Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Risk Factors
2.1. Risk Factors in East Asian Countries
2.2. Risk Factors in Western Countries
2.3. Environmental Carcinogens as Risk Factors
3. Molecular Pathology
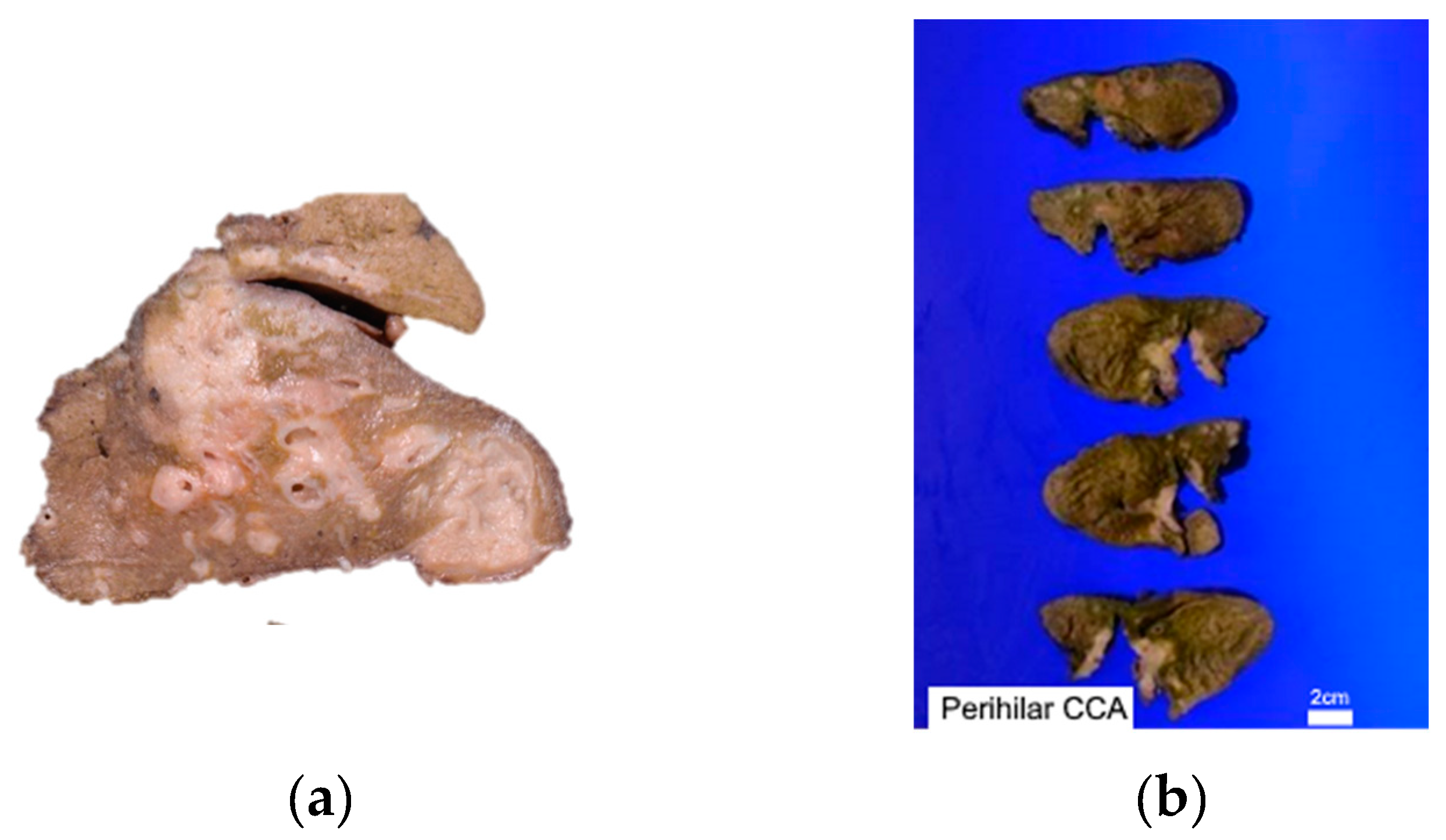
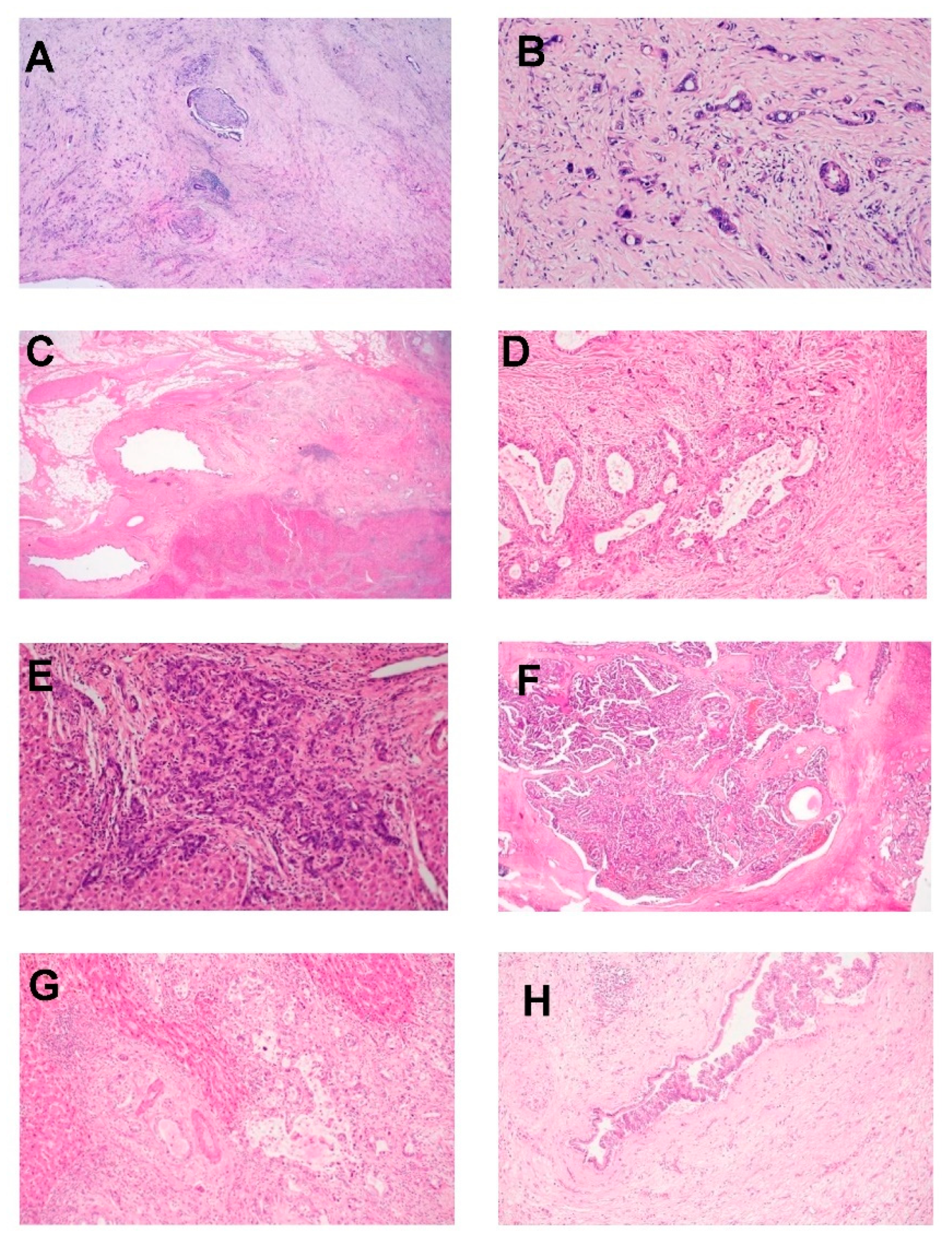
3.1. Pathological Features
3.2. Cholangiocarcinoma Nomenclature according to Location in Biliary Tract Anatomy
3.3. Growth Pattern
3.4. Large and Small Duct Variants of Intrahepatic CCA
3.5. Molecular Genetics
3.6. Diagnosis and Evaluation
3.7. Liquid Biopsy in Diagnostics
4. Current Therapeutic Options
4.1. Surgery
4.2. Chemotherapy
4.3. Adjuvant Chemotherapy
4.4. Second Line Therapy
4.5. Radiation Therapy
5. Emerging Therapeutic Strategies
5.1. Targeted Therapies
5.2. Immunotherapy
| Type of Therapy | Drug | Publication | Target |
|---|---|---|---|
| Targeted therapies | Enasidenib | Reference [145] | IDH2 |
| Tazemetostat | Reference [147] | EZH2 | |
| Bintrafusp alfa | Reference [148] | TGF-β and PD-L1 | |
| Nicotinamide N-methyltransferase inhibitors | Reference [150] | NNMT | |
| Immunotherapies | Pembrolizumab | Reference [163] | PD-1 |
| Nivolumab | Reference [162] | PD-1 | |
| Anti-PD1/PD-L1 + anti-CTLA4 | Reference [165] | PD-1/PD-L1, CTLA4 | |
| Camrelizumab | Reference [167] | PD-1 | |
| Combination | Camrelizumab + gemcitabine + oxaliplatin | Reference [166] | PD-1, DNA synthesis, DNA damage |
5.3. Novel Compounds from Natural Sources
5.4. Modulating Gut Microbiota
5.4.1. Microbiota Dysbiosis in CCA
5.4.2. Modulating Gut Microbiota in CCA Patients
5.4.3. Probiotics in CCA Prevention
5.4.4. Prebiotics in CCA
5.4.5. Fecal Microbiota Transplantation (FMT) in CCA
5.4.6. Implications of Recent Research for the Development of Future CCA Therapeutics
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, M.; Balachandran, V.P.; D’Angelica, M.I. Surgical management of biliary tract cancers. Chin. Clin. Oncol. 2016, 5, 63. [Google Scholar] [CrossRef]
- Fava, G. Molecular mechanisms of cholangiocarcinoma. World J. Gastrointest. Pathophysiol. 2010, 1, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; E Allen, G.; Ng, C.C.Y.; Wong, B.H.; et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef]
- Deng, M.; Ran, P.; Chen, L.; Wang, Y.; Yu, Z.; Cai, K.; Feng, J.; Qin, Z.; Yin, Y.; Tan, S.; et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology 2023, 77, 411–429. [Google Scholar] [CrossRef]
- Bertuccio, P.; Bosetti, C.; Levi, F.; Decarli, A.; Negri, E.; La Vecchia, C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann. Oncol. 2013, 24, 1667–1674. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog Risks Hum. 2012, 100, 1–441. [Google Scholar]
- Jusakul, A.; Kongpetch, S.; Teh, B.T. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr. Opin. Gastroenterol. 2015, 31, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kongpetch, S.; Jusakul, A.; Ong, C.K.; Lim, W.K.; Rozen, S.G.; Tan, P.; Teh, B.T. Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Nagarajan, N.; Lin, S.J.; Korhonen, P.K.; Jex, A.R.; Hall, R.S.; Safavi-Hemami, H.; Kaewkong, W.; Bertrand, D.; Gao, S.; et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. 2014, 5, 4378. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Sripa, B.; Laha, T.; Mulvenna, J.; Gasser, R.B.; Young, N.D.; Bethony, J.M.; Brindley, P.J.; Loukas, A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol. Biosyst. 2011, 7, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Dechakhamphu, S.; Pinlaor, S.; Sitthithaworn, P.; Bartsch, H.; Yongvanit, P. Accumulation of miscoding etheno-DNA adducts and highly expressed DNA repair during liver fluke-induced cholangiocarcinogenesis in hamsters. Mutat. Res. 2010, 691, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Pairojkul, C.; Pinlaor, S.; Khuntikeo, N.; Wongkham, C.; Sripa, B.; Ma, N.; Vaeteewoottacharn, K.; Furukawa, A.; Kobayashi, H.; et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic. Biol. Med. 2013, 65, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Tovar, V.; Moeini, A.; Llovet, J.M. Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for molecular therapies. Oncogene 2013, 32, 4861–4870. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef]
- Söreide, K.; Körner, H.; Havnen, J.; Söreide, J.A. Bile duct cysts in adults. Br. J. Surg. 2004, 91, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Fard-Aghaie, M.H.; Makridis, G.; Reese, T.; Feyerabend, B.; Wagner, K.C.; Schnitzbauer, A.; Bechstein, W.O.; Oldhafer, F.; Kleine, M.; Klempnauer, J.; et al. The rate of cholangiocarcinoma in Caroli Disease a German multicenter study. HPB 2022, 24, 267–276. [Google Scholar] [CrossRef]
- Nordenstedt, H.; Mattsson, F.; El-Serag, H.; Lagergren, J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br. J. Cancer 2012, 106, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Ralphs, S.; Khan, S.A. The role of the hepatitis viruses in cholangiocarcinoma. J. Viral Hepat. 2013, 20, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Mettler, T.A.; Fredericksen, Z.S.; Kim, W.R.; Gores, G.J.; Roberts, R.O.; Olson, J.E.; et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology 2013, 57, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, G.S.; Brennan, T.V.; Warren, R.S. Thorotrast-induced liver neoplasia: A collective review. J. Am. Coll. Surg. 2002, 195, 713–718. [Google Scholar] [CrossRef]
- Brandi, G.; Di Girolamo, S.; Farioli, A.; de Rosa, F.; Curti, S.; Pinna, A.D.; Ercolani, G.; Violante, F.S.; Biasco, G.; Mattioli, S. Asbestos: A hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control. 2013, 24, 911–918. [Google Scholar] [CrossRef]
