Simple Summary
Hodgkin Lymphoma (HL) has a prognostic assessment often relying on PET scans after two ABVD treatment cycles (PET-2). This retrospective, observational, single–center, real-life study, evaluated 212 newly diagnosed HL patients, highlighting the role of IgM levels at diagnosis. IgM ≤ 50 mg/dL at baseline emerges as an independent predictor for progression-free survival in classic HL, offering an early and crucial prognostic marker. Additionally, the combined factors of IgM ≤ 50 mg/dL and the presence of a large nodal mass (<7 cm) serve as predictors of outcomes in classic HL, emphasizing the importance of these parameters in understanding and managing the disease above all in cases with unclear PET-2 and guaranteeing the best of care for the patients with an improved outcome.
Abstract
Hodgkin Lymphoma (HL) is characterized by an inflammatory background in which the reactive myeloid cells may exert an immune-suppressive effect related to the progression of the disease. Immunoglobulin M is the first antibody isotype produced during an immune response, which also plays an immunoregulatory role. Therefore, we investigated if, as a surrogate of defective B cell function, it could have any clinical impact on prognosis. In this retrospective, observational, single–center study, we evaluated 212 newly diagnosed HL patients, including 132 advanced-stage. A 50 mg/dL level of IgM at baseline resulted in 84.1% sensitivity and 45.5% specificity for predicting a complete response in the whole cohort (area under curve (AUC) = 0.62, p = 0.013). In multivariate analysis, baseline IgM ≤ 50 mg/dL and the presence of a large nodal mass (<7 cm) were independent variables able to predict the clinical outcome, while, after two cycles of treatment, IgM ≤ 50 mg/dL at baseline and PET-2 status were independent predictors of PFS. The amount of IgM at diagnosis is a valuable prognostic factor much earlier than PET-2, and it can also provide information for PET-2-negative patients. This can help to identify different HL classes at risk of treatment failure at baseline.
1. Introduction
Hodgkin lymphoma is a B cell malignant neoplasm that is distinguished from all other tumors by its peculiar anatomopathological features [1].
The Reed-Sternberg neoplastic cells are surrounded by an overwhelming inflammatory infiltrate, which plays a key role in the development and progression of the disease [2].
Currently, 18F-Fluoro-2-Deoxy-Glucose Positron Emission Tomography (FDG-PET), performed early after the first two cycles of chemotherapy (PET-2), has been established as the main prognostic factor able to predict clinical outcome and treatment failure [3,4].
A positive PET2 status, as a surrogate of the tumor’s low chemo-sensitivity, reflects the persistence of inflammatory cells with high glycolytic activity [5,6], and it is considered the most valuable tool to set up a risk-adapted strategy [7,8].
However, the negative predictive value of interim-PET is not absolute since about 10% of patients who tested negative failed to achieve a continued complete response.
To improve this risk assessment and avoid therapeutic failures, it would be useful, in clinical management, to identify treatment-independent prognostic factors already at diagnosis and not just after starting treatment.
For this purpose, novel insights in HL biology have been indicated as possible prognostic factors [9], although most of them have not yet been validated in prospective clinical trials.
Until now, most studies carried out have tried to shed light mainly on the controversial role of acquired cell-mediated immunity [10,11,12]; in the meantime, an increasing amount of evidence in Hodgkin Lymphoma suggests that the clonal expansion of altered myeloid progenitors with immunosuppressive activity, known as myeloid-derived suppressed cells (MDSCs) [13] could be the basis for the impairment of the myeloid axis in HL. Previous reports show that granulocytes are dysfunctional in HL, and high levels of immature MDSCs in peripheral blood have a negative predictive value on the patient’s outcome, independently by adopting a therapeutic strategy based on PET-scan results [14,15].
Moreover, an increase in the absolute neutrophil count (ANC) is a frequent finding in HL with a negative prognostic significance [16], although it is not included in the International Prognostic System (IPS) [17]. A high neutrophil-to-lymphocyte ratio (NLR) and a low lymphocyte-to-monocyte ratio (LMR) have been reported as negative prognostic factors by several retrospective studies [18,19], and a more recent report has tested their predictive values in relation to the long-term outcomes of patients treated with a PET-2 risk-adapted strategy [20]; however, the available data in the PET-2 era is still limited, and further efforts should be made in this direction.
On the other hand, the acquired humoral immunity still appears to be almost unexplored.
The origin of the Reed-Sternberg cell has been demonstrated to be from a proliferative B cell of the germinal center, which has acquired some genetic mutations that are able to prevent apoptosis; the proof of this derivation has been provided by the presence of clonal-type rearrangements in the coding genic region for the heavy chains of immunoglobulins, which physiologically are involved in the effector phase of humoral immunity [21].
Starting from this evidence, we investigated if a low amount of immunoglobulin M at baseline, as a surrogate of defective B cell function, could have any clinical impact on prognosis.
Although never tested in the field, baseline values of immunoglobulins could be included among those clinical parameters that, from the beginning, can predict the clinical course of the disease in newly diagnosed HL patients.
