Circulating Tumor Cells: How Far Have We Come with Mining These Seeds of Metastasis?
Abstract
Simple Summary
Abstract
1. Background
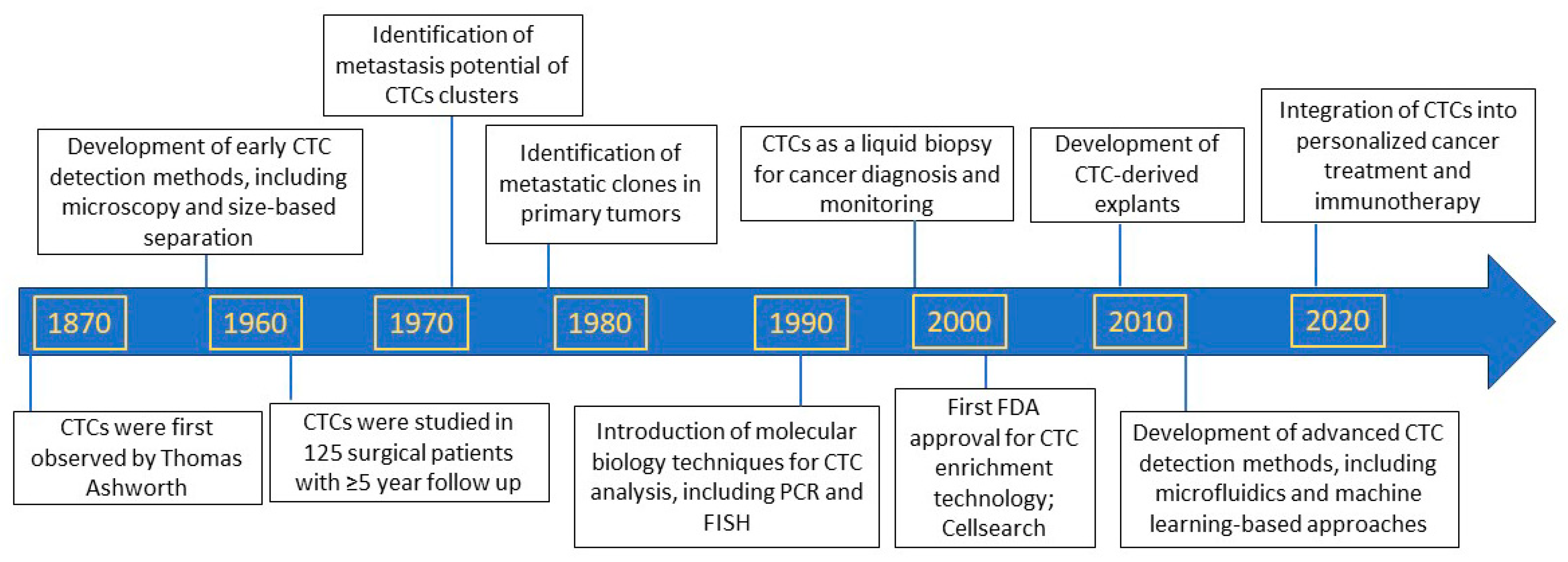
2. Discovery of Circulating Tumor Cells as Precursors of Metastasis
3. Circulating Tumor Cells as Biomarkers to Predict Patient Prognosis
4. Circulating Tumor Cells as Biomarkers to Predict Anti-Cancer Therapy Responses
4.1. Circulating Tumor Cells as Biomarkers to Predict Chemotherapy Responses
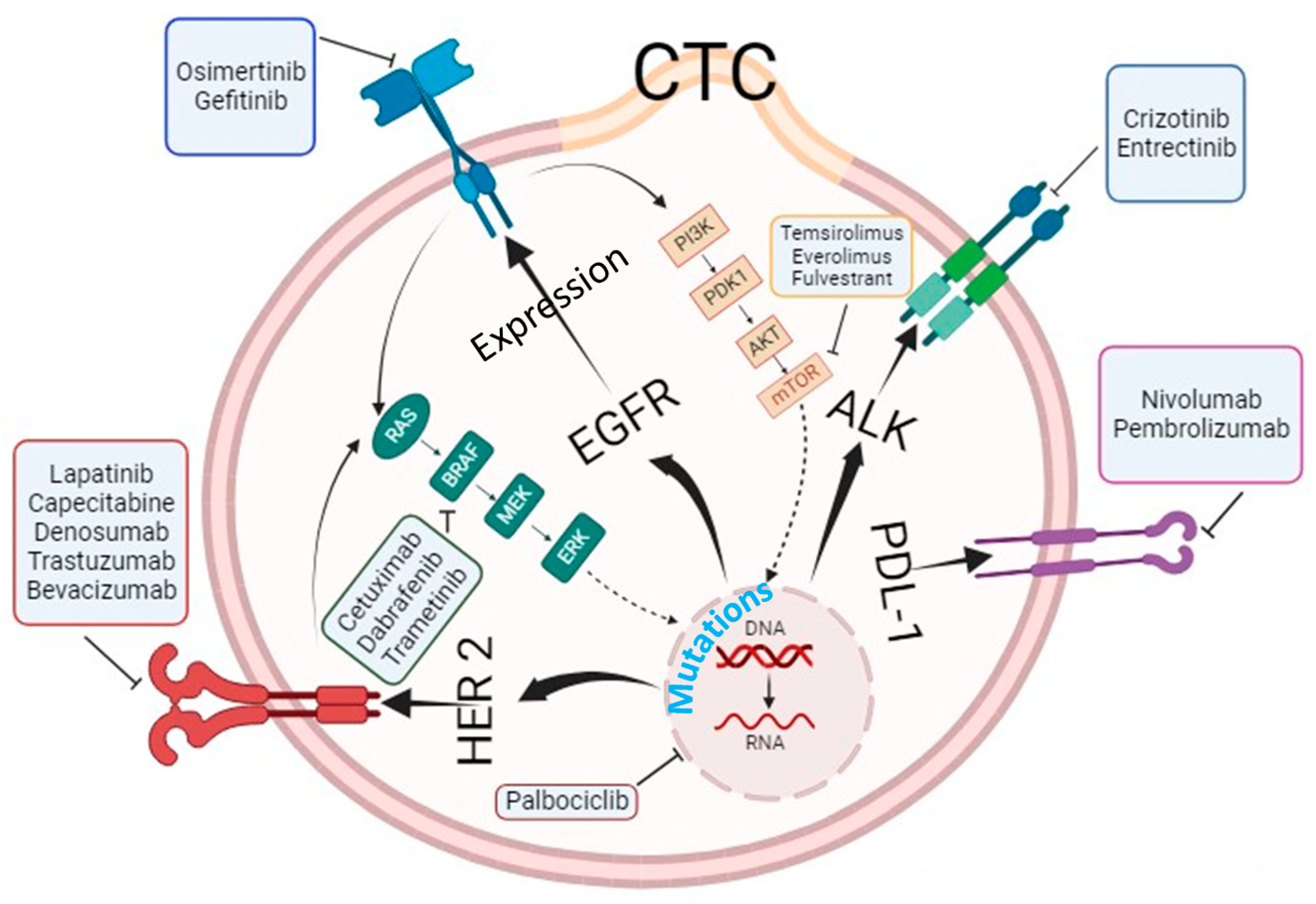
4.2. Circulating Tumor Cells as Biomarkers to Predict Targeted Therapy Responses
4.3. Circulating Tumor Cells as Biomarkers to Predict Immunotherapy Responses
5. Molecular and Genetic Characterization of Circulating Tumor Cells beyond Enumeration to Identify Actionable Mutations
6. Circulating Tumor Cells as Models to Identify Metastasis Competent Signatures
7. Real World Evidence by Circulating-Tumor-Cell-Based Clinical Trials
8. Challenges and Opportunities
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lianidou, E.S. Circulating tumor cells--new challenges ahead. Clin. Chem. 2012, 58, 805–807. [Google Scholar] [CrossRef]
- Roberts, S.; Watne, A.; Mc, G.R.; Mc, G.E.; Cole, W.H. Technique and results of isolation of cancer cells from the circulating blood. JAMA Arch. Surg. 1958, 76, 334–346. [Google Scholar] [CrossRef]
- Engell, H.C. Cancer cells in the blood; a five to nine year follow up study. Ann. Surg. 1959, 149, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.D. The dissemination of cancer cells during operative procedures. Ann. R. Coll. Surg. Engl. 1960, 27, 14–44. [Google Scholar] [PubMed]
- Salgado, I.; Hopkirk, J.F.; Long, R.C.; Ritchie, A.C.; Ritchie, S.; Webster, D.R. Tumour cells in the blood. Can. Med. Assoc. J. 1959, 81, 619–622. [Google Scholar]
- Fidler, I.J.; Kripke, M.L. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977, 197, 893–895. [Google Scholar] [CrossRef]
- Suvilesh, K.N.; Manjunath, Y.; Pantel, K.; Kaifi, J.T. Preclinical models to study patient-derived circulating tumor cells and metastasis. Trends Cancer 2023, 9, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bauerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Lawrence, R.; Watters, M.; Davies, C.R.; Pantel, K.; Lu, Y.J. Circulating tumour cells for early detection of clinically relevant cancer. Nat. Rev. Clin. Oncol. 2023, 20, 487–500. [Google Scholar] [CrossRef]
- Larsson, A.M.; Jansson, S.; Bendahl, P.O.; Levin Tykjaer Jorgensen, C.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Ryden, L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018, 20, 48. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- De Luca, F.; Rotunno, G.; Salvianti, F.; Galardi, F.; Pestrin, M.; Gabellini, S.; Simi, L.; Mancini, I.; Vannucchi, A.M.; Pazzagli, M.; et al. Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget 2016, 7, 26107–26119. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.B.; Gopalan, V.; Matos, M.; Lu, C.T.; Lam, A.K. Genetic Heterogeneity of Single Circulating Tumour Cells in Colorectal Carcinoma. Int. J. Mol. Sci. 2020, 21, 7766. [Google Scholar] [CrossRef]
- Gasch, C.; Plummer, P.N.; Jovanovic, L.; McInnes, L.M.; Wescott, D.; Saunders, C.M.; Schneeweiss, A.; Wallwiener, M.; Nelson, C.; Spring, K.J.; et al. Heterogeneity of miR-10b expression in circulating tumor cells. Sci. Rep. 2015, 5, 15980. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Barnett, E.S.; Schultz, N.; Stopsack, K.H.; Lam, E.T.; Arfe, A.; Lee, J.; Zhao, J.L.; Schonhoft, J.D.; Carbone, E.A.; Keegan, N.M.; et al. Analysis of BRCA2 Copy Number Loss and Genomic Instability in Circulating Tumor Cells from Patients with Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2023, 83, 112–120. [Google Scholar] [CrossRef]
- Lohr, J.G.; Adalsteinsson, V.A.; Cibulskis, K.; Choudhury, A.D.; Rosenberg, M.; Cruz-Gordillo, P.; Francis, J.M.; Zhang, C.Z.; Shalek, A.K.; Satija, R.; et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 2014, 32, 479–484. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Stoney, R.; Frese, K.K.; Simms, N.; Rowe, W.; Pearce, S.P.; Humphrey, S.; Booth, L.; Morgan, D.; Dynowski, M.; et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat. Cancer 2020, 1, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.J.; Trapani, F.; Metcalf, R.L.; Bertolini, G.; Hodgkinson, C.L.; Khandelwal, G.; Kelly, P.; Galvin, M.; Carter, L.; Simpson, K.L.; et al. Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: A clinical case study. Ann. Oncol. 2016, 27, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Frick, M.A.; Feigenberg, S.J.; Jean-Baptiste, S.R.; Aguarin, L.A.; Mendes, A.; Chinniah, C.; Swisher-McClure, S.; Berman, A.; Levin, W.; Cengel, K.A.; et al. Circulating Tumor Cells Are Associated with Recurrent Disease in Patients with Early-Stage Non-Small Cell Lung Cancer Treated with Stereotactic Body Radiotherapy. Clin. Cancer Res. 2020, 26, 2372–2380. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Tartarone, A.; Santarpia, M.; Zhu, Y.; Jiang, G. Circulating tumor cells can predict the prognosis of patients with non-small cell lung cancer after resection: A retrospective study. Transl. Lung Cancer Res. 2021, 10, 995–1006. [Google Scholar] [CrossRef]
- Jan, Y.J.; Chen, J.F.; Zhu, Y.; Lu, Y.T.; Chen, S.H.; Chung, H.; Smalley, M.; Huang, Y.W.; Dong, J.; Chen, L.C.; et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 78–93. [Google Scholar] [CrossRef]
- Lin, M.; Chen, J.F.; Lu, Y.T.; Zhang, Y.; Song, J.; Hou, S.; Ke, Z.; Tseng, H.R. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc. Chem. Res. 2014, 47, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Reategui, E.; Li, W.; Tessier, S.N.; Wong, K.H.; Jensen, A.E.; Thapar, V.; Ting, D.; Toner, M.; Stott, S.L.; et al. Enhanced Isolation and Release of Circulating Tumor Cells Using Nanoparticle Binding and Ligand Exchange in a Microfluidic Chip. J. Am. Chem. Soc. 2017, 139, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Chen, J.F.; Luo, C.H.; Lee, S.; Li, C.H.; Yang, Y.L.; Tsai, Y.H.; Ho, B.C.; Bao, L.R.; Lee, T.J.; et al. Glycan Stimulation Enables Purification of Prostate Cancer Circulating Tumor Cells on PEDOT NanoVelcro Chips for RNA Biomarker Detection. Adv. Healthc. Mater. 2018, 7, 1700701. [Google Scholar] [CrossRef] [PubMed]
- Gallerani, G.; Cocchi, C.; Bocchini, M.; Piccinini, F.; Fabbri, F. Characterization of Tumor Cells Using a Medical Wire for Capturing Circulating Tumor Cells: A 3D Approach Based on Immunofluorescence and DNA FISH. J. Vis. Exp. 2017, 130, 56936. [Google Scholar] [CrossRef]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Black, T.; Redlinger, C.; Romeo, J.; O’Hara, M.; Raj, A.; Carpenter, E.L.; Stanger, B.Z.; et al. A magnetic micropore chip for rapid (<1 hour) unbiased circulating tumor cell isolation and in situ RNA analysis. Lab Chip 2017, 17, 3086–3096. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H. Circulating Tumor Cell Cluster Sorting by Size and Asymmetry. Methods Mol. Biol. 2023, 2679, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yusa, A.; Toneri, M.; Masuda, T.; Ito, S.; Yamamoto, S.; Okochi, M.; Kondo, N.; Iwata, H.; Yatabe, Y.; Ichinosawa, Y.; et al. Development of a new rapid isolation device for circulating tumor cells (CTCs) using 3D palladium filter and its application for genetic analysis. PLoS ONE 2014, 9, e88821. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Marazzini, M.; Hershey, B.; Fardin, A.; Li, Q.; Wang, Z.; Giangreco, G.; Pisati, F.; Marchesi, S.; Disanza, A.; et al. PillarX: A Microfluidic Device to Profile Circulating Tumor Cell Clusters Based on Geometry, Deformability, and Epithelial State. Small 2022, 18, e2106097. [Google Scholar] [CrossRef]