- Farioli, A.; Straif, K.; Brandi, G.; Curti, S.; Kjaerheim, K.; Martinsen, J.I.; Sparen, P.; Tryggvadottir, L.; Weiderpass, E.; Biasco, G.; et al. Occupational exposure to asbestos and risk of cholangiocarcinoma: A population-based case-control study in four Nordic countries. Occup. Environ. Med. 2018, 75, 191–198. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO guidelines for governments and consumers regarding the use of alternative therapies. Rev. Panam. Salud. Publica. 2004, 16, 218–221. [Google Scholar]
- Han, J.; Xian, Z.; Zhang, Y.; Liu, J.; Liang, A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front. Pharmacol. 2019, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, M.R.; Cosyns, J.P.; Arlt, V.M.; Stiborová, M.; Phillips, D.H.; Schmeiser, H.H.; Simmonds, M.S.; Cook, H.T.; Vanherweghem, J.L.; Nortier, J.L.; et al. The epidemiology, diagnosis, and management of aristolochic acid nephropathy: A narrative review. Ann. Intern. Med. 2013, 158, 469–477. [Google Scholar] [CrossRef]
- Sidorenko, V.S.; Yeo, J.E.; Bonala, R.R.; Johnson, F.; Schärer, O.D.; Grollman, A.P. Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res. 2012, 40, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef]
- Poon, S.L.; Pang, S.-T.; McPherson, J.R.; Yu, W.; Huang, K.K.; Guan, P.; Weng, W.-H.; Siew, E.Y.; Liu, Y.; Heng, H.L.; et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci. Transl. Med. 2013, 5, 197ra101. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.C.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Li, C.; Wang, C.; Jiang, W.; Chang, J.; Han, S.; Lu, Z.; Shao, Z.; Wang, Y.; et al. The genomic landscape of cholangiocarcinoma reveals the disruption of post-transcriptional modifiers. Nat. Commun. 2022, 13, 3061. [Google Scholar] [CrossRef]
- Lin, J.; Cao, Y.; Yang, X.; Li, G.; Shi, Y.; Wang, D.; Long, J.; Song, Y.; Mao, J.; Xie, F.; et al. Mutational spectrum and precision oncology for biliary tract carcinoma. Theranostics 2021, 11, 4585–4598. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell. 2022, 40, 70–87.e15. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, Q.; Zhao, L.; Shi, Y.; Wang, N.; Wang, L.; Han, Z. The Mutational Features of Aristolochic Acid-Induced Mouse and Human Liver Cancers. Hepatology 2020, 71, 929–942. [Google Scholar] [CrossRef]
- Marcano-Bonilla, L.; Mohamed, E.A.; Mounajjed, T.; Roberts, L.R. Biliary tract cancers: Epidemiology, molecular pathogenesis and genetic risk associations. Chin. Clin. Oncol. 2016, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Sempoux, C.; Jibara, G.; Ward, S.C.; Fan, C.; Qin, L.; Roayaie, S.; Fiel, M.I.; Schwartz, M.; Thung, S.N. Intrahepatic cholangiocarcinoma: New insights in pathology. Semin. Liver Dis. 2011, 31, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Kakuda, Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Sato, Y.; Harada, K.; Sasaki, M.; Xu, J.; Ikeda, H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010, 2, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Dóra, R.; Kontsek, E.; Gógl, A.; Kiss, A.; Werling, K.; Kovalszky, I.; Schaff, Z.; et al. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol. Oncol. Res. 2020, 26, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sigel, C.S.; Drill, E.; Zhou, Y.; Basturk, O.; Askan, G.; Pak, L.M.; Vakiani, E.; Wang, T.; Boerner, T.; Do, R.K.; et al. Intrahepatic Cholangiocarcinomas Have Histologically and Immunophenotypically Distinct Small and Large Duct Patterns. Am. J. Surg. Pathol. 2018, 42, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.A.; Taddei, T.; Jain, D.; Zhang, X. HNF-1β is a More Sensitive and Specific Marker Than C-Reactive Protein for Identifying Biliary Differentiation in Primary Hepatic Carcinomas. Arch. Pathol. Lab. Med. 2022, 146, 220–226. [Google Scholar] [CrossRef]
- Hayashi, A.; Misumi, K.; Shibahara, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N.; Fukayama, M. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Fernández Moro, C.; Fernandez-Woodbridge, A.; Alistair D’souza, M.; Zhang, Q.; Bozoky, B.; Vasan, S.K.; Catalano, P.; Heuchel, R.; Shtembari, S.; Del Chiaro, M.; et al. Correction: Immunohistochemical Typing of Adenocarcinomas of the Pancreatobiliary System Improves Diagnosis and Prognostic Stratification. PLoS ONE 2017, 12, e0171283. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Martinelli, E.; Melisi, D.; Pinto, C.; Rimassa, L.; Santini, D.; Scarpa, A. Role of molecular genetics in the clinical management of cholangiocarcinoma. ESMO Open 2022, 7, 100505. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Z.; Dong, X.; Zhao, J.; Lin, S.; Hu, X.; Liu, F.-E.; Liu, X.; Zhang, H. Genomic and Transcriptomic Landscape of Tumor Clonal Evolution in Cholangiocarcinoma. Front. Genet. 2020, 11, 195. [Google Scholar] [CrossRef]
- Kongpetch, S.; Jusakul, A.; Lim, J.Q.; Ng, C.C.Y.; Chan, J.Y.; Rajasegaran, V.; Lim, T.H.; Lim, K.H.; Choo, S.P.; Dima, S.; et al. Lack of Targetable FGFR2 Fusions in Endemic Fluke-Associated Cholangiocarcinoma. JCO Glob. Oncol. 2020, 6, 628–638. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Fan, J.; Xu, C.; Guan, B.; Zhang, J.; Guo, B.; Shi, Y.; Wang, P.; Tan, Y.; et al. DNA methylation subtypes guiding prognostic assessment and linking to responses the DNA methyltransferase inhibitor SGI-110 in urothelial carcinoma. BMC Med. 2022, 20, 222. [Google Scholar] [CrossRef]
- Southekal, S.; Shakyawar, S.K.; Bajpai, P.; Elkholy, A.; Manne, U.; Mishra, N.K.; Guda, C. Molecular Subtyping and Survival Analysis of Osteosarcoma Reveals Prognostic Biomarkers and Key Canonical Pathways. Cancers 2023, 15, 2134. [Google Scholar] [CrossRef]
