thyroiditis in a Patient With Breast Cancer. Clin. Nucl. Med. 2021, 46, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Wu, J.; Zhang, C. A Case of Papillary Thyroid Carcinoma with Graves Ophthalmopathy Evaluated by 68Ga-FAPI PET/CT. Endocrine 2022, 76, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wu, J.; Yang, F.; Zhang, C. Comparison of 68 Ga-FAPI and 18 F-FDG PET/CT Inmetastasis of Thyroid Papillary Carcinoma. Hell J. Nucl. Med. 2021, 24, 100–101. [Google Scholar]
- Wu, J.; Wang, Y.; Liao, T.; Rao, Z.; Gong, W.; Ou, L.; Chen, Y.; Zhang, C. Comparison of the Relative Diagnostic Performance of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the Detection of Bone Metastasis in Patients With Different Cancers. Front. Oncol. 2021, 11, 737827. [Google Scholar] [CrossRef]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C.; et al. Imaging Fibroblast Activation Protein in Liver Cancer: A Single-Center Post Hoc Retrospective Analysis to Compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in Patients Presenting with Inconclusive [18F]FDG PET/CT Findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef] [PubMed]
| FAPi Agents | FAPi Tracer Advantages | Histotype | Type of Publication | Limitations |
|---|---|---|---|---|
| [68Ga]FAPi * | Higher detection sensitivity in the liver, bones, and abdominal lymph nodes [40]; capable of localizing an abnormal foci of the uptake at the laryngeal mass, pulmonary nodules, the small nodule adjacent to the pulmonary hilum, which were previously ignored even in a non-contrast CT scan and an iodine scan [42]. | PTC | Interesting image | [68Ga]Ga-FAPi uptake in mDTC lesions is not clearly associated with Tg levels; false positive uptake of [68Ga]Ga-FAPi in myelofibrosis; reactive LNs; arthritis; subcutaneous fibroma [34,42]; thyroiditis [63]; pancreatitis; tuberculous lesions [53]; and low uptake in MTCs [17]. |
| Capable of detecting primary thyroid diffuse large B-cell lymphoma due to the high degree of fibrosis (patient with previous Hashimoto’s thyroiditis) [53]. | Hashimoto’s thyroiditis and primary thyroid diffuse large B-cell lymphoma | Case report | ||
| Lower background value in liver, heart, brain, and gastrointestinal tract compared to [18F]FDG [68]. | PTC | |||
| [18F]FAPi-42 | Comparable diagnostic value with [18F]FDG; and a higher uptake, mainly in patients with a BRAFV600E gene mutation (prediction of mutation status) [35]. | Different histotypes | Comparison between [18F]FAPi-42 and [18F]FDG | N.A. |
| [68Ga]FAPi-04 (otherwise named [68Ga]Ga-DOTA-FAPi-04) | Revealed more metastatic foci than [18F]FDG PET/CT, even if the detection rate rose to 93.1% when performed together [36]; and a higher detection power especially for lung lesions vs. [18F]FDG [39]. | PTC | Comparative study between [68Ga]FAPi-04 and [18F]FDG | Diffuse uptake in chronic thyroiditis and immune-related thyroiditis [64,66] (confirmed also for [68Ga]Ga-FAPi-46 [62]) and also in follicular thyroid adenoma associated with fibrosis and calcification [65]; physiological uptake in myelofibrosis; reactive LNs; arthritis; and subcutaneous fibroma [34]. No statistical significance between the SUVmax of metastatic lesions and Tg level [37]. Faint or absent uptake in lesions less than 1 cm in size or with low Tg levels [37]. |
| Detected hepatic metastases, while [18F]FDG was negative (useful for restaging) [43]; and effective in evaluation of pleural metastasis and, therefore, in restaging [45]. | PTC | Interesting image | ||
| Capable of evaluating immune-mediated disease with activated fibroblast such as Graves ophthalmopathy [67]. | PTC | Case report | ||
| More sensitive than [18F]FDG for neck and distant metastases; and [68Ga]FAPi SUVmax of metastasis is higher than that of [18F]FDG [34]. | Different histotypes | Comparative study between FAPi tracer and [18F]FDG | ||
| Detection rate of 87.5% in metastatic and RR lesions, mainly LNs and distant metastases such as lung, pleura, and bone [37]. | Different histotypes of RR-DTC (22/24 PTC) | Study on detection power of [68Ga]Ga-DOTA-FAPi-04 | ||
| Able to detect bone metastases at an earlier time point compared to [18F]FDG [69]. | Different histotypes | Comparative studies | ||
| Robust detection accuracy in liver lesions; and higher tumor-to-background value compared to [68Ga]Ga-DOTA-TATE [51]. | MTC | Interesting image | ||
| Higher detection power than that of [68Ga]Ga-DOTA-TOC [50]. | Metastatic MTC | Case report | ||
| [68Ga]Ga-DOTA.SA.FAPi | Higher detection power than [18F]FDG PET/CT for lymph nodes, liver, brain, bowel, and lung metastases [38,52]. | RR FCTC | Comparative study between [68Ga]Ga-DOTA.SA.FAPi and [18F]FDG | N.A. |
| [177Lu]Lu-DOTAGA.(SA.FAPi)2 | Overall response rate of 92%, no grade III/IV hematological, renal, and hepatic toxicity [55]. | RR-DTC | Theranostic study | N.A. |
| Partial response [58]. | High-grade MTC | Case report | ||
| Negligible radiotracer uptake in the liver and colon at post-treatment [177Lu]Lu-DOTAGA.Glu.(FAPi)2 scintigraphy [57]. | MTC | Comparative study between [177Lu]Lu-DOTAGA.(SA.FAPi)2 and [177Lu]Lu-DOTAGA.Glu.(FAPi)2 | ||
| [177Lu]Lu-FAPi-46 | Stable disease [56]. | RR-DTC | Interesting image | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guglielmo, P.; Alongi, P.; Baratto, L.; Conte, M.; Abenavoli, E.M.; Buschiazzo, A.; Celesti, G.; Dondi, F.; Filice, R.; Gorica, J.; et al. FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature. Cancers 2024, 16, 839. https://doi.org/10.3390/cancers16040839
Guglielmo P, Alongi P, Baratto L, Conte M, Abenavoli EM, Buschiazzo A, Celesti G, Dondi F, Filice R, Gorica J, et al. FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature. Cancers. 2024; 16(4):839. https://doi.org/10.3390/cancers16040839
Chicago/Turabian StyleGuglielmo, Priscilla, Pierpaolo Alongi, Lucia Baratto, Miriam Conte, Elisabetta Maria Abenavoli, Ambra Buschiazzo, Greta Celesti, Francesco Dondi, Rossella Filice, Joana Gorica, and et al. 2024. "FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature" Cancers 16, no. 4: 839. https://doi.org/10.3390/cancers16040839
APA StyleGuglielmo, P., Alongi, P., Baratto, L., Conte, M., Abenavoli, E. M., Buschiazzo, A., Celesti, G., Dondi, F., Filice, R., Gorica, J., Jonghi-Lavarini, L., Laudicella, R., Librando, M., Linguanti, F., Mattana, F., Miceli, A., Olivari, L., Piscopo, L., Santo, G., ... Evangelista, L. (2024). FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature. Cancers, 16(4), 839. https://doi.org/10.3390/cancers16040839