- Gascoyne, P.R.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef]
- Shim, S.; Stemke-Hale, K.; Tsimberidou, A.M.; Noshari, J.; Anderson, T.E.; Gascoyne, P.R. Antibody-independent isolation of circulating tumor cells by continuous-flow dielectrophoresis. Biomicrofluidics 2013, 7, 11807. [Google Scholar] [CrossRef]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The Parsortix Cell Separation System-A versatile liquid biopsy platform. Cytometry A 2018, 93, 1234–1239. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.Y.; Taran, F.A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.C.; Friedl, T.W.; et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Xu, H.; Wang, Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Okabe, H.; Tsunoda, S.; Hosogi, H.; Hisamori, S.; Tanaka, E.; Tanaka, S.; Sakai, Y. Circulating Tumor Cells as an Independent Predictor of Survival in Advanced Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 3954–3961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawada, T.; Takahashi, H.; Sakakura, K.; Ida, S.; Mito, I.; Toyoda, M.; Chikamatsu, K. Circulating tumor cells in patients with head and neck squamous cell carcinoma: Feasibility of detection and quantitation. Head Neck 2017, 39, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, Y.; Upparahalli, S.V.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G.; Kaifi, J.T. Circulating tumor cell clusters are a potential biomarker for detection of non-small cell lung cancer. Lung Cancer 2019, 134, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, Y.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Deroche, C.B.; Pantel, K.; Li, G.; Kaifi, J.T. Circulating Giant Tumor-Macrophage Fusion Cells Are Independent Prognosticators in Patients With NSCLC. J. Thorac. Oncol. 2020, 15, 1460–1471. [Google Scholar] [CrossRef]
- Manjunath, Y.; Suvilesh, K.N.; Mitchem, J.B.; Avella Patino, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Pantel, K.; Yi, H.; Li, G.; Harris, P.K.; et al. Circulating Tumor-Macrophage Fusion Cells and Circulating Tumor Cells Complement Non-Small-Cell Lung Cancer Screening in Patients With Suspicious Lung-RADS 4 Nodules. JCO Precis. Oncol. 2022, 6, e2100378. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Figlin, R.A.; Burdick, M.D.; Dubinett, S.M.; Elashoff, R.M.; Strieter, R.M. CXCR4 expression on circulating pan-cytokeratin positive cells is associated with survival in patients with advanced non-small cell lung cancer. BMC Cancer 2009, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Taftaf, R.; Reduzzi, C.; Albert, M.K.; Romero-Calvo, I.; Liu, H. Better together: Circulating tumor cell clustering in metastatic cancer. Trends Cancer 2021, 7, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Adorno-Cruz, V.; Chang, Y.F.; Jia, Y.; Kawaguchi, M.; Dashzeveg, N.K.; Taftaf, R.; Ramos, E.K.; Schuster, E.J.; El-Shennawy, L.; et al. EGFR inhibition blocks cancer stem cell clustering and lung metastasis of triple negative breast cancer. Theranostics 2021, 11, 6632–6643. [Google Scholar] [CrossRef]
- Donato, C.; Kunz, L.; Castro-Giner, F.; Paasinen-Sohns, A.; Strittmatter, K.; Szczerba, B.M.; Scherrer, R.; Di Maggio, N.; Heusermann, W.; Biehlmaier, O.; et al. Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells. Cell Rep. 2020, 32, 108105. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; Cheung, E.C.; Blagih, J.; Domart, M.C.; Vousden, K.H. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab. 2019, 30, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 2021, 12, 4867. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112 e114. [Google Scholar] [CrossRef]
- Murlidhar, V.; Reddy, R.M.; Fouladdel, S.; Zhao, L.; Ishikawa, M.K.; Grabauskiene, S.; Zhang, Z.; Lin, J.; Chang, A.C.; Carrott, P.; et al. Poor Prognosis Indicated by Venous Circulating Tumor Cell Clusters in Early-Stage Lung Cancers. Cancer Res. 2017, 77, 5194–5206. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, P.; Martinez-Pena, I.; Pineiro, R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAFs). Cancers 2020, 12, 2861. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Q.; Zhao, Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med. Hypotheses 2016, 87, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, M.L.; Welte, T.; Boral, D.; Liu, H.N.; Yin, W.; Vishnoi, M.; Goswami-Sewell, D.; Li, L.; Pei, G.; Jia, P.; et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int. J. Mol. Sci. 2019, 20, 1916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wong, K.H.K.; Khankhel, A.H.; Zeinali, M.; Reategui, E.; Phillips, M.J.; Luo, X.; Aceto, N.; Fachin, F.; Hoang, A.N.; et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 2017, 17, 3498–3503. [Google Scholar] [CrossRef]
- Jansson, S.; Bendahl, P.O.; Larsson, A.M.; Aaltonen, K.E.; Ryden, L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Meikle, C.K.; Kelly, C.A.; Garg, P.; Wuescher, L.M.; Ali, R.A.; Worth, R.G. Cancer and Thrombosis: The Platelet Perspective. Front. Cell Dev. Biol. 2016, 4, 147. [Google Scholar] [CrossRef]
- Dasgupta, A.; Lim, A.R.; Ghajar, C.M. Circulating and disseminated tumor cells: Harbingers or initiators of metastasis? Mol. Oncol. 2017, 11, 40–61. [Google Scholar] [CrossRef]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef]
- Rack, B.; Schindlbeck, C.; Juckstock, J.; Andergassen, U.; Hepp, P.; Zwingers, T.; Friedl, T.W.; Lorenz, R.; Tesch, H.; Fasching, P.A.; et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst. 2014, 106, dju066. [Google Scholar] [CrossRef]
- Jiang, S.S.; Deng, B.; Feng, Y.G.; Qian, K.; Tan, Q.Y.; Wang, R.W. Circulating tumor cells prior to initial treatment is an important prognostic factor of survival in non-small cell lung cancer: A meta-analysis and system review. BMC Pulm. Med. 2019, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef]
- Conradi, L.C.; Bleckmann, A.; Schirmer, M.; Sprenger, T.; Jo, P.; Homayounfar, K.; Wolff, H.A.; Rothe, H.; Middel, P.; Becker, H.; et al. Thymidylate synthase as a prognostic biomarker for locally advanced rectal cancer after multimodal treatment. Ann. Surg. Oncol. 2011, 18, 2442–2452. [Google Scholar] [CrossRef] [PubMed]