- Hong, J.H.; Yong, C.H.; Heng, H.L.; Chan, J.Y.; Lau, M.C.; Chen, J.; Lee, J.Y.; Lim, A.H.; Li, Z.; Guan, P.; et al. Integrative multiomics enhancer activity profiling identifies therapeutic vulnerabilities in cholangiocarcinoma of different etiologies. Gut 2023, gutjnl-2023-330483. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Y.; Zhang, Q.; Liang, T. Epigenetic dysregulation-mediated COL12A1 upregulation predicts worse outcome in intrahepatic cholangiocarcinoma patients. Clin. Epigenetics. 2023, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Corvera, C.U.; Blumgart, L.H.; Akhurst, T.; DeMatteo, R.P.; D’angelica, M.; Fong, Y.; Jarnagin, W.R. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J. Am. Coll. Surg. 2008, 206, 57–65. [Google Scholar] [CrossRef]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Wintachai, P.; Lim, J.Q.; Techasen, A.; Lert-Itthiporn, W.; Kongpetch, S.; Loilome, W.; Chindaprasirt, J.; Titapun, A.; Namwat, N.; Khuntikeo, N.; et al. Diagnostic and Prognostic Value of Circulating Cell-Free DNA for Cholangiocarcinoma. Diagnostics 2021, 11, 999. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Schwerdel, D.; Dolnik, A.; Beuter, F.; Blätte, T.J.; Schmidt, S.A.; Stanescu-Siegmund, N.; Steinacker, J.; Marienfeld, R.; Kleger, A.; et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci. Rep. 2019, 9, 13261. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Driescher, C.; Fuchs, K.; Haeberle, L.; Goering, W.; Frohn, L.; Opitz, F.V.; Haeussinger, D.; Knoefel, W.T.; Keitel, V.; Esposito, I. Bile-Based Cell-Free DNA Analysis Is a Reliable Diagnostic Tool in Pancreatobiliary Cancer. Cancers 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Zhang, D.; Yin, L.; Qiu, Y.; Liu, J.; Yu, W.; Fu, X.; Zhu, B.; Xu, X.; Duan, A.; et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 2019, 42, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Rullán, M.; Amat, I.; Oyon, D.; Zabalza, L.; Elizalde, M.; Latasa, M.U.; Mercado, M.R.; Ruiz-Clavijo, D.; Saldaña, C.; et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022, 71, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Wasenang, W.; Chaiyarit, P.; Proungvitaya, S.; Limpaiboon, T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenetics 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Toung, J.; Jassowicz, A.; Vijayaraghavan, R.; Kang, H.; Zhang, R.; Kruglyak, K.; Huang, H.; Hinoue, T.; Shen, H.; et al. Targeted methylation sequencing of plasma cell-free DNA for cancer detection and classification. Ann. Oncol. 2018, 29, 1445–1453. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateau, S.; et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014, 60, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallego, C.; Maddalo, D.; Doussot, A.; Kemeny, N.; Kingham, T.P.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Betel, D.; Klimstra, D.; et al. Circulating Plasma Levels of MicroRNA-21 and MicroRNA-221 Are Potential Diagnostic Markers for Primary Intrahepatic Cholangiocarcinoma. PLoS ONE 2016, 11, e0163699. [Google Scholar] [CrossRef]
- Kishimoto, T.; Eguchi, H.; Nagano, H.; Kobayashi, S.; Akita, H.; Hama, N.; Wada, H.; Kawamoto, K.; Tomokuni, A.; Tomimaru, Y.; et al. Plasma miR-21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci. 2013, 104, 1626–1631. [Google Scholar] [CrossRef]
- Soares, K.C.; Jarnagin, W.R. The Landmark Series: Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 4158–4170. [Google Scholar] [CrossRef]
- Hemming, A.W.M.; Reed, A.I.; Howard, R.J.; Fujita, S.; Hochwald, S.N.; Caridi, J.G.; Hawkins, I.F.; Vauthey, J.-N. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003, 237, 686–691. [Google Scholar] [CrossRef]
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef]
- Quinn, L.M.; Dunne, D.F.J.; Jones, R.P.; Poston, G.J.; Malik, H.Z.; Fenwick, S.W. Optimal perioperative care in peri-hilar cholangiocarcinoma resection. Eur. Surg. 2018, 50, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Coelen, R.J.S.; Roos, E.; Wiggers, J.K.; Besselink, M.G.; I Buis, C.; Busch, O.R.C.; Dejong, C.H.C.; van Delden, O.M.; van Eijck, C.H.J.; Fockens, P.; et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: A multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Otsuji, H.; Yokoyama, Y.; Ebata, T.; Igami, T.; Sugawara, G.; Mizuno, T.; Nagino, M. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J. Surg. 2015, 39, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Song, G.W.; Hwang, S.; Ha, T.Y.; Moon, D.B.; Jung, D.H.; Kim, K.H.; Ahn, C.S.; Kim, M.H.; Lee, S.K.; Sung, K.B.; et al. Surgical treatment of hilar cholangiocarcinoma in the new era: The Asan experience. J. Hepatobiliary Pancreat. Sci. 2010, 17, 476–489. [Google Scholar]
- Nishio, H.; Nagino, M.; Nimura, Y. Surgical management of hilar cholangiocarcinoma: The Nagoya experience. HPB 2005, 7, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Hemming, A.W.; Reed, A.I.; Fujita, S.; Foley, D.P.; Howard, R.J. Surgical management of hilar cholangiocarcinoma. Ann. Surg. 2005, 241, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005, 242, 451–458. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B.; Gores, G.J. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004, 10 (Suppl. S2), S65–S68. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef]
- André, T.; Tournigand, C.; Rosmorduc, O.; Provent, S.; Maindrault-Goebel, F.; Avenin, D.; Selle, F.; Paye, F.; Hannoun, L.; Houry, S.; et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: A GERCOR study. Ann. Oncol. 2004, 15, 1339–1343. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J. Hepatobiliary Pancreat. Sci. 2023, 30, 102–110. [Google Scholar] [CrossRef]
- Kim, S.T.; Kang, J.H.; Lee, J.; Lee, H.W.; Oh, S.Y.; Jang, J.S.; Lee, M.A.; Sohn, B.S.; Yoon, S.Y.; Choi, H.J.; et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 2019, 30, 788–795. [Google Scholar] [CrossRef]
- Shroff, R.T.; A Guthrie, K.; Scott, A.J.; Borad, M.J.; Goff, L.W.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; Aghajanian, C.; et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary trac t cancers. J. Clin. Oncol. 2023, 41 (Suppl. S4), LBA490. [Google Scholar] [CrossRef]
