Radiation Therapy for Stage IIA/B Seminoma: Modeling Secondary Cancer Risk for Protons and VMAT versus 3D Photons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
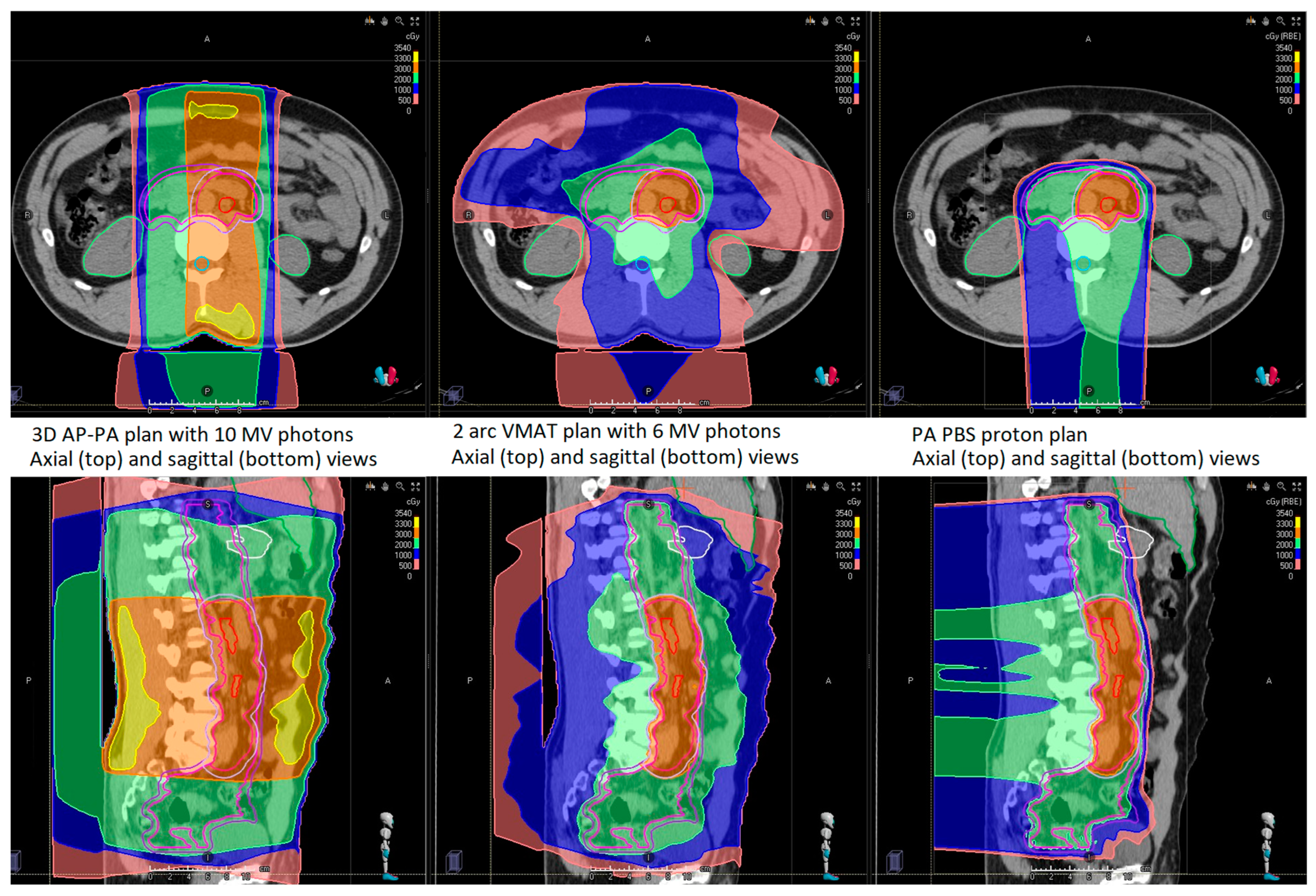
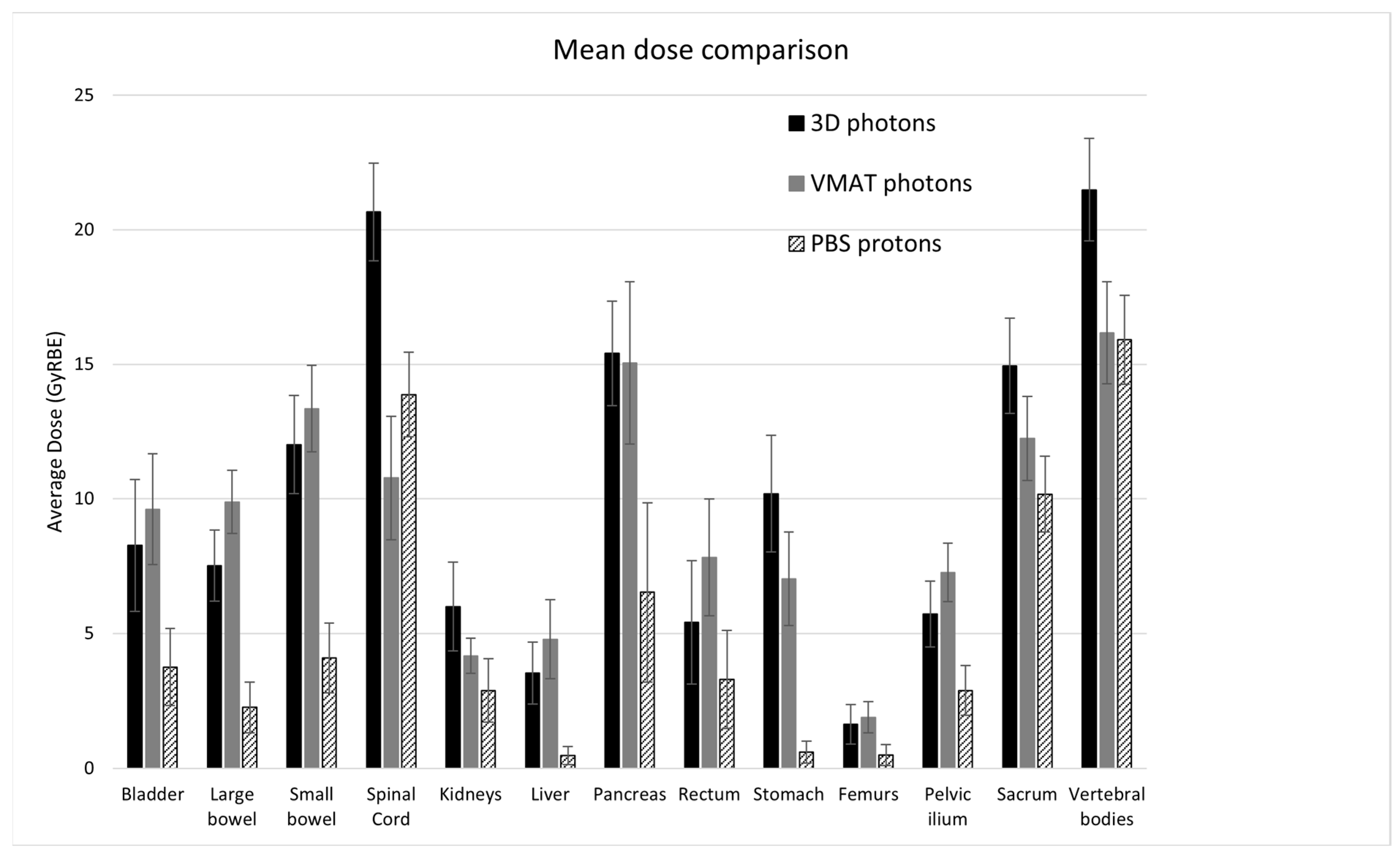
3.1. Dosimetric Comparison
3.2. Secondary Cancer Risk Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 27 September 2021).
- Key Statistics for Testicular Cancer. American Cancer Society. Available online: https://www.cancer.org/cancer/types/testicular-cancer/about/key-statistics.html (accessed on 9 February 2024).