- Troncarelli Flores, B.C.; Souza, E.S.V.; Ali Abdallah, E.; Mello, C.A.L.; Gobo Silva, M.L.; Gomes Mendes, G.; Camila Braun, A.; Aguiar Junior, S.; Thome Domingos Chinen, L. Molecular and Kinetic Analyses of Circulating Tumor Cells as Predictive Markers of Treatment Response in Locally Advanced Rectal Cancer Patients. Cells 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Greystoke, A.; Lancashire, L.; Cummings, J.; Ward, T.; Board, R.; Amir, E.; Hughes, S.; Krebs, M.; Hughes, A.; et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am. J. Pathol. 2009, 175, 808–816. [Google Scholar] [CrossRef]
- Lu, C.Y.; Tsai, H.L.; Uen, Y.H.; Hu, H.M.; Chen, C.W.; Cheng, T.L.; Lin, S.R.; Wang, J.Y. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br. J. Cancer 2013, 108, 791–797. [Google Scholar] [CrossRef]
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Piva, F.; Marinelli, O.; Maggi, F.; Bianchi, F.; Bittoni, A.; Berardi, R.; Giampieri, R.; et al. Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival. Front. Oncol. 2019, 9, 874. [Google Scholar] [CrossRef]
- Petrik, J.; Verbanac, D.; Fabijanec, M.; Hulina-Tomaskovic, A.; Ceri, A.; Somborac-Bacura, A.; Petlevski, R.; Grdic Rajkovic, M.; Rumora, L.; Kruslin, B.; et al. Circulating Tumor Cells in Colorectal Cancer: Detection Systems and Clinical Utility. Int. J. Mol. Sci. 2022, 23, 13582. [Google Scholar] [CrossRef]
- Mohamed, B.M.; Ward, M.P.; Bates, M.; Spillane, C.D.; Kelly, T.; Martin, C.; Gallagher, M.; Heffernan, S.; Norris, L.; Kennedy, J.; et al. Ex vivo expansion of circulating tumour cells (CTCs). Sci. Rep. 2023, 13, 3704. [Google Scholar] [CrossRef]
- Shuel, S.L. Targeted cancer therapies: Clinical pearls for primary care. Can. Fam. Physician 2022, 68, 515–518. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Maheswaran, S.; Sequist, L.V.; Nagrath, S.; Ulkus, L.; Brannigan, B.; Collura, C.V.; Inserra, E.; Diederichs, S.; Iafrate, A.J.; Bell, D.W.; et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 2008, 359, 366–377. [Google Scholar] [CrossRef]
- Tzanikou, E.; Markou, A.; Politaki, E.; Koutsopoulos, A.; Psyrri, A.; Mavroudis, D.; Georgoulias, V.; Lianidou, E. PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: A direct comparison study. Mol. Oncol. 2019, 13, 2515–2530. [Google Scholar] [CrossRef]
- Lopes, C.; Piairo, P.; Chicharo, A.; Abalde-Cela, S.; Pires, L.R.; Corredeira, P.; Alves, P.; Muinelo-Romay, L.; Costa, L.; Dieguez, L. HER2 Expression in Circulating Tumour Cells Isolated from Metastatic Breast Cancer Patients Using a Size-Based Microfluidic Device. Cancers 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yuan, X.; Tian, Y.; Wu, H.; Xu, H.; Hu, G.; Wu, K. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J. Hematol. Oncol. 2015, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Musella, V.; Pietrantonio, F.; Di Buduo, E.; Iacovelli, R.; Martinetti, A.; Sottotetti, E.; Bossi, I.; Maggi, C.; Di Bartolomeo, M.; de Braud, F.; et al. Circulating tumor cells as a longitudinal biomarker in patients with advanced chemorefractory, RAS-BRAF wild-type colorectal cancer receiving cetuximab or panitumumab. Int. J. Cancer 2015, 137, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D. Circulating cancer cells. Ann. Oncol. 2010, 21 (Suppl. 7), vii95–vii100. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, G.H.; Jeon, H.K.; Park, S.J. Clinical Application of Circulating Tumor Cells in Gastric Cancer. Gut Liver 2019, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kiniwa, Y.; Nakamura, K.; Mikoshiba, A.; Ashida, A.; Akiyama, Y.; Morimoto, A.; Okuyama, R. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAF mutation. BMC Cancer 2021, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Cancer Immunotherapy, Part 1: Current Strategies and Agents. Pharm. Ther. 2017, 42, 375–383. [Google Scholar]
- Pilard, C.; Ancion, M.; Delvenne, P.; Jerusalem, G.; Hubert, P.; Herfs, M. Cancer immunotherapy: It’s time to better predict patients’ response. Br. J. Cancer 2021, 125, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, S. Quantifying PD-L1 Expression to Monitor Immune Checkpoint Therapy: Opportunities and Challenges. Cancers 2020, 12, 3173. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.A.; Bottcher, L.M.; et al. Determination of PD-L1 Expression in Circulating Tumor Cells of NSCLC Patients and Correlation with Response to PD-1/PD-L1 Inhibitors. Cancers 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; Del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Botticelli, A.; Cirillo, A.; Strigari, L.; Valentini, F.; Cerbelli, B.; Scagnoli, S.; Cerbelli, E.; Zizzari, I.G.; Rocca, C.D.; D’Amati, G.; et al. Anti-PD-1 and Anti-PD-L1 in Head and Neck Cancer: A Network Meta-Analysis. Front. Immunol. 2021, 12, 705096. [Google Scholar] [CrossRef] [PubMed]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
- Anantharaman, A.; Friedlander, T.; Lu, D.; Krupa, R.; Premasekharan, G.; Hough, J.; Edwards, M.; Paz, R.; Lindquist, K.; Graf, R.; et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016, 16, 744. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Li, J.; Tong, B.; Xu, Y.; Chen, M.; Liu, X.; Gao, X.; Shi, Y.; Zhao, J.; et al. Circulating tumor cells PD-L1 expression detection and correlation of therapeutic efficacy of immune checkpoint inhibition in advanced non-small-cell lung cancer. Thorac. Cancer 2023, 14, 470–478. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells 2019, 8, 809. [Google Scholar] [CrossRef]
- Sho, S.; Court, C.M.; Winograd, P.; Lee, S.; Hou, S.; Graeber, T.G.; Tseng, H.R.; Tomlinson, J.S. Precision oncology using a limited number of cells: Optimization of whole genome amplification products for sequencing applications. BMC Cancer 2017, 17, 457. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Silvestris, E.; Felici, C.; Quaresmini, D.; Cafforio, P.; Silvestris, F. Next-generation Sequencing (NGS) Analysis on Single Circulating Tumor Cells (CTCs) with No Need of Whole-genome Amplification (WGA). Cancer Genom. Proteom. 2017, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Barbirou, M.; Miller, A.; Manjunath, Y.; Ramirez, A.B.; Ericson, N.G.; Staveley-O’Carroll, K.F.; Mitchem, J.B.; Warren, W.C.; Chaudhuri, A.A.; Huang, Y.; et al. Single Circulating-Tumor-Cell-Targeted Sequencing to Identify Somatic Variants in Liquid Biopsies in Non-Small-Cell Lung Cancer Patients. Curr. Issues Mol. Biol. 2022, 44, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.A.; Agelaki, S. Single-Cell RNA Sequencing Uncovers Heterogeneous Circulating Tumor Cell Subsets in Breast Cancer. Cancers 2022, 14, 1314. [Google Scholar] [CrossRef]