- Ishihara, S.; Horiguchi, A.; Miyakawa, S.; Endo, I.; Miyazaki, M.; Takada, T. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat. Sci 2016, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Ruo, L.; Little, S.A.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Wagman, R.; Blumgart, L.H.; Fong, Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: Implications for adjuvant therapeutic strategies. Cancer 2003, 98, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Todaka, A.; Yanagimoto, H.; Morinaga, S.; Kobayashi, S.; et al. Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2023, 401, 195–203. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Zheng, Y.; Tu, X.; Zhao, P.; Jiang, W.; Liu, L.; Tong, Z.; Zhang, H.; Yan, C.; Fang, W.; Wang, W. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br. J. Cancer 2018, 119, 291–295. [Google Scholar] [CrossRef]
- Kim, B.J.; Yoo, C.; Kim, K.-P.; Hyung, J.; Park, S.J.; Ryoo, B.-Y.; Chang, H.-M. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: Retrospective analysis of 321 patients. Br. J. Cancer 2017, 116, 561–567. [Google Scholar] [CrossRef]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.P.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Patel, S.H.; Kooby, D.A.; Weber, S.; Bloomston, M.; Cho, C.; Hatzaras, I.; Schmidt, C.; Winslow, E.; Staley III, C.A.; et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: A multi-institution analysis. HPB 2012, 14, 514–522. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ikai, I.; Fujii, H.; Hatano, E.; Shimahara, Y. Surgical resection of hilar cholangiocarcinoma: Analysis of survival and postoperative complications. World J. Surg. 2007, 31, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Koerkamp, B.G.; Wiggers, J.K.; Gonen, M.; Doussot, A.; Allen, P.J.; Besselink, M.G.H.; Blumgart, L.H.; Busch, O.R.C.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann. Oncol. 2015, 26, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, C.; Jia, H.-D.; Diao, Y.-K.; Xing, H.; Pawlik, T.M.; Lau, W.Y.; Shen, F.; Huang, D.-S.; Zhang, C.-W.; et al. Prognostic factors of resectable perihilar cholangiocarcinoma: A systematic review and meta-analysis of high-quality studies. Ther. Adv. Gastrointest. Endosc. 2021, 14, 2631774521993065. [Google Scholar] [CrossRef]
- Nakahashi, K.; Ebata, T.; Yokoyama, Y.; Igami, T.; Mizuno, T.; Yamaguchi, J.; Onoe, S.; Watanabe, N.; Nagino, M. How long should follow-up be continued after R0 resection of perihilar cholangiocarcinoma? Surgery 2020, 168, 617–624. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Wu, L.; Wan, T. Survival after surgical resection of distal cholangiocarcinoma: A systematic review and meta-analysis of prognostic factors. Asian J. Surg. 2017, 40, 129–138. [Google Scholar] [CrossRef]
- Komaya, K.; Ebata, T.; Shirai, K.; Ohira, S.; Morofuji, N.; Akutagawa, A.; Yamaguchi, R.; Nagino, M.; Aoba, T.; Kaneoka, Y.; et al. Nagoya Surgical Oncology Group. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br. J. Surg. 2017, 104, 426–433. [Google Scholar] [CrossRef]
- Ren, B.; Guo, Q.; Yang, Y.; Liu, L.; Wei, S.; Chen, W.; Tian, Y. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat. Oncol. 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- BonetBeltrán, M.; Allal, A.S.; Gich, I.; Solé, J.M.; Carrió, I. Is adjuvant radiotherapy needed after curative resection of extrahepatic biliary tract cancers? A systematic review with a meta-analysis of observational studies. Cancer Treat. Rev. 2012, 38, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Lin, N.; Deng, M.; Wang, L.; Zeng, Y.; Liu, J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229292. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Q.; Zhang, J.Y.; Tian, H.; Tang, L.N.; Li, A.L. Role of adjuvant (chemo)radiotherapy for resected extrahepatic cholangiocarcinoma: A meta-analysis. J. Zhejiang Univ. Sci. B 2020, 21, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.A.; Frassica, D.A.; Yeo, C.J.; Riall, T.S.; Lillemoe, K.D.; Cameron, J.L.; Donehower, R.C.; Laheru, D.A.; Hruban, R.H.; Abrams, R.A. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Nassour, I.; Mokdad, A.A.; Porembka, M.R.; Choti, M.A.; Polanco, P.M.; Mansour, J.C.; Minter, R.M.; Wang, S.C.; Yopp, A.C. Adjuvant Therapy Is Associated with Improved Survival in Resected Perihilar Cholangiocarcinoma: A Propensity Matched Study. Ann. Surg. Oncol. 2018, 25, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bednar, F.; Cho, C.S.; Nathan, H. Survival benefit with adjuvant radiotherapy after resection of distal cholangiocarcinoma: A propensity-matched National Cancer Database analysis. Cancer 2021, 127, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Ben-Josef, E.; Guthrie, K.A.; El-Khoueiry, A.B.; Corless, C.L.; Zalupski, M.M.; Lowy, A.M.; Thomas, C.R., Jr.; Alberts, S.R.; Dawson, L.A.; Micetich, K.C.; et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J. Clin. Oncol. 2015, 33, 2617–2622. [Google Scholar] [CrossRef]
- CSCO. 2020 Guidelines of Chinese Society of Clinical Oncology (CSCO) for Biliary Tract Cancer; Liang, H.J., Shen, F., Qin, S.Q., Eds.; CSCO: Beijing, China, 2020; Volume 1, pp. 1–39. (In Chinese) [Google Scholar]
- Gómez-España, M.A.; Montes, A.F.; Garcia-Carbonero, R.; Mercadé, T.M.; Maurel, J.; Martín, A.M.; Pazo-Cid, R.; Vera, R.; Carrato, A.; Feliu, J. SEOM clinical guidelines for pancreatic and biliary tract cancer (2020). Clin. Transl. Oncol. 2021, 23, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Chinese Chapter of International Hepato-PancreatoBiliary Association; Hepatic Surgery Group. Chinese Society of Surgery, Chinese Medical Association. Diagnosis and treatment of cholangiocarcinoma:surgical expert consensus. J. Clin. Hepatol. 2015, 31, 12–16. [Google Scholar]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef]