- Kollmannsberger, C.; Tyldesley, S.; Moore, C.; Chi, K.N.; Murray, N.; Daneshmand, S.; Black, P.; Duncan, G.; Hayes-Lattin, B.; Nichols, C. Evolution in management of testicular seminoma: Population-based outcomes with selective utilization of active therapies. Ann. Oncol. 2011, 22, 808–814. [Google Scholar] [CrossRef]
- Boujelbene, N.; Cosinschi, A.; Boujelbene, N.; Khanfir, K.; Bhagwati, S.; Herrmann, E.; Mirimanoff, R.O.; Ozsahin, M.; Zouhair, A. Pure seminoma: A review and update. Radiat. Oncol. 2011, 6, 90. [Google Scholar] [CrossRef]
- Paly, J.J.; Lin, C.C.; Gray, P.J.; Hallemeier, C.L.; Beard, C.; Sineshaw, H.; Jemal, A.; Efstathiou, J.A. Management and outcomes of clinical stage IIA/B seminoma: Results from the National Cancer Data Base 1998–2012. Pract. Radiat. Oncol. 2016, 6, e249–e258. [Google Scholar] [CrossRef]
- Jonska-Gmyrek, J.; Peczkowski, P.; Michalski, W.; Poniatowska, G.; Zolciak-Siwinska, A.; Kotowicz, B.; Wiechno, P.; Golawska, M.; Kowalska, M.; Demkow, T. Radiotherapy in testicular germ cell tumours—A literature review. Contemp. Oncol. 2017, 21, 203–208. [Google Scholar] [CrossRef]
- Wilder, R.B.; Buyyounouski, M.K.; Efstathiou, J.A.; Beard, C.J. Radiotherapy treatment planning for testicular seminoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e445–e452. [Google Scholar] [CrossRef]
- Stein, M.E.; Zidan, J.; Charas, T.; Ben-Yosef, R. Radiotherapy for Stage IIA seminoma: The Northern Israel Oncology Center Experience, 1971–2010. Rep. Pract. Oncol. Radiother. 2014, 19, 281–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Musio, D.; Gravina, G.L.; Marampon, F.; Tombolini, V. Adjuvant radiation therapy in stage I seminoma: 20 years of oncologic results. Oncotarget 2016, 7, 80077–80082. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Powell, M.E. Intensity-modulated radiotherapy—What is it? Cancer Imaging 2004, 26, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef]
- Efstathiou, J.A.; Paly, J.J.; Lu, H.M.; Athar, B.S.; Moteabbed, M.; Niemierko, A.; Adams, J.A.; Bekelman, J.E.; Shipley, W.U.; Zietman, A.L.; et al. Adjuvant radiation therapy for early stage seminoma: Proton versus photon planning comparison and modeling of second cancer risk. Radiother. Oncol. 2012, 103, 12–17. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Kramer, K.; O’Meara, W.P.; Bekelman, J.E.; Belard, A.; McDonough, J.; O’Connell, J. Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 242–249. [Google Scholar] [CrossRef]
- Choo, R.; Kazemba, B.; Choo, C.S.; Lester, S.C.; Whitaker, T. Proton Therapy for Stage IIA-B Seminoma: A New Standard of Care for Treating Retroperitoneal Nodes. Int. J. Part. Ther. 2018, 5, 50–57. [Google Scholar] [CrossRef]
- Pasalic, D.; Prajapati, S.; Ludmir, E.B.; Tang, C.; Choi, S.; Kudchadker, R.; Frank, S.J. Outcomes and Toxicities of Proton and Photon Radiation Therapy for Testicular Seminoma. Int. J. Part. Ther. 2020, 7, 11–20. [Google Scholar] [CrossRef]
- Maxwell, R.; Chang, Y.; Paul, C.; Vaughn, D.J.; Christodouleas, J.P. Cancer Control, Toxicity, and Secondary Malignancy Risks of Proton Radiation Therapy for Stage I-IIB Testicular Seminoma. Adv. Radiat. Oncol. 2023, 8, 101259. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Schneider, U.; Zwahlen, D.; Ross, D.; Kaser-Hotz, B. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Kaser-Hotz, B. Radiation risk estimates after radiotherapy: Application of the organ equivalent dose concept to plateau dose-response relationships. Radiat. Environ. Biophys. 2005, 44, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Kaser-Hotz, B. A simple dose-response relationship for modeling secondary cancer incidence after radiotherapy. Z Med. Phys. 2005, 15, 31–37. [Google Scholar] [CrossRef]
- Schneider, U.; Lomax, A.; Besserer, J.; Pemler, P.; Lombriser, N.; Kaser-Hotz, B. The impact of dose escalation on secondary cancer risk after radiotherapy of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Sumila, M.; Robotka, J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor. Biol. Med. Model. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Pierce, D.A.; Shimizu, Y.; Cullings, H.M.; Fujita, S.; Funamoto, S.; Kodama, K. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat. Res. 2004, 162, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Walsh, L. Cancer risk estimates from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Radiat. Environ. Biophys. 2008, 47, 253–263. [Google Scholar] [CrossRef] [PubMed]
- BEIR: Health Risks from Exposure to Low Levels of Ionizing Radiation, BEIR VII, Phase 2; National Research Council: Ottawa, ON, Canada; National Academy of Science: Washington, DC, USA, 2006.