- Suvilesh, K.N.; Nussbaum, Y.I.; Radhakrishnan, V.; Manjunath, Y.; Avella, D.M.; Staveley-O’Carroll, K.F.; Kimchi, E.T.; Chaudhuri, A.A.; Shyu, C.R.; Li, G.; et al. Tumorigenic circulating tumor cells from xenograft mouse models of non-metastatic NSCLC patients reveal distinct single cell heterogeneity and drug responses. Mol. Cancer 2022, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Pradines, A.; Farella, M.; Casanova, A.; Gouin, S.; Keller, L.; Favre, G.; Mazieres, J. Monitoring KRAS mutations in circulating DNA and tumor cells using digital droplet PCR during treatment of KRAS-mutated lung adenocarcinoma. Lung Cancer 2016, 100, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Detection of EGFR Mutations in Plasma cfDNA and Paired CTCs of NSCLC Patients before and after Osimertinib Therapy Using Crystal Digital PCR. Cancers 2021, 13, 2736. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Halabi, S.; Kemeny, G.; Anand, M.; Giannakakou, P.; Nanus, D.M.; George, D.J.; Gregory, S.G.; Armstrong, A.J. Circulating Tumor Cell Genomic Evolution and Hormone Therapy Outcomes in Men with Metastatic Castration-Resistant Prostate Cancer. Mol. Cancer Res. 2021, 19, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Gasch, C.; Bauernhofer, T.; Pichler, M.; Langer-Freitag, S.; Reeh, M.; Seifert, A.M.; Mauermann, O.; Izbicki, J.R.; Pantel, K.; Riethdorf, S. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem. 2013, 59, 252–260. [Google Scholar] [CrossRef]
- Gorges, T.M.; Kuske, A.; Rock, K.; Mauermann, O.; Muller, V.; Peine, S.; Verpoort, K.; Novosadova, V.; Kubista, M.; Riethdorf, S.; et al. Accession of Tumor Heterogeneity by Multiplex Transcriptome Profiling of Single Circulating Tumor Cells. Clin. Chem. 2016, 62, 1504–1515. [Google Scholar] [CrossRef]
- Ortega, F.G.; Lorente, J.A.; Garcia Puche, J.L.; Ruiz, M.P.; Sanchez-Martin, R.M.; de Miguel-Perez, D.; Diaz-Mochon, J.J.; Serrano, M.J. miRNA in situ hybridization in circulating tumor cells--MishCTC. Sci. Rep. 2015, 5, 9207. [Google Scholar] [CrossRef]
- Magbanua, M.J.; Sosa, E.V.; Scott, J.H.; Simko, J.; Collins, C.; Pinkel, D.; Ryan, C.J.; Park, J.W. Isolation and genomic analysis of circulating tumor cells from castration resistant metastatic prostate cancer. BMC Cancer 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, L.; Gao, Y.; Wang, Y.; Liu, Y.; Zhang, H.; Wang, Q.; Hu, F.; Li, J.; Tan, J.; et al. Role of aneuploid circulating tumor cells and CD31(+) circulating tumor endothelial cells in predicting and monitoring anti-angiogenic therapy efficacy in advanced NSCLC. Mol. Oncol. 2021, 15, 2891–2909. [Google Scholar] [CrossRef]
- Davis, A.A.; Zhang, Q.; Gerratana, L.; Shah, A.N.; Zhan, Y.; Qiang, W.; Finkelman, B.S.; Flaum, L.; Behdad, A.; Gradishar, W.J.; et al. Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res. 2019, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dumenil, C.; Julie, C.; Giraud, V.; Dumoulin, J.; Labrune, S.; Chinet, T.; Emile, J.F.; He, B.; Giroux Leprieur, E. Molecular characterization of circulating tumor cells in lung cancer: Moving beyond enumeration. Oncotarget 2017, 8, 109818–109835. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.V.; Bardia, A.; Wittner, B.S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T.K.; Licausi, J.A.; Desai, R.; et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016, 537, 102–106. [Google Scholar] [CrossRef]
- Franken, A.; Kraemer, A.; Sicking, A.; Watolla, M.; Rivandi, M.; Yang, L.; Warfsmann, J.; Polzer, B.M.; Friedl, T.W.P.; Meier-Stiegen, F.; et al. Comparative analysis of EpCAM high-expressing and low-expressing circulating tumour cells with regard to their clonal relationship and clinical value. Br. J. Cancer 2023, 128, 1742–1752. [Google Scholar] [CrossRef]
- Abouleila, Y.; Onidani, K.; Ali, A.; Shoji, H.; Kawai, T.; Lim, C.T.; Kumar, V.; Okaya, S.; Kato, K.; Hiyama, E.; et al. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Sci. 2019, 110, 697–706. [Google Scholar] [CrossRef]
- Aoki, M.; Shoji, H.; Kashiro, A.; Takeuchi, K.; Shimizu, Y.; Honda, K. Prospects for Comprehensive Analyses of Circulating Tumor Cells in Tumor Biology. Cancers 2020, 12, 1135. [Google Scholar] [CrossRef]
- Cardinali, B.; De Luca, G.; Tasso, R.; Coco, S.; Garuti, A.; Buzzatti, G.; Sciutto, A.; Arecco, L.; Villa, F.; Carli, F.; et al. Targeting PIK3CA Actionable Mutations in the Circulome: A Proof of Concept in Metastatic Breast Cancer. Int. J. Mol. Sci. 2022, 23, 6320. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Del Grammastro, M.; Felicioni, L.; Malatesta, S.; Filice, G.; Centi, I.; De Pas, T.; Santoro, A.; Chella, A.; Brandes, A.A.; et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: Toward a real-time liquid biopsy for treatment. PLoS ONE 2014, 9, e103883. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.L.; Freeman, J.B.; Millward, M.; Ziman, M.; Gray, E.S. Detection of BRAF-V600E and V600K in melanoma circulating tumour cells by droplet digital PCR. Clin. Biochem. 2015, 48, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Catelain, C.; Pailler, E.; Oulhen, M.; Faugeroux, V.; Pommier, A.L.; Farace, F. Detection of Gene Rearrangements in Circulating Tumor Cells: Examples of ALK-, ROS1-, RET-Rearrangements in Non-Small-Cell Lung Cancer and ERG-Rearrangements in Prostate Cancer. Adv. Exp. Med. Biol. 2017, 994, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra148. [Google Scholar] [CrossRef] [PubMed]
- Balcik-Ercin, P.; Cayrefourcq, L.; Soundararajan, R.; Mani, S.A.; Alix-Panabieres, C. Epithelial-to-Mesenchymal Plasticity in Circulating Tumor Cell Lines Sequentially Derived from a Patient with Colorectal Cancer. Cancers 2021, 13, 5408. [Google Scholar] [CrossRef] [PubMed]
- Tehranian, C.; Fankhauser, L.; Harter, P.N.; Ratcliffe, C.D.H.; Zeiner, P.S.; Messmer, J.M.; Hoffmann, D.C.; Frey, K.; Westphal, D.; Ronellenfitsch, M.W.; et al. The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol. 2022, 24, 213–225. [Google Scholar] [CrossRef]
- Savelieva, O.E.; Tashireva, L.A.; Kaigorodova, E.V.; Buzenkova, A.V.; Mukhamedzhanov, R.K.; Grigoryeva, E.S.; Zavyalova, M.V.; Tarabanovskaya, N.A.; Cherdyntseva, N.V.; Perelmuter, V.M. Heterogeneity of Stemlike Circulating Tumor Cells in Invasive Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2780. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.C.; Feng, W.N.; Zhang, L.; Liu, X.Q.; Guo, W.B.; Deng, Y.M.; Zou, Q.F.; Yang, J.J.; Zhou, Q.; et al. Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients. Ther. Adv. Med. Oncol. 2023, 15, 17588359231167818. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Z.; Zhang, X.; Gong, J.; Shen, L. Value of serum human epithelial growth factor receptor 2 extracellular domain and circulating tumor cells in evaluating therapeutic response in advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2017, 20, 1293–1299. [Google Scholar]
- Cayrefourcq, L.; Thomas, F.; Mazard, T.; Assenat, E.; Assou, S.; Alix-Panabieres, C. Selective treatment pressure in colon cancer drives the molecular profile of resistant circulating tumor cell clones. Mol. Cancer 2021, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Park, J.; Mittal, A.; Abou-Alfa, G.K.; El Dika, I.; Epstein, A.S.; Ilson, D.H.; Kelsen, D.P.; Ku, G.Y.; Li, J.; et al. Circulating tumor and invasive cell expression profiling predicts effective therapy in pancreatic cancer. Cancer 2022, 128, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Helissey, C.; Berger, F.; Cottu, P.; Dieras, V.; Mignot, L.; Servois, V.; Bouleuc, C.; Asselain, B.; Pelissier, S.; Vaucher, I.; et al. Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: The observational step of the CirCe01 phase III trial. Cancer Lett. 2015, 360, 213–218. [Google Scholar] [CrossRef]