2. Patients and Methods
2.1. Patient Selection
In this retrospective, observational, single-center study, we evaluated 212 newly diagnosed HL patients who accessed the Hematology Division of the University of Catania consecutively. The HL diagnosis was the only criterion needed to include patients in the study, regardless of age and the histological subtype. Exclusion criteria were known active infective diseases (including HIV and tuberculosis infection), the presence of autoimmune diseases, the use of oral or parenteral corticosteroids, or other immunosuppressant therapy less than 3 months before diagnosis.
All patients provided their written informed consent in accordance with the Declaration of Helsinki.
For all patients, the diagnosis was made via histological evaluation, following an excisional biopsy of the involved lymph node; an immunophenotype study was performed for histological classification. Bone marrow aspiration was performed to evaluate the disease extent and define the correct staging. Before starting treatment, a blood sample was collected for a complete blood count (CBC) evaluation and routine biochemical examinations.
White blood cell (WBC) counts and types (neutrophils, eosinophils, basophils, lymphocytes, and monocytes) were determined using an electrical impedance method in an automatic blood counter device. The quantification of immunoglobulins (IgG, IgA, IgM) at baseline was performed via nephelometry, as part of standard initial clinical work-up. The baseline characteristics of patients included in the study are shown in Table 1.

Table 1.
Baseline characteristics of patients included in the study (n = 212).
2.2. Staging, Risk Assessment, and Treatment
For each patient, we assessed the stage of disease at diagnosis according to the Ann-Arbor classification system, classifying patients as affected by an early (stage I–II) or advanced-stage disease (stage IIB, III–IV). The risk assessment was carried out by calculating the International Prognostic Score (IPS). Functional and morphological evaluation of the patient at baseline was obtained both with Positron Emission Tomography (PET) and Computed tomography (CT) scans.
For patients with advanced disease (Ann-Arbor stage ≥IIB), treatment consisted of six cycles according to the ABVD regimen (Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine), followed by involved field radiotherapy, if clinically indicated. Positive PET-2 patients were shifted to a BEACOPP regimen (Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, and Prednisone) for eight cycles and, in the case of persistent disease, autologous stem cell transplant was performed. Early-stage disease patients received two, four, or six cycles of ABVD chemotherapy plus involved field radiotherapy, as clinically indicated.
Both baseline PET and PET-2 were performed using a standard technique, and a semi-quantitative Deauville score was attributed to each image. A minimally positive scan was defined as any scan with any residual FDG uptake outside the physiological areas of tracer concentration [22].
2.3. Statistical Analysis
Qualitative results were summarized as counts and percentages. Descriptive statistics were generated for analyzing the results, and a p-value under 0.05 was considered significant.
The primary endpoint of the study was to establish if baseline immunoglobulins could have any impact on the progression-free survival (PFS) of newly diagnosed HL patients. The secondary endpoint included the evaluation of baseline immunoglobulins to predict overall survival (OS) or PET-2 status in the whole cohort or only in the cohort of advanced-stage patients.
The correlation of baseline IgM with other established prognostic factors in HL was assessed with Pearson’s chi-square test (or Fisher’s Exact Test) for categorical parameters and with a Mann–Whitney or Wilcoxon signed rank test for continuous parameters.
Progression-free survival (PFS) was calculated from the time of inclusion of patients in the study until the date of progression, relapse, or death or until the date at which the patient was last known to be in remission. The positivity of PET-2 was not considered an event. PFS was analyzed using the Kaplan–Meier Test. Standard errors were calculated using the Greenwood method, and the 95% confidence intervals were computed as 1.96 times the standard error in each direction.
The Cox Proportional Hazards Model was used to evaluate IgM at diagnosis as a prognostic marker for PFS and to assess and adjust with other known prognostic factors.
All calculations were performed using MedCalc Statistical Software version 19.1.6 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; accessed on 10 October 2023) and Addinsoft (2024). XLSTAT statistical and data analysis solution, New York, NY, USA, https://www.xlstat.com/en (accessed on 10 October 2023), was used.
3. Results
3.1. Baseline IgM Is Reduced in cHL
In this series of 212 consecutive cHL patients, the median age was 31.8 (range, 14.8–76.9), and half of the patients were males (Table 1).
The median ANC, ALC, and NLR at diagnosis were 7.24 × 103/μL, 1.46 × 103/μL, and 4.79, respectively. The median baseline values for IgM, IgA, and IgG were 103.00 (interquartile range (IQR), 50.00–336.00), 211.00 (IQR, 36.00–690.00), and 1169.00 (IQR, 312.00–2763.0) mg/dL, respectively. Low levels of IgM (≤50 mg/dL) and IgG (≤1000 mg/dL) were not associated with age, gender, advanced stage, high WBC, low ALC counts, high IPS, positive PET-2, or increased LDH (Table 2).

Table 2.
Evaluation of the strength of the association between baseline levels of IgA, IgG, and IgM and established prognostic factors in HL (Fisher exact test).
3.2. Low Baseline IgM Concentrations Are Associated with Clinical Outcomes
After two ABVD cycles, out of 212 patients included in the study, 33 (16%) had positive PET-2 (17 with a Deauville score of 4 and 16 with a Deauville score of 5) and 179 (84%) had a negative PET-2 (Figure 1).