- Paganetti, H.; Depauw, N.; Johnson, A.; Forman, R.B.; Lau, J.; Jimenez, R. The risk for developing a secondary cancer after breast radiation therapy: Comparison of photon and proton techniques. Radiother. Oncol. 2020, 149, 212–218. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Australia, 2015; Available online: https://www.r-project.org (accessed on 15 February 2022).
- Haque, W.; Wages, C.; Zhu, X.R.; Choi, S.; Pugh, T.J.; Frank, S.J.; Lee, A.; Mahmood, U. Proton therapy for seminoma: Case report describing the technique, efficacy, and advantages of proton-based therapy for seminoma. Pract. Radiat. Oncol. 2015, 5, 135–140. [Google Scholar] [CrossRef]
- Lui, A.; Zeng, J.; Chen, J.; Weg, E.S.; Ellis, W.; Psutka, S.P.; Nyame, Y.A.; Yezefski, T.; Lin, D.; Schade, G.; et al. Proton Radiation Therapy for Stage IIA/IIB Testicular Seminoma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e411–e412. [Google Scholar] [CrossRef]
- Chung, C.S.; Yock, T.I.; Nelson, K.; Xu, Y.; Keating, N.L.; Tarbell, N.J. Incidence of second malignancies among patients treated with proton versus photon radiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 46–52. [Google Scholar] [CrossRef]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]
- Travis, L.B.; Andersson, M.; Gospodarowicz, M.; van Leeuwen, F.E.; Bergfeldt, K.; Lynch, C.F.; Curtis, R.E.; Kohler, B.A.; Wiklund, T.; Storm, H.; et al. Treatment-associated leukemia following testicular cancer. J. Natl. Cancer Inst. 2000, 92, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Agosteo, S.; Pedroni, E.; Besserer, J. Secondary neutron dose during proton therapy using spot scanning. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Haciislamoglu, E.; Cinar, Y.; Gurcan, F.; Canyilmaz, E.; Gungor, G.; Yoney, A. Secondary cancer risk after whole-breast radiation therapy: Field-in-field versus intensity modulated radiation therapy versus volumetric modulated arc therapy. Br. J. Radiol. 2019, 92, 20190317. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419. [Google Scholar] [CrossRef]
- Nogueira, L.M.; Jemal, A.; Yabroff, K.R.; Efstathiou, J.A. Assessment of Proton Beam Therapy Use Among Patients with Newly Diagnosed Cancer in the US, 2004–2018. JAMA Netw Open. 2022, 5, e229025. [Google Scholar] [CrossRef]
- American Society for Radiation Oncology. Proton Beam Therapy, June 2017. Available online: https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Reimbursement/Model_Policies/Content_Pieces/ASTROPBTModelPolicy.pdf (accessed on 21 February 2023).