- Behbakht, K.; Sill, M.W.; Darcy, K.M.; Rubin, S.C.; Mannel, R.S.; Waggoner, S.; Schilder, R.J.; Cai, K.Q.; Godwin, A.K.; Alpaugh, R.K. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: A Gynecologic Oncology Group study. Gynecol. Oncol. 2011, 123, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Smerage, J.B.; Budd, G.T.; Doyle, G.V.; Brown, M.; Paoletti, C.; Muniz, M.; Miller, M.C.; Repollet, M.I.; Chianese, D.A.; Connelly, M.C.; et al. Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer. Mol. Oncol. 2013, 7, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Pierga, J.Y.; Bidard, F.C.; Cropet, C.; Tresca, P.; Dalenc, F.; Romieu, G.; Campone, M.; Mahier Ait-Oukhatar, C.; Le Rhun, E.; Goncalves, A.; et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: The LANDSCAPE trial. Ann. Oncol. 2013, 24, 2999–3004. [Google Scholar] [CrossRef]
- Kalykaki, A.; Agelaki, S.; Kallergi, G.; Xyrafas, A.; Mavroudis, D.; Georgoulias, V. Elimination of EGFR-expressing circulating tumor cells in patients with metastatic breast cancer treated with gefitinib. Cancer Chemother. Pharmacol. 2014, 73, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Berger, F.; Cottu, P.; Loirat, D.; Rampanou, A.; Brain, E.; Cyrille, S.; Bourgeois, H.; Kiavue, N.; Deluche, E.; et al. Clinical utility of circulating tumour cell-based monitoring of late-line chemotherapy for metastatic breast cancer: The randomised CirCe01 trial. Br. J. Cancer 2021, 124, 1207–1213. [Google Scholar] [CrossRef]
- Kaifi, J.T.; Kunkel, M.; Das, A.; Harouaka, R.A.; Dicker, D.T.; Li, G.; Zhu, J.; Clawson, G.A.; Yang, Z.; Reed, M.F.; et al. Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: A prospective trial with different detection techniques. Cancer Biol. Ther. 2015, 16, 699–708. [Google Scholar] [CrossRef]
- Frithiof, H.; Welinder, C.; Larsson, A.M.; Ryden, L.; Aaltonen, K. A novel method for downstream characterization of breast cancer circulating tumor cells following CellSearch isolation. J. Transl. Med. 2015, 13, 126. [Google Scholar] [CrossRef]
- Narbe, U.; Bendahl, P.O.; Aaltonen, K.; Ferno, M.; Forsare, C.; Jorgensen, C.L.T.; Larsson, A.M.; Ryden, L. The Distribution of Circulating Tumor Cells Is Different in Metastatic Lobular Compared to Ductal Carcinoma of the Breast-Long-Term Prognostic Significance. Cells 2020, 9, 1718. [Google Scholar] [CrossRef] [PubMed]
- Agelaki, S.; Kalykaki, A.; Markomanolaki, H.; Papadaki, M.A.; Kallergi, G.; Hatzidaki, D.; Kalbakis, K.; Mavroudis, D.; Georgoulias, V. Efficacy of Lapatinib in Therapy-Resistant HER2-Positive Circulating Tumor Cells in Metastatic Breast Cancer. PLoS ONE 2015, 10, e0123683. [Google Scholar] [CrossRef]
- Milojkovic Kerklaan, B.; Pluim, D.; Bol, M.; Hofland, I.; Westerga, J.; van Tinteren, H.; Beijnen, J.H.; Boogerd, W.; Schellens, J.H.; Brandsma, D. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2016, 18, 855–862. [Google Scholar] [CrossRef]
- Childs, A.; Vesely, C.; Ensell, L.; Lowe, H.; Luong, T.V.; Caplin, M.E.; Toumpanakis, C.; Thirlwell, C.; Hartley, J.A.; Meyer, T. Expression of somatostatin receptors 2 and 5 in circulating tumour cells from patients with neuroendocrine tumours. Br. J. Cancer 2016, 115, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Caplin, M.; Khan, M.S.; Toumpanakis, C.; Shetty, S.; Ramage, J.K.; Houchard, A.; Higgs, K.; Shah, T. Circulating tumour cells and tumour biomarkers in functional midgut neuroendocrine tumours. J. Neuroendocrinol. 2022, 34, e13096. [Google Scholar] [CrossRef]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef]
- Salgia, R.; Weaver, R.W.; McCleod, M.; Stille, J.R.; Yan, S.B.; Roberson, S.; Polzer, J.; Flynt, A.; Raddad, E.; Peek, V.L.; et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: Exploratory analysis of a phase II study. Investig. New Drugs 2017, 35, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Beinse, G.; Berger, F.; Cottu, P.; Dujaric, M.E.; Kriegel, I.; Guilhaume, M.N.; Dieras, V.; Cabel, L.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cell count and thrombosis in metastatic breast cancer. J. Thromb. Haemost. 2017, 15, 1981–1988. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, V.; Leroy, S.; Cohen, C.; Heeke, S.; Cattet, F.; Bence, C.; Lalvee, S.; Mouroux, J.; Marquette, C.H.; et al. Use of circulating tumor cells in prospective clinical trials for NSCLC patients—Standardization of the pre-analytical conditions. Clin. Chem. Lab. Med. 2018, 56, 980–989. [Google Scholar] [CrossRef]
- Ilie, M.; Mazieres, J.; Chamorey, E.; Heeke, S.; Benzaquen, J.; Thamphya, B.; Boutros, J.; Tiotiu, A.; Fayada, J.; Cadranel, J.; et al. Prospective Multicenter Validation of the Detection of ALK Rearrangements of Circulating Tumor Cells for Noninvasive Longitudinal Management of Patients With Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 807–816. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Litiere, S.; Rothe, F.; Riethdorf, S.; Proudhon, C.; Fehm, T.; Aalders, K.; Forstbauer, H.; Fasching, P.A.; Brain, E.; et al. Trastuzumab versus observation for HER2 nonamplified early breast cancer with circulating tumor cells (EORTC 90091-10093, BIG 1-12, Treat CTC): A randomized phase II trial. Ann. Oncol. 2018, 29, 1777–1783. [Google Scholar] [CrossRef]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018, 7, e1438111. [Google Scholar] [CrossRef]
- Buscail, E.; Alix-Panabieres, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef]
- Jacot, W.; Cottu, P.; Berger, F.; Dubot, C.; Venat-Bouvet, L.; Lortholary, A.; Bourgeois, H.; Bollet, M.; Servent, V.; Luporsi, E.; et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: The CirCe T-DM1 trial. Breast Cancer Res. 2019, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Orden, V.; Martinez, A.; Bando, I.; Balbin, M.; Bellosillo, B.; Palanca, S.; Peligros Gomez, M.I.; Mediero, B.; Llovet, P.; et al. Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naive Metastatic Colorectal Cancer Patients Prospectively Collected. Clin. Color. Cancer 2020, 19, e110–e116. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Fonseca, P.; Sastre, J.; Garcia-Alfonso, P.; Gomez-Espana, M.A.; Salud, A.; Gil, S.; Rivera, F.; Reina, J.J.; Quintero, G.; Valladares-Ayerbes, M.; et al. Association of Circulating Tumor Cells and Tumor Molecular Profile With Clinical Outcomes in Patients With Previously Untreated Metastatic Colorectal Cancer: A Pooled Analysis of the Phase III VISNU-1 and Phase II VISNU-2 Randomized Trials. Clin. Color. Cancer 2023, 22, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; Vieitez, J.M.; Gomez-Espana, A.; Gil Calle, S.; Salud-Salvia, A.; Grana, B.; Garcia-Alfonso, P.; Rivera, F.; Quintero-Aldana, G.A.; Reina-Zoilo, J.J.; et al. FOLFOXIRI plus bevacizumab versus FOLFOX plus bevacizumab for patients with metastatic colorectal cancer and >/=3 circulating tumour cells: The randomised phase III VISNU-1 trial. ESMO Open 2020, 5, e000944. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Gomez-Caamano, A.; Cabeza Rodriguez, M.A.; Muinelo-Romay, L.; Martin de Vidales, C.; Abalo, A.; Calvo Crespo, P.; Leon Mateos, L.; Olivier, C.; Vega Piris, L.V. Detection and dynamics of circulating tumor cells in patients with high-risk prostate cancer treated with radiotherapy and hormones: A prospective phase II study. Radiat. Oncol. 2020, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Hovaguimian, F.; Braun, J.; Z’Graggen, B.R.; Schlapfer, M.; Dumrese, C.; Ewald, C.; Dedes, K.J.; Fink, D.; Rolli, U.; Seeberger, M.; et al. Anesthesia and Circulating Tumor Cells in Primary Breast Cancer Patients: A Randomized Controlled Trial. Anesthesiology 2020, 133, 548–558. [Google Scholar] [CrossRef]