- Marinelli, I.; Guido, A.; Fuccio, L.; Farioli, A.; Panni, V.; Giaccherini, L.; Arcelli, A.; Ercolani, G.; Brandi, G.; Cammelli, S.; et al. Clinical Target Volume in Biliary Carcinoma: A Systematic Review of Pathological Studies. Anticanc. Res. 2017, 37, 955–961. [Google Scholar]
- Jung, W.; Kim, K.; Min, S.K.; Nam, E.M.; Lee, J.K. Mapping of local recurrence after pancreaticoduodenectomy for distal extrahepatic cholangiocarcinoma: Implications for adjuvant radiotherapy. Br. J. Radiol. 2019, 92, 20190285. [Google Scholar] [CrossRef]
- Wang, N.; Huang, A.; Kuang, B.; Xiao, Y.; Xiao, Y.; Ma, H. Progress in Radiotherapy for Cholangiocarcinoma. Front. Oncol. 2022, 12, 868034. [Google Scholar] [CrossRef]
- Zhou, W.; Qian, L.; Rong, Y.; Zhou, Q.; Shan, J.; Li, P.; Shi, L.; Liu, H.; Sun, X. Prognostic factors and patterns of recurrence after curative resection for patients with distal cholangiocarcinoma. Radiother. Oncol. 2020, 147, 111–117. [Google Scholar] [CrossRef]
- Sudan, D.; DeRoover, A.; Chinnakotla, S.; Fox, I.; Shaw, B.; McCashland, T.; Sorrell, M.; Tempero, M.; Langnas, A.B.S., Jr. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transplant. 2002, 2, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Murad, S.D.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Jones, C.M.; Duffy, J.P.; Petrowsky, H.; Farmer, D.G.; French, S.; Finn, R.; Durazo, F.A.; Saab, S.; Tong, M.J.; et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: A 24-year experience in a single center. Arch. Surg. 2011, 146, 683–689. [Google Scholar] [CrossRef]
- Gkika, E.; Hallauer, L.; Kirste, S.; Adebahr, S.; Bartl, N.; Neeff, H.P.; Fritsch, R.; Brass, V.; Nestle, U.; Grosu, A.L.; et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer 2017, 17, 781. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Dagoglu, N.; Mancias, J.; Raven, K.; Khwaja, K.; Tseng, J.F.; Ng, K.; Enzinger, P.; Miksad, R.; Bullock, A.; et al. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J. Cancer. 2015, 6, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, W.S.; Koom, W.S.; Rim, C.H. Efficacy of stereotactic body radiotherapy for unresectable or recurrent cholangiocarcinoma: A meta-analysis and systematic review. Strahlenther. Onkol. 2019, 195, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.T.; Tan, Y.; Miller, E.D.; Williams, T.M.; Alexandra Diaz, D. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Transl. Radiat. Oncol. 2019, 19, 66–71. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Chan-On, W.; Nairismägi, M.L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. IDH inhibitors in advanced cholangiocarcinoma: Another arrow in the quiver? Cancer Treat. Res. Commun. 2021, 27, 100356. [Google Scholar] [CrossRef]
- Vaquero, J.; Lobe, C.; Tahraoui, S.; Clapéron, A.; Mergey, M.; Merabtene, F.; Wendum, D.; Coulouarn, C.; Housset, C.; Desbois-Mouthon, C.; et al. The IGF2/IR/IGF1R Pathway in Tumor Cells and Myofibroblasts Mediates Resistance to EGFR Inhibition in Cholangiocarcinoma. Clin. Cancer Res. 2018, 24, 4282–4296. [Google Scholar] [CrossRef]
- Bekric, D.; Neureiter, D.; Ablinger, C.; Dobias, H.; Beyreis, M.; Ritter, M.; Jakab, M.; Bischof, J.; Koller, U.; Kiesslich, T.; et al. Evaluation of Tazemetostat as a Therapeutically Relevant Substance in Biliary Tract Cancer. Cancers 2023, 15, 1569. [Google Scholar] [CrossRef]
- Yoo, C.; Oh, D.Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer. 2020, 8, e000564. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ke, S.; Wang, C.; Xu, Y.; Li, Z.; Song, K.; Bai, M.; Zhou, M.; Yu, H.; Yin, B.; et al. NNMT promotes the progression of intrahepatic cholangiocarcinoma by regulating aerobic glycolysis via the EGFR-STAT3 axis. Oncogenesis 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Fan, J.; Oh, D.-Y.; Choi, H.J.; Kim, J.W.; Chang, H.-M.; Bao, L.; Sun, H.-C.; Macarulla, T.; Xie, F.; et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): A multicentre, single-arm, phase 2b study. Lancet Oncol. 2023, 24, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Thongprasert, S.; Napapan, S.; Charoentum, C.; Moonprakan, S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann. Oncol. 2005, 16, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Ann. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, L.; Xie, X.; Jiang, G.; Xie, H.; Zheng, S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J. Surg. Oncol. 2009, 100, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Li, S.-H.; Fu, X.; Yan, X.-P.; Chen, J.; Qiu, Y.-D.; Guo, R.-P. Relationship between PD-L1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021, 21, 371. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest. Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Wan, L.; Wang, Z.; Wang, H.; Ge, C.; Liu, Y.; Hao, Y.; Zhang, D.; Shi, G.; et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E..; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.K.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer. 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Y.; Zhao, Y.; Yang, Q.; Dong, L.; Liu, J.; Li, X.; Zhao, Z.; Mei, Q.; Han, W. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: Results from a phase II study. J. Immunother. Cancer. 2020, 8, e000367. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients with Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Wu, H.; Gu, Y.; Shao, Y.; Shao, Q.; Zhu, F.; Li, X.; Qian, X.; Hu, J.; et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: A single-arm, open-label, phase II trial. J. Immunother. Cancer. 2020, 8, e001240. [Google Scholar] [CrossRef]