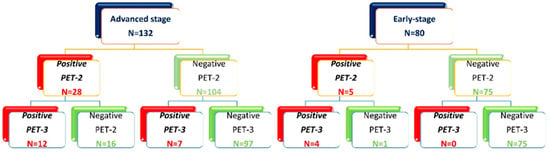
Figure 1.
Allocation of patients based on the PET-2 and PET-3 scan.
Advanced-stage patients with positive PET-2 (28/132) were allocated to an escalated BEACOPP program (n = 18, not completed for toxicity in five cases), or they completed the program with a total of six cycles outside of clinical trials (n = 10), followed by high-dose chemotherapy (IGEV or DHAP) and autologous stem cell transplantation; 104 patients with negative PET-2 continued with ABVD for an additional four cycles, followed by consolidation radiotherapy on the initial bulky nodal site of disease in 24 cases (Figure 1). One early death due to infection occurred among advanced-stage patients with negative PET-2. A third PET evaluation was performed at the end of planned treatment (PET-3), and a treatment failure within 2 years was registered in 7/104 patients with negative PET-2 as compared to 12/28 with positive PET-2 (Figure 1).
In 80 early-stage patients, therapy was not modified according to the PET-2 results. Based on disease extension, ABVD cycles were followed by involved field 30 Gy radiotherapy (ifRT), except in four cases where treatment for medical conditions and the patient’s will was extended to six cycles of ABVD. Two of them did not achieve a complete response and were treated with salvage-regimen IGEV and autologous stem cell transplantation (Figure 1). All five patients with positive PET2 progressed within 2 years and received high-dose chemotherapy (IGEV or DHAP) and autologous stem cell transplantation.
After a median follow-up of 70.2 months (range 4.8–214.0 months), 188 patients (88.7%) were in continued complete remission (cCR), 6 patients (3.0%) progressed during therapy or immediately after (during the first six months), and 18 patients relapsed (8.4%) within two years from the end of treatment (Table 1). Among 132 advanced-stage patients, 28 (27.7%) had a positive PET-2 scan. Among 80 early-stage patients, 5 (6%) had a positive PET-2 scan, as summarized in Figure 1. As expected, a positive PET-2 scan was associated with an inferior PFS, as shown in Figure 2.
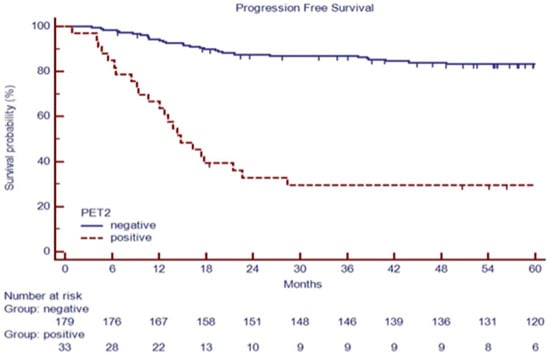
Figure 2.
PFS based on PET-2 status in 212 consecutive cHL patients.
In addition, 49/212 (23.1%) patients had a baseline IgM value lower than 50 mg/dL (Table 1). Achieving and maintaining a complete response (cCR) was associated with lower median IgM than treatment failure (respectively, 90 versus 48 mg/dL, p = 0.0015). A Receiver Operating Characteristic (ROC) Curve analysis identified a level of IgM of 50 mg/dL to discriminate patients with 84.1% sensitivity and 45.5% specificity (area under the curve (AUC), 0.624; p = 0.013, Figure 3), while IgG and IgA amounts at baseline failed to identify patients in complete remission (AUC, respectively, 0.55; p = 0.28 and 0.52, p = 0.61).
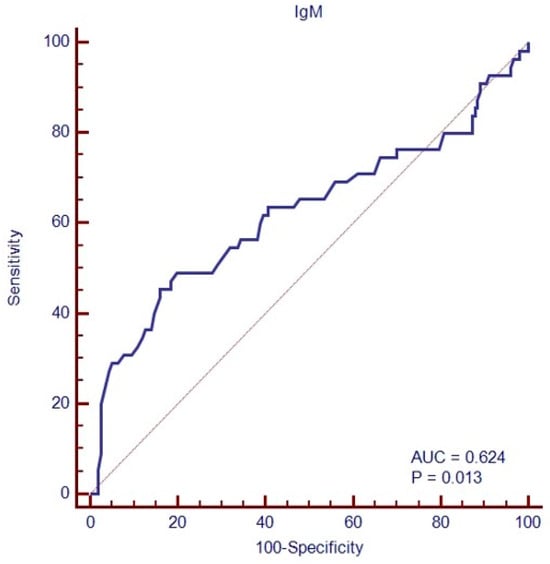
Figure 3.
ROC analysis of baseline immunoglobulin (IgM class) amounts to predict treatment failure.