- Brady, L.; Hayes, B.; Sheill, G.; Baird, A.M.; Guinan, E.; Stanfill, B.; Vlajnic, T.; Casey, O.; Murphy, V.; Greene, J.; et al. Platelet cloaking of circulating tumour cells in patients with metastatic prostate cancer: Results from ExPeCT, a randomised controlled trial. PLoS ONE 2020, 15, e0243928. [Google Scholar] [CrossRef]
- Bidard, F.C.; Jacot, W.; Kiavue, N.; Dureau, S.; Kadi, A.; Brain, E.; Bachelot, T.; Bourgeois, H.; Goncalves, A.; Ladoire, S.; et al. Efficacy of Circulating Tumor Cell Count-Driven vs Clinician-Driven First-line Therapy Choice in Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: The STIC CTC Randomized Clinical Trial. JAMA Oncol. 2021, 7, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Galardi, F.; De Luca, F.; Biagioni, C.; Migliaccio, I.; Curigliano, G.; Minisini, A.M.; Bonechi, M.; Moretti, E.; Risi, E.; McCartney, A.; et al. Circulating tumor cells and palbociclib treatment in patients with ER-positive, HER2-negative advanced breast cancer: Results from a translational sub-study of the TREnd trial. Breast Cancer Res. 2021, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.C.F.; Barlow, W.E.; Somlo, G.; Gralow, J.R.; Schott, A.F.; Hayes, D.F.; Kuhn, P.; Hicks, J.B.; Welter, L.; Dy, P.A.; et al. A Randomized Trial of Fulvestrant, Everolimus, and Anastrozole for the Front-line Treatment of Patients with Advanced Hormone Receptor-positive Breast Cancer, SWOG S1222. Clin. Cancer Res. 2022, 28, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Kontopodis, E.; Ntzifa, A.; Jordana-Ariza, N.; Karachaliou, N.; Pantazaka, E.; Charalambous, H.A.; Psyrri, A.; Tsaroucha, E.; Boukovinas, I.; et al. Effect of Osimertinib on CTCs and ctDNA in EGFR Mutant Non-Small Cell Lung Cancer Patients: The Prognostic Relevance of Liquid Biopsy. Cancers 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Schott, D.; Pizon, M.; Drozdz, S.; Wendt, T.; Wittig, A.; Pachmann, K. Increased Circulating Epithelial Tumor Cells (CETC/CTC) over the Course of Adjuvant Radiotherapy Is a Predictor of Less Favorable Outcome in Patients with Early-Stage Breast Cancer. Curr. Oncol. 2022, 30, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Jin, D.; Luo, J.; Shi, Y.; Zhang, Y.; Wu, L.; Song, Y.; Su, D.; Pan, Z.; et al. Mu-opioid receptor agonist facilitates circulating tumor cell formation in bladder cancer via the MOR/AKT/Slug pathway: A comprehensive study including randomized controlled trial. Cancer Commun. 2023, 43, 365–386. [Google Scholar] [CrossRef]
- Fehm, T.; Mueller, V.; Banys-Paluchowski, M.; Fasching, P.A.; Friedl, T.W.P.; Hartkopf, A.; Huober, J.; Loehberg, C.; Rack, B.; Riethdorf, S.; et al. Efficacy of Lapatinib in Patients with HER2-Negative Metastatic Breast Cancer and HER2-Positive Circulating Tumor Cells-The DETECT III Clinical Trial. Clin. Chem. 2024, 70, 307–318. [Google Scholar] [CrossRef]
| Name | Enrichment Technique | Type (Physical or Biological) | Key Findings |
|---|---|---|---|
| Herringbone (HB)-Chip | Surface affinity | Biological | CTCs were detected in 93% of patients with metastatic disease [13]. |
| Nano Velcro | Cell affinity | Biological | Capable of detecting, isolating, and purifying CTCs from blood samples with high efficiency for subsequent molecular analyses [28,29]. |
| Nanoparticle-herringbone microfluidic chip (NP-HBCTC-Chip) | Surface affinity | Biological | Enhanced capture efficiency and recovery of isolated CTCs [30]. |
| PEDOT Nano Velcro Chips | Cell affinity | Biological | Ability to achieve high cell purity as well as preserve the integrity of RNA transcripts from the purified cells [31]. |
| CaTCh FISH | Magnetic separation/fluorescence in situ hybridization | Physical | Capture CTCs for in situ RNA analysis [32,33]. |
| Two-stage microfluidic chip | Size and asymmetry based capturing | Physical | High rate (99%) CTC clusters recovery with 87% viability [34,35]. |
| Bait-trap chip | In situ rolling circle amplification (RCA) method | Physical | Accurate and ultrasensitive capture of live CTCs from peripheral blood [35]. |
| 3D Palladium Filter | Lithography plus electroforming process | Physical | Enumeration and isolation of CTCs for genetic analysis [36]. |
| Pillar-X | Bimodular microfluidic device | Biophysical | Efficiently captures both single cells and clusters and sorts them based on size, cohesiveness, and epithelial identity [37]. |
| Dielectrophoretic field-flow-fractionation (DEP-FFF) | Batch-mode microfluidic di-electrophoresis method | Physical | 70–75% capture efficiency [38,39]. |
| Parsortix™ Cell Separation System | Microfluidic particle separation technology | Biophysical | High capture efficiency and viable CTCs for downstream analyses [40]. |
| Name | Advantages | Limitations | References |
|---|---|---|---|
| RT-PCR |
|
| [14,110] |
| RNA in situ hybridization |
|
| [15,111] |
| Single-cell RNA sequencing |
|
| [16,25] |
| Fluorescence In Situ Hybridization (FISH) |
|
| [17,112] |
| Integrated immunostaining fluorescence in situ hybridization (iFISH) |
|
| [113] |
| Targeted DNA sequencing |
|
| [12,114] |
| Single-cell exome/genome sequencing |
|
| [18,115,116] |
| Bulk mass spectroscopy |
|
| [117,118] |
| Single-cell mass spectroscopy |
|
| [119,120] |
| Trial Number, Year of Completion, Study Type and Phase. | Name | Cancer Type | Cancer Stage and Other Information | Key Findings |
|---|---|---|---|---|
| NCT00429793. 2012. Interventional. Phase 2. | NA | Ovarian Cancer | Advanced. Grade 1,2,3. Tumor types-adenocarcinoma, clear cell carcinoma, endometrioid adenocarcinoma and serous adenocarcinoma. | Positive CTC pre-treatment showed lack of response to mTOR inhibitor, temsirolimus and high expression of apoptosis marker in CTCs was associated with longer progression-free survival [135]. |
| NCT00156273. 2008. Observational. | NA | Breast cancer | Advanced (Stage IV). Metastatic breast cancer. ECOG status 0–2. | In patients with elevated CTC, higher levels of CTC-apoptosis were associated with worse prognosis, while higher CTC-BCL-2 levels correlated with better outcomes [136]. |
| NCT00967031. 2012. Interventional. Phase 2. | LANDSCAPE | Breast cancer | Advanced. Brain metastases overexpressing HER2. ECOG performance status of 0–2. | After 21 days of lapatinib treatment, a disappearance of CTC was observed in 11 of 36 patients. The 1-year overall survival rate was 83.9% in patients with no CTC at day 21 versus 42.9% in patients with ≥1 CTC [137]. |