| Clinical Stage | Age at Treatment | Treatment Modality | Laterality | Number of LN | Largest LN Dimension (cm) | LN Location |
|---|---|---|---|---|---|---|
| IIA | 41 | PBS | Left | 2 | 1.4 | Left common iliac, level L4/L5; left para-aortic, level L2/L3 |
| IIA | 38 | PBS | Left | 4 | 1.0 | Left para-aortic, level L3; left external iliac, levels L2 and L5/S1 and S5 |
| IIA | 48 | PBS | Right | 1 | 2.0 | Anterior to right psoas, level L5/S1 |
| IIA | 38 | PBS | Left | >5 | 1.0 | Left para-aortic, top of L2 to bottom L3 and level L4 |
| IIA | 41 | 3D | Left | 1 | 1.2 | Left para-aortic, level L2/L3 |
| IIB | 41 | PBS | Right | >5 | 2.3 | Aortocaval, level L1/L2; multiple, level L3 |
| IIB | 32 | 3D | Right | 1 | 2.7 | Right external iliac, levels S3–S5 |
| IIB | 54 | 3D | Right | 1 | 3.8 | Below the renal veins, level L3 |
| IIB | 38 | 3D | Left | 1 | 2.1 | Left para-aortic, level L1/L2 |
| IIB | 33 | 3D | Left | 2 | 3.3 | Left para-aortic, level mid-L2; anterior to left psoas, level L4/L5 |
| Organs | a | EUD 3D (GyRBE) | EUD VMAT (GyRBE) | EUD PBS (GyRBE) | Mean Dose 3D (GyRBE) | Mean Dose VMAT (GyRBE) | Mean Dose PBS (GyRBE) |
|---|---|---|---|---|---|---|---|
| Bladder | 2 | 11.3 (8.8, 13.8) | 11.1 (9.0, 13.2) | 7.4 (5.4, 9.5) † | 8.3 (5.8, 10.7) | 9.6 (7.6, 11.7) * | 3.8 (2.3, 5.2) † |
| Large bowel | 5 | 19.4 (18.0, 20.8) | 15.5 (14.0, 17.0) * | 13.1 (11.0, 15.2) † | 7.5 (6.2, 8.8) | 9.9 (8.7, 11.1) * | 2.3 (1.3, 3.2) † |
| Small bowel | 5 | 22.4 (20.3, 24.4) | 19.4 (17.6, 21.2) * | 16.8 (14.8, 18.8) † | 12.0 (10.2, 13.8) | 13.4 (11.7, 15.0) | 4.1 (2.8, 5.4) † |
| Spinal cord | 20 | 27.7 (24.9, 30.5) | 18.0 (15.0, 20.9) * | 20.1 (17.4, 22.9) † | 20.7 (18.8, 22.5) | 10.8 (8.5, 13.1) * | 13.9 (12.3, 15.4) † |
| Kidneys | 1.5 | 7.6 (5.7, 9.5) | 4.5 (3.7, 5.2) * | 4.5 (3.1, 6.1) | 6.0 (4.3, 7.6) | 4.2 (3.5, 4.8) * | 2.9 (1.7, 4.1) † |
| Liver | 0.8 | 2.9 (1.9, 3.9) | 4.5 (3.1, 5.9) * | 0.25 (0.05, 0.45) † | 3.5 (2.4, 4.7) | 4.8 (3.3, 6.2) * | 0.48 (0.15, 0.81) † |
| Pancreas | 5 | 20.4 (18.0, 22.8) | 17.7 (14.7, 20.8) * | 14.7 (11.4, 18.0) † | 15.4 (13.5, 17.4) | 15.1 (12.0, 18.1) | 6.5 (3.2, 9.9) † |
| Rectum | 5 | 11.9 (9.4, 14.4) | 11.3 (8.8, 13.8) | 10.0 (7.4, 12.6) † | 5.4 (3.1, 7.7) | 7.8 (5.6, 10.0) * | 3.3 (1.5, 5.1) † |
| Stomach | 7 | 19.9 (17.2, 22.7) | 11.1 (8.8, 13.3) * | 7.8 (5.3, 10.3) † | 10.2 (8.0, 12.4) | 7.0 (5.3, 8.8) * | 0.60 (0.20, 1.0) † |
| Femurs | 3 | 5.9 (4.0, 7.8) | 4.6 (3.3, 5.8) * | 3.0 (1.4, 4.6) † | 1.6 (0.9, 2.4) | 1.9 (1.3, 2.5) | 0.49 (0.10, 0.88) † |
| Pelvic ilium | 3 | 13.1 (11.3, 15.0) | 9.8 (8.6, 11.0) * | 9.1 (7.6, 10.7) | 5.7 (4.5, 6.9) | 7.3 (6.2, 8.3) * | 2.9 (2.0, 3.8) † |
| Sacrum | 3 | 18.9 (17.0, 20.8) | 14.0 (12.4, 15.6) * | 14.6 (13.0, 16.2) | 14.9 (13.2, 16.7) | 12.2 (10.7, 13.8) * | 10.1 (8.8, 11.6) † |