Based on ROC analysis, a cut-off level of 50 mg/dL of IgM at baseline was chosen to predict clinical outcomes at 36 or 60 months in a further analysis. Patient allocation based on the clinical outcome and basal amount of IgM is shown in Figure 4.
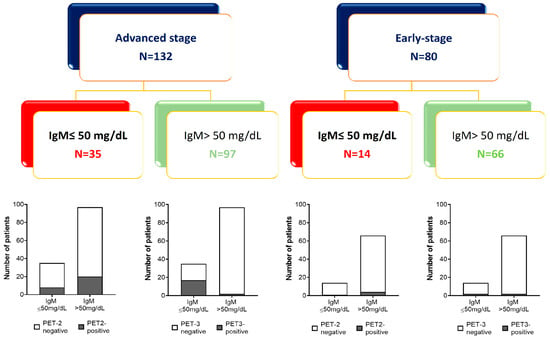
Figure 4.
Patient allocation based on the amount of baseline IgM and clinical outcome (PET2 and PET3 scans).
PFS at 60 months was 54.1% versus 81.1% in patients with IgM ≤ 50 mg/dL or IgM > 50 mg/dL, respectively (p < 0.001, Figure 5).
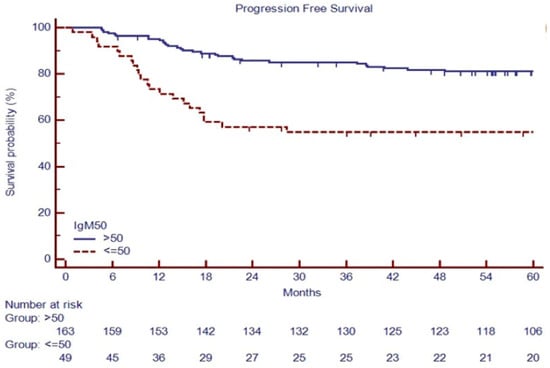
Figure 5.
PFS based on baseline IgM in 212 consecutive cHL patients.
A white blood cell (WBC) count ≥ 15,000 cells/μL (p = 0.005), IPS ≥ 3 (p = 0.01), large nodal mass > 7 cm (p = 0.02), extranodal disease (p = 0.03), positive PET-2 (p < 0.0001), NLR ≥ 6 (p = 0.008), and IgM ≤ 50 mg/dL (p < 0.0001) were predictors of 5-year progression-free survival (PFS) in the univariate analysis (Table 3).

Table 3.
Univariate analysis of PFS of all patients included in the study (n = 212).
To overcome statistical bias due to the retrospective analysis in a limited series, we split randomly, at 50:50, the cohort into a training set (n = 106) and a validation set (n = 106, Supplementary Table S1) to test the contribution of IgM in the risk stratification. In the training set, the 5-year PFS estimates were 54.9% for patients carrying low versus 72.5% for patients carrying normal IgM at baseline (p = 0.02, as shown in Supplementary Table S2), which was confirmed in the validation set (53.8 vs. 86.6%, Supplementary Table S3). Univariate analysis of PFS in training and validation further confirmed the positive PET-2 (p < 0.001 in both settings, shown in Supplementary Figure S1, panels A and C) and IgM ≤ 50 mg/dL (respectively, p = 0.02 and p < 0.001, in the training and validation set, Supplementary Figure S1, panels B–D) as predictors of 5-year PFS.
3.3. Baseline IgM Can Predict Clinical Outcomes in Advanced-Stage HL Patients
Advanced-stage patients with positive PET-2 had an inferior outcome, with a 5-year PFS of 25.2% versus 80.5% for negative PET-2 patients (p < 0.0001). After two cycles of treatment, patients with negative PET-2 carrying IgM ≤ 50 mg/dL had poor outcomes, with a PFS at 60 months of 68.0% versus 84.7% for cases with IgM > 50 mg/dL, p = 0.0068 (Figure 6), the only predictor of the outcome in the univariate analysis (Table 4).
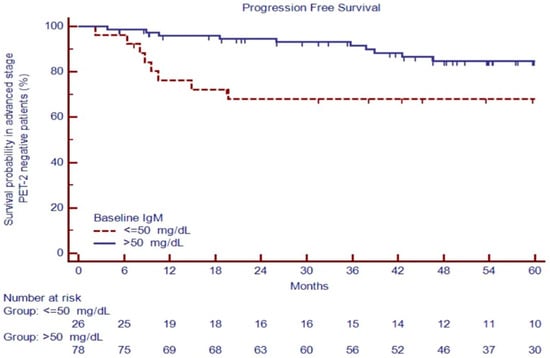
Figure 6.
PFS in PET-2 negative patients based on IgM evaluation at diagnosis.

Table 4.
Univariate analysis of PFS in advanced-stage patients (n = 132).
Thus, in advanced-stage patients, WBC count ≥ 15,000 cells/μL (p = 0.012), IPS ≥ 3 (p = 0.01), large nodal mass > 7 cm (p = 0.02), extranodal disease (p = 0.04), positive PET-2 (p < 0.0001), NLR ≥ 6 (p = 0.002), and IgM ≤ 50 mg/dL (p = 0.0002) were predictors of 5-year PFS in the univariate analysis (Table 4).