| NCT00428896. 2008. Interventional. Phase 2. | NA | Breast Cancer | Advanced. Metastatic breast cancer with EGFR expression. | A median reduction of 96.4 and 94.1% in CTC count was observed in 11 (64.7%) and 12 (70.6%) of patients after the first and the second gefitinib treatment cycles, respectively. Treatment-resistant CTCs could be eliminated by gefitinib in metastatic breast cancer, and EGFR expression on CTCs merits further validation as a potential biomarker for specific and effective targeting of CTCs [138]. |
| NCT00382018. 2017. Interventional. Phase 3. | SWOG S0500 | Breast Cancer | Advanced. Metastatic breast cancer. ECOG status 0–2. Patients enrolled before initiation of first line of chemotherapy. ER-positive, HER2-negative, triple-negative and HER2-positive patients were included in the study. | Prognostic significance of CTCs in patients with metastatic breast cancer receiving first-line chemotherapy was confirmed. For patients with persistently increased CTCs after 21 days of first-line chemotherapy, early switching to an alternate cytotoxic therapy was not effective in prolonging overall survival [130]. |
| NCT01349842. 2018. Interventional. Phase 3. | CirCe01 | Breast Cancer | Advanced (Stage III–IV). Metastatic lobular or ductal adenocarcinoma. Eastern Cooperative Oncology Group (ECOG) status 0–4. | Early changes in CTC count were correlated with first cycle of third line chemotherapy treatment outcome. Among patients with <5 CTC/7.5 mL at baseline showed better prognostication for progression-free survival [134]. However, due to the limited accrual and compliance, this trial failed to demonstrate the clinical utility of CTC monitoring in third- and fourth-lines chemotherapy [139]. |
| NCT01722903. 2015. Observational. | NA | Colorectal Cancer | Advanced (Stage IV). Colorectal cancer with resectable metastases limited to liver and lungs. | CTCs were quantified in blood of patients collected at incision, during resection, 30 min after resection, and on postoperative day 1 by EpCAM-based CellSearch and size-based isolation method. CTC quantity was significantly higher with size-based filtration method than CellSearch at all points of blood collection [140]. |
| NCT01322893. 2016. Observational. | CTC-MBC | Breast Cancer | Advanced (Stage IV). Metastatic breast cancer with estrogen receptor alpha and HER2 expression. Invasive lobular and ductal carcinoma of no special type. ECOG status 0–2. | Study demonstrated the feasibility to ascertain the status of important predictive biomarkers expressed in breast cancer CTCs using the newly developed CTC-DropMount technique [141]. Patients with a continuous presence of apoptotic or CTC clusters in follow up during systemic therapy had worse prognosis than patients without similar CTC characteristics [66]. Longitudinal evaluation of CTC and CTC clusters were shown to improve prognostication and monitoring in patients with metastatic breast cancer starting first-line systemic therapy [10]. The number of CTCs were found to be higher in invasive lobular carcinoma compared to invasive ductal carcinoma highlighting the importance of different CTC cut-off considerations in different breast cancer types [142]. |
| NCT00694252. 2011. Interventional. Phase 2. | NA | Breast Cancer | Advanced (Stage IIIB and IV). ECOG status 0–2. | Lapatinib treatment is effective in decreasing HER2-positive CTCs in patients with metastatic breast cancer irrespective of the HER2 status of the primary tumor [143]. |
| NCT01713699. 2017. Interventional. | NA | Leptomeningeal metastases from 9 tumor types * | Advanced. Patients treated for advanced EpCAM-positive solid tumors. ECOG status 0–4. | EpCAM-based flow cytometry assay to detect CTCs in cerebrospinal fluid is superior to cytology for the diagnosis of leptomeningeal metastases in patients with a clinical suspicion of metastases but a negative or inconclusive MRI [144]. |
| NCT02075606. 2017. Interventional. Phase 4. | CALMNET | Neuroendocrine cancers # Midgut neuroendocrine cancers % | Early and advanced. Only patients with well or moderately differentiated tumors with a Ki67 proliferation index of <20% was recruited. | Somatostatin receptors 2 and 5 were detected on CTCs in patients with neuroendocrine tumors which might be a useful biomarker for evaluating somatostatin receptor-targeted therapies [145]. Patients without CTC at baseline may be more likely to achieve a symptomatic response following lanreotide autogel treatment than patients with CTC [146]. |
| NCT01577511. 2017. Observational. | NA | Colorectal Cancer | Advanced (Stage IV). Chemotherapy-naïve patients with metastatic colorectal cancer. | Patient-derived colorectal CTC lines contain functional cancer stem cells and express high levels of drug metabolism genes rendering them resistant to conventional therapies [147]. |
| NCT01439568. 2016. Interventional. Phase 2. | NA | SCLC | Advanced. A total of 60–70% of patients had extensive-stage disease. | Weak positive correlation at baseline between CXCR4 expression in tumor tissue and CTCs was observed in patients treated with CXCR4 peptide antagonist LY2510924 plus carboplatin-etoposide. Baseline CXCR4+ CTCs ≥ 7% was prognostic of shorter progression-free survival [148]. |
| NCT00898014. 2010. Observational. | IC2006-04 | Breast Cancer | Advanced (Stage IV). No prior chemotherapy for metastatic disease. | Detectable CTC was the only factor observed to be significantly associated with an increased risk of arterial thrombotic events [149]. |
| NCT01625702. 2015. Interventional. | NA | Gastric cancer | Advanced gastric adenocarcinoma. Karnofsky performance status ≥ 60. | CTC number was found to be significantly correlated to prognosis in histologically HER2-negative patients treated with fluorouracil-based chemotherapy. In patients that are histologically HER2-positive, CTC number was not obviously correlated to the progression-free or overall survival during combined anti-HER2-targeted therapy [131]. |
| NCT02372448. 2019. Interventional. | STALKLUNG01 | NSCLC | Early and advanced. Lung adenocarcinoma with ALK rearrangement on tumor tissue was included. | As a part of standardization of the pre-analytical conditions for CTC-based clinical trials, study found out that blood processed after 24 h and 48 h in BCT tubes showed stable CTCs counts and integrity, whereas CTCs in K3EDTA tubes showed an altered morphology in all patients. Moreover, CTCs recovered in BCT or K3EDTA tubes were evaluable for MET expression, ALK rearrangement studies [150]. CTCs can be used as a complementary tool to a tissue biopsy for the detection of ALK rearrangements. Longitudinal analyses of CTCs are promising for real-time patient monitoring and improved delivery of molecularly guided therapy [151]. |
| NCT01548677. 2017. Interventional. Phase 2. | TREAT-CTC | Breast Cancer | Early. HER2-negative primary non metastatic adenocarcinoma of the breast. | Study aimed to assess whether trastuzumab treatment decreases the detection rate of CTCs in HER2 nonamplified, early breast cancer patients and found that Trastuzumab does not decrease the detection rate of CTCs [152]. |