| Vertebral bodies | 8 | 26.6 (24.1, 29.1) | 21.9 (19.6, 24.3) * | 21.5 (19.1, 24.0) | 21.5 (19.6, 23.4) | 16.2 (14.3, 18.1) * | 15.9 (14.2, 17.6) |
| Organ | 3D EAR | VMAT EAR | PBS EAR | 3D LAR (%) | VMAT LAR (%) | PBS LAR (%) |
|---|---|---|---|---|---|---|
| Bladder | 2.4 (2.2, 2.6) | 3.0 (2.5, 3.6) | 0.86 (0.66, 1.05) † | 0.34 (0.29, 0.38) | 0.42 (0.34, 0.51) | 0.12 (0.09, 0.15) † |
| Large bowel | 3.0 (2.7, 3.3) | 1.8 (1.6, 1.9) * | 0.41 (0.26, 0.55) † | 0.22 (0.20, 0.25) | 0.13 (0.12, 0.14) * | 0.03 (0.02, 0.04) † |
| Small bowel | 2.6 (2.4, 2.8) | 1.4 (1.2, 1.7) * | 0.59 (0.48, 0.71) † | 0.19 (0.17, 0.21) | 0.10 (0.09, 0.12) * | 0.04 (0.03, 0.05) † |
| Spinal cord | 8.2 (7.7, 8.7) | 5.9 (5.2, 6.6) * | 6.1 (5.6, 6.7) | 1.15 (1.03, 1.27) | 0.82 (0.70, 0.95) * | 0.85 (0.76, 0.95) |
| Kidneys | 3.5 (3.2, 3.8) | 3.4 (3.1, 3.8) | 0.81 (0.64, 0.98) † | 0.26 (0.23, 0.28) | 0.25 (0.23, 0.28) | 0.06 (0.05, 0.07) † |
| Liver | 1.17 (1.04, 1.30) | 1.70 (1.62, 1.79) * | 0.10 (0.05, 0.15) † | 0.13 (0.11, 0.15) | 0.19 (0.18, 0.21) * | 0.012 (0.005, 0.018) † |
| Pancreas | 1.84 (1.66, 2.01) | 1.17 (0.98, 1.36) * | 1.14 (1.01, 1.28) | 0.14 (0.12, 0.15) | 0.09 (0.07, 0.10) * | 0.08 (0.07, 0.10) |
| Rectum | 3.2 (1.9, 4.6) | 4.7 (3.4, 6.0) * | 2.0 (0.9, 3.0) † | 0.46 (0.25, 0.66) | 0.67 (0.46, 0.87) * | 0.28 (0.12, 0.44) † |
| Stomach | 3.2 (3.0, 3.3) | 3.6 (3.4, 3.7) * | 0.32 (0.18, 0.46) † | 0.49 (0.43, 0.54) | 0.55 (0.48, 0.61) * | 0.05 (0.02, 0.07) † |
| Total | 29.1 | 26.7 | 12.3 | 3.4% | 3.2% | 1.52% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pursley, J.; Remillard, K.; Depauw, N.; Lee, G.; Grassberger, C.; Paganetti, H.; Efstathiou, J.A.; Kamran, S.C. Radiation Therapy for Stage IIA/B Seminoma: Modeling Secondary Cancer Risk for Protons and VMAT versus 3D Photons. Cancers 2024, 16, 784. https://doi.org/10.3390/cancers16040784
Pursley J, Remillard K, Depauw N, Lee G, Grassberger C, Paganetti H, Efstathiou JA, Kamran SC. Radiation Therapy for Stage IIA/B Seminoma: Modeling Secondary Cancer Risk for Protons and VMAT versus 3D Photons. Cancers. 2024; 16(4):784. https://doi.org/10.3390/cancers16040784
Chicago/Turabian StylePursley, Jennifer, Kyla Remillard, Nicolas Depauw, Grace Lee, Clemens Grassberger, Harald Paganetti, Jason A. Efstathiou, and Sophia C. Kamran. 2024. "Radiation Therapy for Stage IIA/B Seminoma: Modeling Secondary Cancer Risk for Protons and VMAT versus 3D Photons" Cancers 16, no. 4: 784. https://doi.org/10.3390/cancers16040784
APA StylePursley, J., Remillard, K., Depauw, N., Lee, G., Grassberger, C., Paganetti, H., Efstathiou, J. A., & Kamran, S. C. (2024). Radiation Therapy for Stage IIA/B Seminoma: Modeling Secondary Cancer Risk for Protons and VMAT versus 3D Photons. Cancers, 16(4), 784. https://doi.org/10.3390/cancers16040784