In the multivariate analysis, among parameters available at baseline, only IgM title ≤ 50 mg/dL and a large nodal mass > 7 cm were independent predictors of treatment failure, while when including the PET-2 status, only IgM ≤ 50 mg/dL retained its independent prognostic relevance (Table 5).

Table 5.
Multivariate analysis of PFS in patients included in the study, according to clinical predictors, including the PET-2 status.
3.4. Combining Baseline IgM and the Presence of Large Nodal Mass Can Predict Clinical Outcomes in Advanced-Stage HL Patients Independently of the PET-2 Status
Based on previous findings, we stratified advanced-stage patients into three groups based on clinical variables available at diagnosis: the low-risk group was defined as the absence of a large nodal mass (LNM) and baseline IgM >50 mg/dL (n = 63, 48%); the standard-risk group by either the presence of an LNM or baseline IgM ≤ 50 mg/dL (n = 59, 45%); the high-risk group by the concurrent presence of an LNM and IgM ≤ 50 mg/dL (n = 10, 7%).
The PFSs at 60 months were significantly different among the three risk groups, at 83.5%, 59.5%, and 40.0%, respectively, p < 0.0001 (Figure 7).
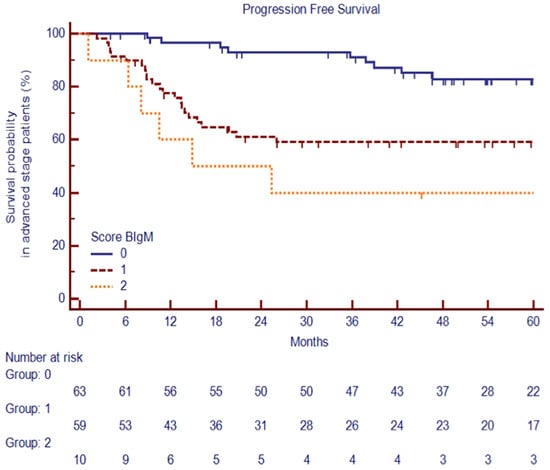
Figure 7.
PFS at 60 months among the three risk groups of advanced-stage cHL patients.
4. Discussion
This work illustrates the results of a study aimed at demonstrating, for the first time in the field, the pathogenetic involvement of a reduction in immunoglobulins at baseline in clinical outcomes of newly diagnosed HL patients.
In the era of risk-adapted therapy in HL, an appropriate risk classification is needed in order to identify those patients for whom aggressive treatment is required.
The prognostic model based on the International Prognostic System (IPS) has insubstantial clinical utility because only 19% of patients with scores of 4 and 5 had a probability of 7-year-progression-free survival (PFS) < 50% [7].
Currently, PET-2 is the most valid tool for predicting poor outcomes and setting a risk-adapted strategy for treatment, and PET-2-derived information is currently used in clinical practice to switch to intensified or de-escalate treatment [8,23].
However, some issues are still open: PET-2 evaluation provides quite late information because it is available only during treatment when it is likely to believe that the biological mechanisms leading to chemoresistance have already been activated. Moreover, its negative predictive value is not absolute, as about 10% of patients with a negative PET-2 are still at risk of treatment failure [8,23].
For these reasons, the search for prognostic factors able to identify high-risk patients at diagnosis and not during treatment remains an objective that is not fully achieved yet.
From this perspective, several biomarkers have been proposed during the last few years as putative predictors of clinical outcomes, although some of them could not be included because of a lack of reproducibility [12,19,24,25]. Recently, pretreatment and on-treatment circulating tumor DNA (ctDNA) levels have shown a potential role in the dynamic monitoring of classic HL, supporting the utility of noninvasive strategies [26].
Thymus and activation-regulated chemokines (TARCs) and Arginase-1 (Arg1) stand out among the soluble factors that reflect the immune system dysregulation and which can be easily tested at diagnosis with a peripheral blood sample [27,28,29]. These factors are associated with therapeutic success and negativity of PET-2 [30], but their predictive role has not yet been demonstrated in a prospective series of patients treated upfront with a risk-adapted strategy.
The role played by the acquired cell-mediated immunity (especially the regulatory function of T cells) in the development and progression of disease has been largely investigated by an increasing number of studies. Instead, as the side of acquired humoral immunity has been much less explored, one might ask if B cell aggregates can play an active role in intratumoral immunity or if they are mere bystanders in the HL microenvironment. Indeed, so-called immunoparesis, a condition that describes the suppression of uninvolved immunoglobulins, has been recently reported as a precursor marker of the absence of an immune response to the SARS-CoV-2 vaccine in lymphoproliferative disorders [31,32].
In view of these results and because of the B cell derivation of HL, we supposed that reduced levels of immunoglobulins at diagnosis, as a marker of humoral immunity impairment, could contribute to immunological dysfunction, which appears to be the main pathogenetic issue and which has important effects on the clinical outcomes of these patients.