- Chen, X.; Qin, S.; Gu, S.; Ren, Z.; Chen, Z.; Xiong, J.; Liu, Y.; Meng, Z.; Zhang, X.; Wang, L.; et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int. J. Cancer 2021, 149, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Atractylodes lancea for cholangiocarcinoma: Modulatory effects on CYP1A2 and CYP3A1 and pharmacokinetics in rats and biodistribution in mice. PLoS ONE 2022, 17, e0277614. [Google Scholar] [CrossRef]
- Hahnvajanawong, C.; Boonyanugomol, W.; Nasomyon, T.; Loilome, W.; Namwat, N.; Anantachoke, N.; Tassaneeyakul, W.; Sripa, B.; Namwat, W.; Reutrakul, V. Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines. World J. Gastroenterol. 2010, 16, 2235–2243. [Google Scholar] [CrossRef]
- Janeklang, S.; Nakaew, A.; Vaeteewoottacharn, K.; Seubwai, W.; Boonsiri, P.; Kismali, G.; Suksamrarn, A.; Okada, S.; Wongkham, S. In vitro and in vivo antitumor activity of tiliacorinine in human cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 7473–7478. [Google Scholar] [CrossRef]
- Decharchoochart, P.; Suthiwong, J.; Samatiwat, P.; Kukongviriyapan, V.; Yenjai, C. Cytotoxicity of compounds from the fruits of Derris indica against cholangiocarcinoma and HepG2 cell lines. J. Nat. Med. 2014, 68, 730–736. [Google Scholar] [CrossRef]
- Hemtasin, C.; Kanokmedhakul, S.; Kanokmedhakul, K.; Hahnvajanawong, C.; Soytong, K.; Prabpai, S.; Kongsaeree, P. Cytotoxic pentacyclic and tetracyclic aromatic sesquiterpenes from Phomopsis archeri. J. Nat. Prod. 2011, 74, 609–613. [Google Scholar] [CrossRef]
- Khumkomkhet, P.; Kanokmedhakul, S.; Kanokmedhakul, K.; Hahnvajanawong, C.; Soytong, K. Antimalarial and cytotoxic depsidones from the fungus Chaetomium brasiliense. J. Nat. Prod. 2009, 72, 1487–1491. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [CrossRef]
- Kwak, T.W.; Shin, H.J.; Jeong, Y.-I.; Han, M.-E.; Oh, S.-O.; Kim, H.-J.; Kim, D.H.; Kang, D.H. Anticancer activity of streptochlorin, a novel antineoplastic agent, in cholangiocarcinoma. Drug Des. Devel. Ther. 2015, 9, 2201–2214. [Google Scholar] [CrossRef][Green Version]
- Lang, M.; Henson, R.; Braconi, C.; Patel, T. Epigallocatechin-gallate modulates chemotherapy-induced apoptosis in human cholangiocarcinoma cells. Liver Int. 2009, 29, 670–677. [Google Scholar] [CrossRef]
- Deng, T.; Li, J.; He, B.; Chen, B.; Liu, F.; Chen, Z.; Zheng, J.; Shi, Z.; Zhang, T.; Deng, L.; et al. Gut microbiome alteration as a diagnostic tool and associated with inflammatory response marker in primary liver cancer. Hepatol. Int. 2022, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, S.; Jin, C.; Lin, Z.; Deng, T.; Xie, X.; Deng, L.; Li, X.; Ma, J.; Ding, X.; et al. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients With Cholangiocarcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 751795. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, J.; Su, F.; Ding, X.; Zhang, Y.; Dou, L.; Liu, Y.; Ke, Y.; Liu, X.; Liu, Y.; et al. Characteristics of bile microbiota in cholelithiasis, perihilar cholangiocarcinoma, distal cholangiocarcinoma, and pancreatic cancer. Am. J. Transl. Res. 2022, 14, 2962–2971. [Google Scholar]
- Rao, B.C.; Zhang, G.Z.; Zou, Y.W.; Ren, T.; Ren, H.Y.; Liu, C.; Yu, Z.J.; Ren, Z.G. Alterations in the human oral microbiome in cholangiocarcinoma. Mil. Med Res. 2022, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Brasse-Lagnel, C.; Tuech, J.; Montialoux, H.; Papet, E.; Tortajada, P.; Bekri, S.; Schwarz, L. Influence of Probiotics Administration Before Liver Resection in Patients with Liver Disease: A Randomized Controlled Trial. World J. Surg. 2022, 46, 656–665. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Fuller, R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J. Nutr. 2000, 130 (Suppl. S2), 391S–395S. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Bouter, K.E.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef]
| East Asian Countries | Western Countries |
|---|---|
| Opisthorchis viverrini (Ov) | Primary sclerosing cholangitis (PSC) |
| Clonorchis sinensis | Choledochal cysts |
| Caroli disease | |
| Caroli syndrome | |
| Liver cirrhosis | |
| Cholelithiasis | |
| Choledocholithiasis | |
| Hepatitis B virus (HBV) | |
| Hepatitis C virus (HCV) | |
| Non-alcoholic fatty liver disease (NAFLD) | |
| Type 2 diabetes mellitus | |
| Inflammatory bowel disease | |
| Alcohol consumption | |
| Tobacco smoking | |
| Obesity | |
| Hypertension |
| Genetic Aberrations | Intrahepatic CCA (%) | Extrahepatic CCA (%) | Normal Function of Gene | Mechanisms |
|---|---|---|---|---|
| Inactivating mutations | ||||
| TP53 | 20–40 | 30–60 | Tumor suppressor | Transcription factor. |
| ARID1A | 7–36 | 12–20 | Tumor suppressor | Transcription factor. |
| CDKN2A/B | 6–27 | 9–28 | Tumor suppressor | Cell cycle. |
| BAP1 | 13–21 | 1 | Tumor suppressor | Transcription factor. |
| SMAD4 | 4–17 | 10–22 | Tumor suppressor | Transcription factor |
| RNF43 | 9 | 1 | Tumor suppressor | Wnt signaling. |
| PTEN | 1–11 | 1 | Tumor suppressor | Membrane binding. |
| Activating mutations | ||||
| KRAS | 7–24 | 8–45 | Proto-oncogene | Signaling cascade, e.g., MAPK pathway. |
| IDH1/2 | 10–30 | 0–7 | Proto-oncogene | Glucose metabolism, cellular defense against oxidative stress. |
| PIK3CA | 3–9 | 5–7 | Proto-oncogene | Cell growth, survival, and motility. |
| BRAF | 3–7 | 3–7 | Proto-oncogene | Signaling cascade, e.g., RAS/MAPK pathway. |
| Amplifications | ||||
| ERBB2/3 | 4–8 | 5–17 | Proto-oncogene | Signaling cascade, e.g., PI3K/AKT and ERK signaling. |
| MET | 2–7 | 1 | Proto-oncogene | Tyrosine kinase. |
| Fusions | ||||
| FGFR1-3 | 10–45 | 1 | Proto-oncogene | Cell proliferation, differentiation, migration, and apoptosis |
| NTRK | 4 | 4 | Proto-oncogene | Receptor tyrosine kinases. |
| Location | USG | CT | MRI |
|---|---|---|---|