| NCT02937116. 2020. Interventional. Phase 1. | IBI308 | Ten types of gastrointestinal tumors @ | Advanced (Stage IIIB-IV). ECOG status 0–1. | Abundance of PD-L1high CTCs at baseline serve as a predictor to screen patients for PD-1/PD-L1 blockade therapies and measuring the dynamic changes in CTC indicate the therapeutic response at early time [153]. |
| NCT03032913. 2017. Observational. | PANC-CTC | Pancreatic cancer | Early (Stage I, IIb and III). Pancreatic ductal adenocarcinoma. | Combined CTC and exosome detection displayed 100% of sensitivity and 80% of specificity, with a negative predictive value of 100%. High levels of exosomes and/or CTC presence were significantly correlated with progression-free survival and with overall survival when CTC clusters were found [154]. |
| NCT01975142. 2019. Interventional. Phase 2. | CirCe T-DM1 | Breast Cancer | Advanced. Metastatic breast cancer. HER2-negative primary tumor. ECOG status of 0–2. | CTC with HER2 amplification can be detected in a limited subset of HER2-negative metastatic breast cancer patients indicating the importance of clonal evolutionary changes within the tumor [155]. |
| NCT01640444 (VISNU-2). 2018. Interventional. Phase 2. NCT01640405 (VISNU-1). 2018. Interventional. Phase 3. | VISNÚ-1/2 | Colorectal Cancer | Advanced. Metastatic colorectal adenocarcinoma. ECOG status of 0–1. | Elevated baseline CTCs and RAS mutations were associated with clinicopathologic features known to be associated with poor prognosis [156]. Patients with baseline CTC ≥ 3 count had poor prognosis [157]. First-line 5-fluorouracil/leucovorin, oxaliplatin, irinotecan plus bevacizumab treatment significantly improved progression-free survival in patients with ≥3 CTCs at baseline compared to 5-fluorouracil/leucovorin, oxaliplatin plus bevacizumab doublet therapy [158]. |
| NCT01800058. 2018. Observational. | NA | Prostate Cancer | Early (Stage II and III). Prostate adenocarcinoma. Karnofsky performance score of ≥70. | Positive CTC status at diagnosis, following neoadjuvant androgen deprivation therapy, at the end of radiotherapy, and 9 months after radiotherapy was not significantly associated with any clinical or pathologic factors and overall survival [159]. |
| NCT02005770. 2018. Interventional. Phase 4. | NA | Breast Cancer | Early (Stage 0–III). Primary preinvasive and invasive breast cancer without metastases. | Study evaluated the association of different types of anesthesia with postoperative CTC counts in surgically resectable breast cancer patients and found that there was no difference between sevoflurane and propofol with respect to CTC counts over time [160]. |
| NCT02453139. 2017. Interventional. | ExPeCT | Prostate Cancer | Advanced. Prostate adenocarcinoma participants were stratified based on body mass index. | Platelet cloaking of CTCs was observed in the patient population for the first time but without any significant correlation with clinico-pathological information [161]. |
| NCT01710605. 2018. Interventional. Phase 3. | STIC CTC | Breast Cancer | Advanced. Metastatic ductal adenocarcinoma. | CTC count was found to be a reliable biomarker method for guiding the choice between chemotherapy and endocrine therapy as the first-line treatment in hormone receptor-positive, HER2-negative metastatic breast cancer patients [162]. |
| NCT01596790. 2019. Interventional. | NA | Colorectal Cancer | Advanced. Colon or rectum adenocarcinoma, visceral metastases. WHO performance status 0, 1 or 2. | Differential gene expression pattern was observed in CTCs of same patient during first- and second-line chemotherapy treatments and disease progression highlighting the CTCs adaptability to escape treatment pressure [132]. |
| NCT02549430. 2017. Interventional. Phase 2. | TREnd | Breast Cancer | Advanced. Endocrine resistant ER-positive, HER2-negative advanced breast adenocarcinoma. | CTC count was found to be a promising modality in monitoring palbociclib response in patients with ER-positive, HER2-negative advanced breast cancer [163]. |
| NCT02137837. 2019. Interventional. Phase 3. | SWOG1222 | Breast Cancer | Advanced. Invasive breast carcinoma with ER-positive and HER-2-negative status. | An association was observed of baseline CTC and ctDNA with poorer survival [164]. |
| NCT02771314. 2020. Interventional. Phase 2. | NA | NSCLC | Early and advanced. Patients with histologically documented EGFR-mutant NSCLC. | The decrease in both CTCs and ctDNA occurring early during osimertinib treatment in EGFR Mutant NSCLC patients was found to be predictive of better outcome [165]. |
| NCT03033927. 2024 (estimated). Observational. | NA | Pancreatic cancer | Advanced pancreatic adenocarcinoma. | Chemosensitivity assay profiling of CTCs was found to be a promising tool for guiding therapy in advanced pancreatic adenocarcinoma [133]. |
| NCT03935802. 2018. Observational. | NA | Breast Cancer | Early (Stage I–III). Invasive ductal carcinoma, Invasive lobular carcinoma. | Increase in CTC numbers over the course of adjuvant radiotherapy signified a potential predictive biomarker to judge relative risk or benefit in patients with early breast cancer [166]. |
| NCT04358718. 2021. Interventional. | NA | Bladder cancer | Early | μ-opioid receptor agonists used for pain treatment both during and after surgery in blader cancer patients was associated with high CTCs and CTC cluster counts [167]. |
| NCT01740804. 2026. Observational. | POLICE | NSCLC | Advanced (Stage IIIb and IV). Adenocarcinoma, squamous cell carcinoma and Mixed NSCLC ECOG status of 0–1. | CTC persistent presence during treatment represented poor prognosis and resistance to chemotherapy in advanced NSCLC [129]. |
| NCT01619111. 2022. Interventional. Phase 3. | DETECT III | Breast Cancer | Advanced. HER2+ metastatic breast cancer. ECOG Score < 2. | Study demonstrated that phenotyping of CTCs has clinical utility for stratification of metastatic breast cancer patients irrespective of HER-2-positive or -negative status for targeted therapy. Study highlighted the phenotypic changes in tumor cells during disease progression [168]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, V.; Kaifi, J.T.; Suvilesh, K.N. Circulating Tumor Cells: How Far Have We Come with Mining These Seeds of Metastasis? Cancers 2024, 16, 816. https://doi.org/10.3390/cancers16040816
Radhakrishnan V, Kaifi JT, Suvilesh KN. Circulating Tumor Cells: How Far Have We Come with Mining These Seeds of Metastasis? Cancers. 2024; 16(4):816. https://doi.org/10.3390/cancers16040816
Chicago/Turabian StyleRadhakrishnan, Vijay, Jussuf T. Kaifi, and Kanve N. Suvilesh. 2024. "Circulating Tumor Cells: How Far Have We Come with Mining These Seeds of Metastasis?" Cancers 16, no. 4: 816. https://doi.org/10.3390/cancers16040816
APA StyleRadhakrishnan, V., Kaifi, J. T., & Suvilesh, K. N. (2024). Circulating Tumor Cells: How Far Have We Come with Mining These Seeds of Metastasis? Cancers, 16(4), 816. https://doi.org/10.3390/cancers16040816