Indeed, in recent years, evidence has emerged to suggest that humoral immunity may contribute to the immune response in cHL, and several immunohistochemical studies have been carried out to assess the immunophenotypic characteristics of these B cell infiltrates; particularly, increased numbers of CD20+ B cells in the tumor infiltrate were shown to favor a good prognosis regardless of the disease stage. Therefore, these results might suggest an active role of B cells in the anti-tumor immune response [33,34].
However, this observation appears to be controversial, as targeting B cells with Rituximab led to a better outcome in cHL patients; moreover, B cells may trigger Hodgkin and Reed-Sternberg cell (HRS) growth through the expression of CD30L and CD40L [35].
Interestingly, high numbers of CD38+ B-cells (including transitional B cells, regulatory B cells, memory B cells, and post-germinal center cells) are associated with worse survival outcomes [34]. In this regard, CD38 signaling can decrease the differentiation of IgM-producing plasma cells [36], a specific cell subset for which a positive effect on the prognosis of these patients has been shown [34].
In our study, we evaluated the survival analyses that have been carried out for the whole cohort of patients, as well as, subsequently, only for patients with the advanced stage of disease, for whom we expected a worse outcome.
Our findings show that the IgM amount at diagnosis is a valuable prognostic factor much earlier than PET-2 and can also provide information for negative PET-2 patients. Therefore, it could be added to known predictive factors in order to identify different HL classes for the risk of treatment failure at baseline.
5. Conclusions
In HL, the IgM level at the time of diagnosis could serve as a valuable prognostic indicator before the conclusion of the first two chemotherapy cycles, even offering insights into PET-2-negative patients. This early assessment proves to be beneficial for identifying distinct classes that may be at risk of treatment failure from the outset.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16040826/s1.
Author Contributions
Conceptualization, A.D. and A.R.; formal analysis, A.D. and A.R.; data collection and curation, G.S., A.C., A.F., G.M., A.L.C., A.P., M.I. and C.C.; writing—original draft preparation, A.D., G.S. and A.R.; writing—review and editing, A.D., A.R. and F.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Policlinico di Catania (study IEO1355, approved on 20 September 2021, n.220/2021/PO).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mathas, S.; Hartmann, S.; Küppers, R. Hodgkin Lymphoma: Pathology and Biology. Semin. Hematol. 2016, 53, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Celegato, M.; Casagrande, N. Microenvironmental Interactions in Classical Hodgkin Lymphoma and Their Role in Promoting Tumor Growth, Immune Escape and Drug Resistance. Cancer Lett. 2016, 380, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, L.; Puccini, B.; Zinzani, P.L.; Biggi, A.; Castagnoli, A.; Merli, F.; Balzarotti, M.; Stelitano, C.; Spina, M.; Vitolo, U.; et al. The Prognostic Value of Positron Emission Tomography Performed after Two Courses (INTERIM-PET) of Standard Therapy on Treatment Outcome in Early Stage Hodgkin Lymphoma: A Multicentric Study by the Fondazione Italiana Linfomi (FIL). Am. J. Hematol. 2015, 90, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Barrington, S.F.; Biggi, A.; Chauvie, S.; Kostakoglu, L.; Gregianin, M.; Meignan, M.; Mikhaeel, G.N.; Loft, A.; Zaucha, J.M.; et al. The Predictive Role of Interim Positron Emission Tomography for Hodgkin Lymphoma Treatment Outcome Is Confirmed Using the Interpretation Criteria of the Deauville Five-Point Scale. Haematologica 2014, 99, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Kostakoglu, L. Interim FDG-PET in Hodgkin Lymphoma: A Compass for a Safe Navigation in Clinical Trials? Blood 2012, 120, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Strauss, L.; Consonni, F.M.; Travelli, C.; Genazzani, A.; Porta, C. Metabolic Regulation of Suppressive Myeloid Cells in Cancer. Cytokine Growth Factor. Rev. 2017, 35, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Marcheselli, R.; Sacchi, S.; Re, A.; Pagani, C.; Tucci, A.; Botto, B.; Vitolo, U.; Molinari, A.L.; Puccini, B.; et al. The Classic Prognostic Factors in Advanced Hodgkin ’ s Lymphoma Patients Are Losing Their Meaning at the Time of Pet-Guided Treatments. Ann. Hematol. 2019, 99, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F.; et al. Early Interim 2-[18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography Is Prognostically Superior to International Prognostic Score in Advanced-Stage Hodgkin’s Lymphoma: A Report from a Joint Italian-Danish Study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef]