| Intrahepatic | Mass with irregular margins, which may be hypo- or hyperechoic or may have mixed echogenicity. | Hypodense lesion in the liver, which can be well-defined or infiltrative, with bile duct dilatation. Capsular retraction may be seen in 20% of cases. Peripheral rim enhancement in both the arterial and venous phases. | T1 hypointense and T2 hyperintense mass with proximal ductal dilatation with intense delayed contrast enhancement. |
| Perihilar | Ductal dilatation of both intrahepatic ducts or nonunion of the right and left hepatic ducts | Ductal dilatation of both intrahepatic ducts or nonunion of the right and left hepatic ducts. | Presence of mass on the central hepatic ducts, prominent intrahepatic duct dilatation. Best visualized on delayed imaging (obtained 1–5 min after contrast administration). |
| Distal | Indirect signs: ductal dilatation | Dilatation of intrahepatic and extrahepatic ducts with distended gall bladder. Abrupt change in ductal diameter. | Circumferential thickening and delayed enhancement of the bile duct wall. Abrupt change in the ductal diameter. Best visualized on delayed imaging (obtained 1–5 min after contrast administration). |
| Current | Emerging |
|---|---|
| Surgical Resection | Targeted therapies - Enasidenib - Tazemetostat - Bintrafusp alfa - Nicotinamide N-methyltransferase inhibitors |
| Chemotherapy - Cisplatin + gemcitabine - Gemcitabine + S-1 - Cisplatin + gemcitabine + S1 - Gemcitabine, cisplatin, and nab-paclitaxel | Immunotherapy - Pembrolizumab - Durvalumab - Anti-PD1/PD-L1 + anti-CTLA4 - Camrelizumab |
| Adjuvant Chemotherapy - Capecitabine - S-1 | Combination - Camrelizumab + gemcitabine + oxaliplatin - Immunotherpay + VEGF inhibitors - Immunotherapy + PARP inhibitors |
| Second line therapy - Oxaliplatin + 5-FU - 5FU - Capecitabine + irinotecan | Novel compounds |
| Radiation therapy (including neoadjuvant) | Gut microbiota |
| Targeted therapies - Pemigatinib (Pemazyre) - Infigratinib (Truseltiq) - Futibatinib (Lytgobi) - Ivosidenib (Tibsovo) | |
| Combination - Durvalumab + gemcitabine + cisplatin |
| Trial | Arm | No. of Patients | Biliary Cancer Subtype | Outcome Median OS, HR |
|---|---|---|---|---|
| ABC-02 [87] | Gemcitabine 1000 mg/m2, cisplatin 25 mg/m2 on days 1 and 8, every 3 weeks × 8 cycles vs. gemcitabine 1000 mg/m2 days 1, 8, and 15, every 4 weeks × 6 cycles. | 410 | 58% Cholagiocarcinoma | 11.7 months vs. 8.1 months 0.640 (0.52–0.80), p < 0.001 |
| FUGA-BT (JCOG1113) [89] | Gemcitabine 1000 mg/m2, cisplatin 25 mg/m2 on days 1 and 8, every 3 weeks vs. gemcitabine 1000 mg/m2 infusion on days 1 and 8 and S-1 orally twice daily 60–80 mg/day on days 1–14, every 3 weeks till progression. | 354 | 57% Cholangiocarcinoma | 13.4 months vs. 15.1 months 0.945 (0.78–1.15) Non-inferior |
| Kim et al. [93] | Gemcitabine 1000 mg/m2 on days 1 and 8, and oxaliplatin 100 mg/m2 on day 1, every 3 weeks × 8 cycles vs. capecitabine 1000 mg/m2, twice daily, on days 1–14 and oxaliplatin 130 mg/m2 on day 1, every 3 weeks × 8 cycles. | 222 | 74% Cholangiocarcinoma | 10.4 months vs. 10.6 months p = 0.131 |
| KHBO1401-MITSUBA [92] | Gem Cis vs. Gem Cis S1 gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 infusion on day 1. gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 infusion on day 1, and 80 mg/m2 of S-1 on days 1–7, every 2 weeks. | 246 | 64–65% Cholangiocarcinoma | 13.5 months vs. 12.6 months 0.79 (0.628–0.996), p = 0.046 |
| SWOG 1815 [94] | Gemcitabine 800 mg/m2, cisplatin 25 mg/m2, Nab paclitaxel 100 mg/m2 on days 1 and 8, every 3 weeks vs. gemcitabine 1000 mg/m2, cisplatin 25 mg/m2 on days 1 and 8, every 3 weeks till progression. | 441 | 84% Cholangiocarcinoma | 14.0 months vs. 12.7 months 0.93, (0.74–1.19), p = 0.58 |
| Trial | Experimental | Control | No of Patients | Biliary Cancer Subtype | Outcome |
|---|---|---|---|---|---|
| BCAT [98] | Gemcitabine 1000 mg/m2 D1, 8, and 15, every 4 weeks × 6 cycles. | Observation | 117 108 | All extrahepatic cholangiocarcinoma | Primary outcome: OS not significant (p = 0.964), median survival 62.3 vs. 63.8 months, HR = 1.01. |
| PRODIGE12/ACCORD18 [99] | Gemcitabine 1000 mg/m2 day 1 + oxaliplatin 85 mg/m2 day 2, every 2 weeks × 12 cycles. | Observation | 73 82 | 80% Intra and extrahepatic cholangiocarcinoma | Primary outcome: RFS not significant (p = 0.48), median relapse-free survival 30.4 vs. 18.5 months, HR = 0.88. |
| BILCAP [100] | Capecitabine 1250 mg/m2 day 1–14, every 3 weeks × 8 cycles. | Observation | 210 220 | 82% Intra and extrahepatic cholangiocarcinoma | Primary Outcome: OS Significant by per-protocol analysis (p = 0.028), median survival 53 vs. 36 months, HR = 0.75. |
| JCOG 1202 [101] | S-1 40 mg, 50 mg, or 60 mg according to body surface area, orally administered twice daily for 4 weeks, followed by 2 weeks of rest for four cycles. | Observation | 218 222 | 69% Intra and extrahepatic cholangiocarcinoma | Primary outcome: OS significant (p = 0.0080), median overall survival was not estimable vs. 6.1 years, HR: 0.69. |
| Compounds (Class) | Source | Doses | Pharmacological Effects | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Tiliacorinine (bisbenzylisoquinoline alkaloid) | Tiliacoratriandra(Colebr.) Diels (roots and stems) | CCA-xenografted mice dosage: 10 mg/kg body weight, once daily for 3 consecutive days. Route: intraperitoneal injection. | Reduced tumor volumes 45.16 ± 12.52 mm3 and tumor weight 0.07 ± 0.02 g compared to the control group (injected with 0.01% DMSO) 80.22 ± 18.75 mm3 and 0.13 ± 0.04 g, respectively. | apoptosis induction via cleavage of PARP-1 by caspase, BAX -BclxL and XIAP | Janeklang et al. [170] |
| Thymoquinone (1,4-benzoquinones) | Nigella sativa L. oil | Xenografted nude mice. dosage: 2, 4, or 8 mg/mouse for 20 days. Route: intragastric intubation | Reduced tumor size compared to control (PBS) groups. | PI3K/Akt and NF-κB and regulated gene products, including p-AKT, p65, XIAP, Bcl-2, COX-2, and VEGF. | Xu et al. [174] |
| Streptochlorin (indole alkaloid) | Marine Streptomyces sp. | HuCCT-1-xenografted nude mouse. Dosage: 5 mg/kg for 22 days. Route: subcutaneously injection. | Inhibited tumor growth (tumor volume 6.6 times and 5.4 times smaller than control (phosphate-buffered saline) and vehicle (thermosensitive gel) groups). Inhibited invasion and migration of CCA cells. Regulated tumor metastasis of HuCCT-1 cells in mouse liver metastasis. | Apoptosis induction -Bcl-2 expression Bax, Bad, and cytochrome c activation of caspase-3. -NFkB, VEGF, and Notch 1 | Kwak et al. [175] |
| Epigallocatechin gallate (EGCG) (catechin) | Green tea | Mz-ChA-1 cells xenografted nude mice. Dosage: 20 mg/kg EGCG for 10 days and a combination study of i.p. injections of EGCG (20 mg/kg) for 10 days, and gemcitabine (120 mg/kg) for 3 h following EGCG injections on days 1, 4, and 7 only. Route: intraperitoneal injections. | Reduced the growth of tumor and increased the sensitivity to Gemcitabineof Mz-ChA-1 cell xenografts in nude mice EGCG alone showed a significant reduction in tumor growth compared to gemcitabine. | Induction of apoptosis. | Lang et al. [176] |
| Hepatobiliary Diseases | Bacteria | Location | Metabolites | Associated Cancer Mechanism | Reference |
|---|---|---|---|---|---|
| CCA patients (n = 46), hepatocellular carcinoma (HCC) patients (n = 143). | Enriched in Faecalibacterium, Klebsiella, Ruminococcus Gnavus group, Lactobacillus, Dorea, Veillonella, Burkholderia, Caballeronia, Paraburkholderia, and Citrobacter genera. | gut | NA | The microbial biomarker of CCA | Deng et al. [177] |
| pCCA (n = 14), dCCA (n = 9), Pancreatic cancer (PC) (n = 8). and cholelithiasis (n = 22). | The top three biomarkers for pCCA in the genus level were Pseudomonas, Sphingomonas, and Halomonas; for dCCA, were Streptococcus, Prevotella, and Halomonas; and for PC were Pseudomonas, Chloroplast, and Acinetobacter. | bile | Differences in the metabolic pathways in different groups when compared to healthy controls. | The correlation between bile microbiome and the progression of pCCA, dCCA, and PC. | Li et al. [180] |
| CCA (n = 74), HCC (n = 35). | The diagnostic biomarkers for CCA were Lautropia, Alloprevotella, and Actinomyces. | oral microbiome | NA | The oral microbial markers for non-invasive diagnostic tools for CCA | Rao et al. [181] |
| CCA patients (n = 53), cholelithiasis (n = 47). | Enriched in Burkholderia, Cabelleronia, Paraburkholderia, Faecalibacterium, and Ruminococcus-1 genera. | gut | NA | The gut microbiota served as a non-invasive diagnostic biomarker for early diagnosis of CCA. | Zhang et al. [178] |
| iCCA patients. | Enriched in Lactobacillus, Actinomyces, Peptostreptococcaceae, Alloscardovia, and Bifidobacteriaceae family. | gut | Increased in six conjugated bile acids (BAs) and decreased in chenodeoxycholic acid in plasma of iCCA patients. | Altered gut microbiota correlated with the BAs metabolism and inflammatory cytokines. | Jia et al. [179] |
| Condition/Disease | Sample Size (n) | Intervention | Outcomes | Status (Location) | NCT Number | References |
|---|---|---|---|---|---|---|
| ||||||
| 46 | The oral probiotic (Lactobacillus rhamnosus) administered one time a day during the immunotherapy treatment within 6 months. | NA | Recruiting (Jiangxi Provincial Cancer Hospital) | NCT05032014 | - |
| 280 | Orally administered 5 mL of probiotics (containing L.casei, L. palntarum, Strptococcus faecalis, and Bifidobacterium) every 12 h for 10 consecutive days per month. Duration of treatment: 2 cycles of continuous treatment per year during a 3-year period (6 cycles). | NA | Not yet recruiting: Austral University, Argentina | NCT03853928 | - |
| 664 | Administered active substance mixture of Bifidobacterium lactis LA303, L.plantanum 301, L.salivarius LA, and Bifidobacterium lactis 304. 2 capsules per day for 14 days. | The administration of probiotics promoted an immune response in patients in the post-operative period. | Completed: University Hospital Rouen, Haute Normandie, France | NCT02021253 | Roussel et al. [182]. |
| ||||||
| Colon cancer liver metastasis | 250 | Oral administered 4 soft gelatin capsules: 1 capsule containing 1 g pure eicosapentaenoic acid (EPA). | NA | Recruiting: University of Leeds | NCT04682665 | - |
| ||||||
| HCC | 12 | Combination: FMT with Atezolizumab plus Bevacizumab. After a single FMT, patients will continue to receive atezolizumab/bevacizumab every 21-days according to protocol. | NA | Not recruiting yet: Medical University of Vienna | NCT05750030 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosla, D.; Misra, S.; Chu, P.L.; Guan, P.; Nada, R.; Gupta, R.; Kaewnarin, K.; Ko, T.K.; Heng, H.L.; Srinivasalu, V.K.; et al. Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers 2024, 16, 801. https://doi.org/10.3390/cancers16040801
Khosla D, Misra S, Chu PL, Guan P, Nada R, Gupta R, Kaewnarin K, Ko TK, Heng HL, Srinivasalu VK, et al. Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers. 2024; 16(4):801. https://doi.org/10.3390/cancers16040801
Chicago/Turabian StyleKhosla, Divya, Shagun Misra, Pek Lim Chu, Peiyong Guan, Ritambhra Nada, Rajesh Gupta, Khwanta Kaewnarin, Tun Kiat Ko, Hong Lee Heng, Vijay Kumar Srinivasalu, and et al. 2024. "Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches" Cancers 16, no. 4: 801. https://doi.org/10.3390/cancers16040801
APA StyleKhosla, D., Misra, S., Chu, P. L., Guan, P., Nada, R., Gupta, R., Kaewnarin, K., Ko, T. K., Heng, H. L., Srinivasalu, V. K., Kapoor, R., Singh, D., Klanrit, P., Sampattavanich, S., Tan, J., Kongpetch, S., Jusakul, A., Teh, B. T., Chan, J. Y., & Hong, J. H. (2024). Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers, 16(4), 801. https://doi.org/10.3390/cancers16040801