- Agostinelli, C.; Gallamini, A.; Stracqualursi, L.; Agati, P.; Tripodo, C.; Fuligni, F.; Sista, M.T.; Fanti, S.; Biggi, A.; Vitolo, U.; et al. The Combined Role of Biomarkers and Interim PET Scan in Prediction of Treatment Outcome in Classical Hodgkin’s Lymphoma: A Retrospective, European, Multicentre Cohort Study. Lancet Haematol. 2016, 3, e467–e479. [Google Scholar] [CrossRef]
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic Impact of Tumour-Infiltrating Th2 and Regulatory T Cells in Classical Hodgkin Lymphoma. Hematol. Oncol. 2009, 27, 31–39. [Google Scholar] [CrossRef]
- Greaves, P.; Clear, A.; Owen, A.; Iqbal, S.; Lee, A.; Matthews, J.; Wilson, A.; Calaminici, M.; Gribben, J.G. Defining Characteristics of Classical Hodgkin Lymphoma Microenvironment T-Helper Cells. Blood 2013, 122, 2856–2863. [Google Scholar] [CrossRef]
- Marshall, N.A.; Christie, L.E.; Munro, L.R.; Culligan, D.J.; Johnston, P.W.; Barker, R.N.; Vickers, M.A. Immunosuppressive Regulatory T Cells Are Abundant in the Reactive Lymphocytes of Hodgkin Lymphoma. Blood 2004, 103, 1755–1762. [Google Scholar] [CrossRef]
- Sica, A.; Porta, C.; Morlacchi, S.; Banfi, S.; Strauss, L.; Rimoldi, M.; Totaro, M.G.; Riboldi, E. Origin and Functions of Tumor-Associated Myeloid Cells (TAMCs). Cancer Microenviron. 2012, 5, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Trellakis, S.; Bruderek, K.; Schmaltz, D.; Steller, G.; Elian, M.; Suttmann, H.; Schenck, M.; Welling, J.; Zabel, P.; et al. Myeloid-Derived Suppressor Cells in the Peripheral Blood of Cancer Patients Contain a Subset of Immature Neutrophils with Impaired Migratory Properties. J. Leukoc. Biol. 2011, 89, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Forte, S.; Chiarenza, A.; Figuera, A.; Motta, G.; Palumbo, G.A.; Ippolito, M.; Consoli, U.; et al. Circulating Myeloid-Derived Suppressor Cells Correlate with Clinical Outcome in Hodgkin Lymphoma Patients Treated up-Front with a Risk-Adapted Strategy. Br. J. Haematol. 2015, 168, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Tibullo, D.; Giallongo, C.; La Cava, P.; Chiarenza, A.; Motta, G.; Caruso, A.L.; Villari, L.; et al. The Prognostic Value of the Myeloid-Mediated Immunosuppression Marker Arginase-1 in Classic Hodgkin Lymphoma. Oncotarget 2016, 7, 67333–67346. [Google Scholar] [CrossRef] [PubMed]
- Hasenclever, D.; Diehl, V. A Prognostic Score for Advanced Hodgkin’s Disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Porrata, L.F.; Ristow, K.; Colgan, J.P.; Habermann, T.M.; Witzig, T.E.; Inwards, D.J.; Ansell, S.M.; Micallef, I.N.; Johnston, P.B.; Nowakowski, G.S.; et al. Peripheral Blood Lymphocyte/Monocyte Ratio at Diagnosis and Survival in Classical Hodgkin’s Lymphoma. Haematologica 2012, 97, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Marcheselli, R.; Bari, A.; Tadmor, T.; Marcheselli, L.; Cox, M.C.; Pozzi, S.; Ferrari, A.; Baldini, L.; Gobbi, P.; Aviv, A.; et al. Neutrophil-Lymphocyte Ratio at Diagnosis Is an Independent Prognostic Factor in Patients with Nodular Sclerosis Hodgkin Lymphoma: Results of a Large Multicenter Study Involving 990 Patients. Hematol. Oncol. 2017, 35, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Pavoni, C.; Di Raimondo, F.; Tarella, C.; Viviani, S.; Rossi, A.; Patti, C.; Picardi, M.; Cantonetti, M.; La Nasa, G.; et al. The Neutrophil to Lymphocyte Ratio (NLR) and the Presence of Large Nodal Mass Are Independent Predictors of Early Response: A Subanalysis of the Prospective Phase II PET-2-adapted HD0607 Trial. Cancer Med. 2020, 9, 8735. [Google Scholar] [CrossRef]
- Kuppers, R.; Rajewsky, K.; Zhao, M.; Simons, G.; Laumann, R.; Fischer, R.; Hansmann, M.L. Hodgkin Disease: Hodgkin and Reed-Sternberg Cells Picked from Histological Sections Show Clonal Immunoglobulin Gene Rearrangements and Appear to Be Derived from B Cells at Various Stages of Development. Proc. Natl. Acad. Sci. USA 1994, 91, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A. Positron Emission Tomography Scanning: A New Paradigm for the Management of Hodgkin’s Lymphoma. Haematologica 2010, 95, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. FDG-PET after Two Cycles of Chemotherapy Predicts Treatment Failure and Progression-Free Survival in Hodgkin Lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef]
- Cattaruzza, L.; Gloghini, A.; Olivo, K.; Di Francia, R.; Lorenzon, D.; De Filippi, R.; Carbone, A.; Colombatti, A.; Pinto, A.; Aldinucci, D. Functional Coexpression of Interleukin (IL)-7 and Its Receptor (IL-7R) on Hodgkin and Reed-Sternberg Cells: Involvement of IL-7 in Tumor Cell Growth and Microenvironmental Interactions of Hodgkin’s Lymphoma. Int. J. Cancer 2009, 125, 1092–1101. [Google Scholar] [CrossRef]
- Greaves, P.; Clear, A.; Coutinho, R.; Wilson, A.; Matthews, J.; Owen, A.; Shanyinde, M.; Lister, T.A.; Calaminici, M.; Gribben, J.G. Expression of FOXP3, CD68, and CD20 at Diagnosis in the Microenvironment of Classical Hodgkin Lymphoma Is Predictive of Outcome. J. Clin. Oncol. 2013, 31, 256–262. [Google Scholar] [CrossRef]
- Alig, S.K.; Esfahani, M.S.; Garofalo, A.; Li, M.Y.; Rossi, C.; Flerlage, T.; Flerlage, J.E.; Adams, R.; Binkley, M.S.; Shukla, N.; et al. Distinct Hodgkin Lymphoma Subtypes Defined by Noninvasive Genomic Profiling. Nature 2024, 625, 778–787. [Google Scholar] [CrossRef]
- Van Den Berg, A.; Visser, L.; Poppema, S. High Expression of the CC Chemokine TARC in Reed-Sternberg Cells. A Possible Explanation for the Characteristic T-Cell Infiltratein Hodgkin’s Lymphoma. Am. J. Pathol. 1999, 154, 1685–1691. [Google Scholar] [CrossRef]
- Niens, M.; Visser, L.; Nolte, I.M.; Van Der Steege, G.; Diepstra, A.; Cordano, P.; Jarrett, R.F.; Te Meerman, G.J.; Poppema, S.; Van Den Berg, A. Serum Chemokine Levels in Hodgkin Lymphoma Patients: Highly Increased Levels of CCL17 and CCL22. Br. J. Haematol. 2008, 140, 527–536. [Google Scholar] [CrossRef]
- Di Stasi, A.; De Angelis, B.; Rooney, C.M.; Zhang, L.; Mahendravada, A.; Foster, A.E.; Heslop, H.E.; Brenner, M.K.; Dotti, G.; Savoldo, B. T Lymphocytes Coexpressing CCR4 and a Chimeric Antigen Receptor Targeting CD30 Have Improved Homing and Antitumor Activity in a Hodgkin Tumor Model. Blood 2009, 113, 6392–6402. [Google Scholar] [CrossRef] [PubMed]
- Farina, L.; Rezzonico, F.; Spina, F.; Dodero, A.; Mazzocchi, A.; Crippa, F.; Alessi, A.; Dalto, S.; Viviani, S.; Corradini, P. Serum Thymus and Activation-Regulated Chemokine Level Monitoring May Predict Disease Relapse Detected by PET Scan after Reduced-Intensity Allogeneic Stem Cell Transplantation in Patients with Hodgkin Lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 1982–1988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duminuco, A.; Nardo, A.; Orofino, A.; Giunta, G.; Conticello, C.; Del Fabro, V.; Chiarenza, A.; Parisi, M.S.; Figuera, A.; Leotta, S.; et al. Efficacy and Safety of Tixagevimab-Cilgavimab versus SARS-CoV-2 Breakthrough Infection in the Hematological Conditions. Cancer 2024, 130, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Duminuco, A.; Romano, A.; Leotta, D.; La Spina, E.; Cambria, D.; Bulla, A.; Del Fabro, V.; Tibullo, D.; Giallongo, C.; Palumbo, G.A.; et al. Clinical Outcome of SARS-CoV-2 Infections Occurring in Multiple Myeloma Patients after Vaccination and Prophylaxis with Tixagevimab/Cilgavimab. Front. Oncol. 2023, 13, 1157610. [Google Scholar] [CrossRef]
- Chetaille, B.; Bertucci, F.; Finetti, P.; Esterni, B.; Stamatoullas, A.; Picquenot, J.M.; Copin, M.C.; Morschhauser, F.; Casasnovas, O.; Petrella, T.; et al. Molecular Profiling of Classical Hodgkin Lymphoma Tissues Uncovers Variations in the Tumor Microenvironment and Correlations with EBV Infection and Outcome. Blood 2009, 113, 2765–2775. [Google Scholar] [CrossRef]
- Tudor, C.S.; Distel, L.V.; Eckhardt, J.; Hartmann, A.; Niedobitek, G.; Buettner, M. B Cells in Classical Hodgkin Lymphoma Are Important Actors Rather than Bystanders in the Local Immune Reaction. Hum. Pathol. 2013, 44, 2475–2486. [Google Scholar] [CrossRef]
- Clodi, K.; Asgari, Z.; Younes, M.; Palmer, J.L.; Cabanillas, F.; Carbone, A.; Andreeff, M.; Younes, A. Expression of CD40 Ligand (CD154) in B and T Lymphocytes of Hodgkin Disease: Potential Therapeutic Significance. Cancer 2002, 94, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Manjarrez-Orduño, N.; Moreno-García, M.E.; Fink, K.; Santos-Argumendo, L. CD38 Cross-Linking Enhances TLR-Induced B Cell Proliferation but Decreases IgM Plasma Cell Differentiation. Eur. J. Immunol. 2007, 37, 358–367. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).