Promising Results of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Perihilar Cholangiocarcinoma in a Systematic Review and Single-Arm Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Study Selection and Data Extraction
2.4. Quality Assessment (Bias)
2.5. Definition of Extracted Data
2.5.1. Demographic and Baseline Characteristics
2.5.2. Surgical Technique
2.5.3. Postoperative Complications and Mortality
2.5.4. Oncological Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Studies
3.2. Quality Assessment
3.3. Demographic and Baseline Characteristics
3.4. Surgical Technique
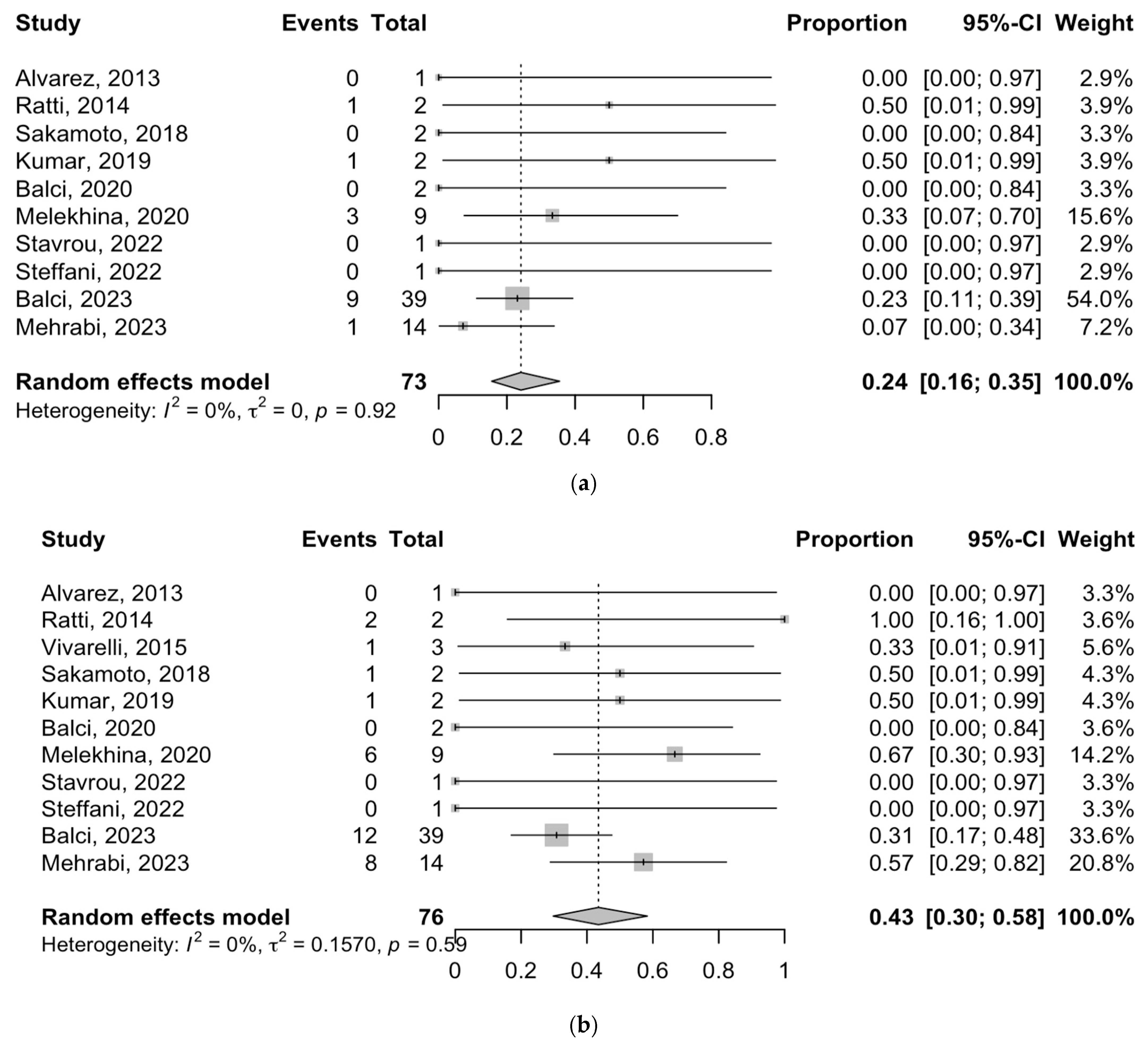
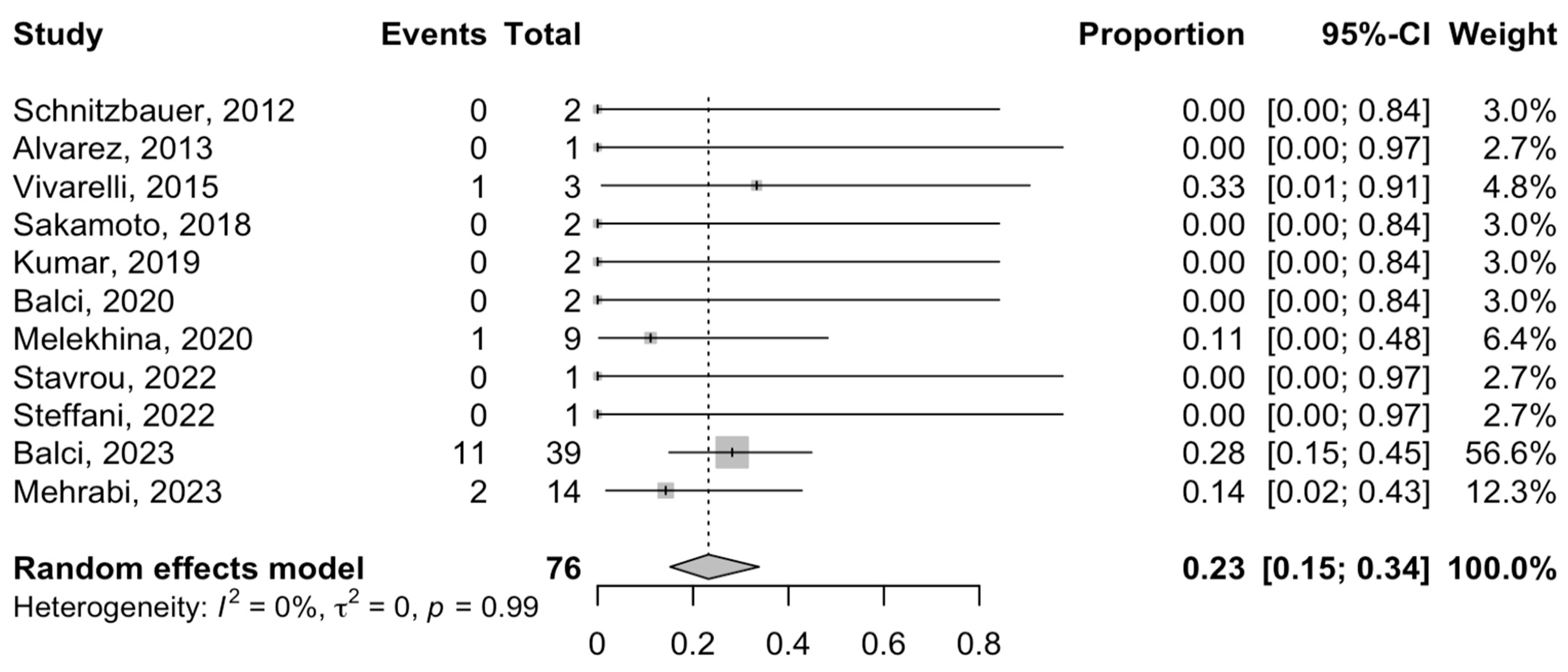
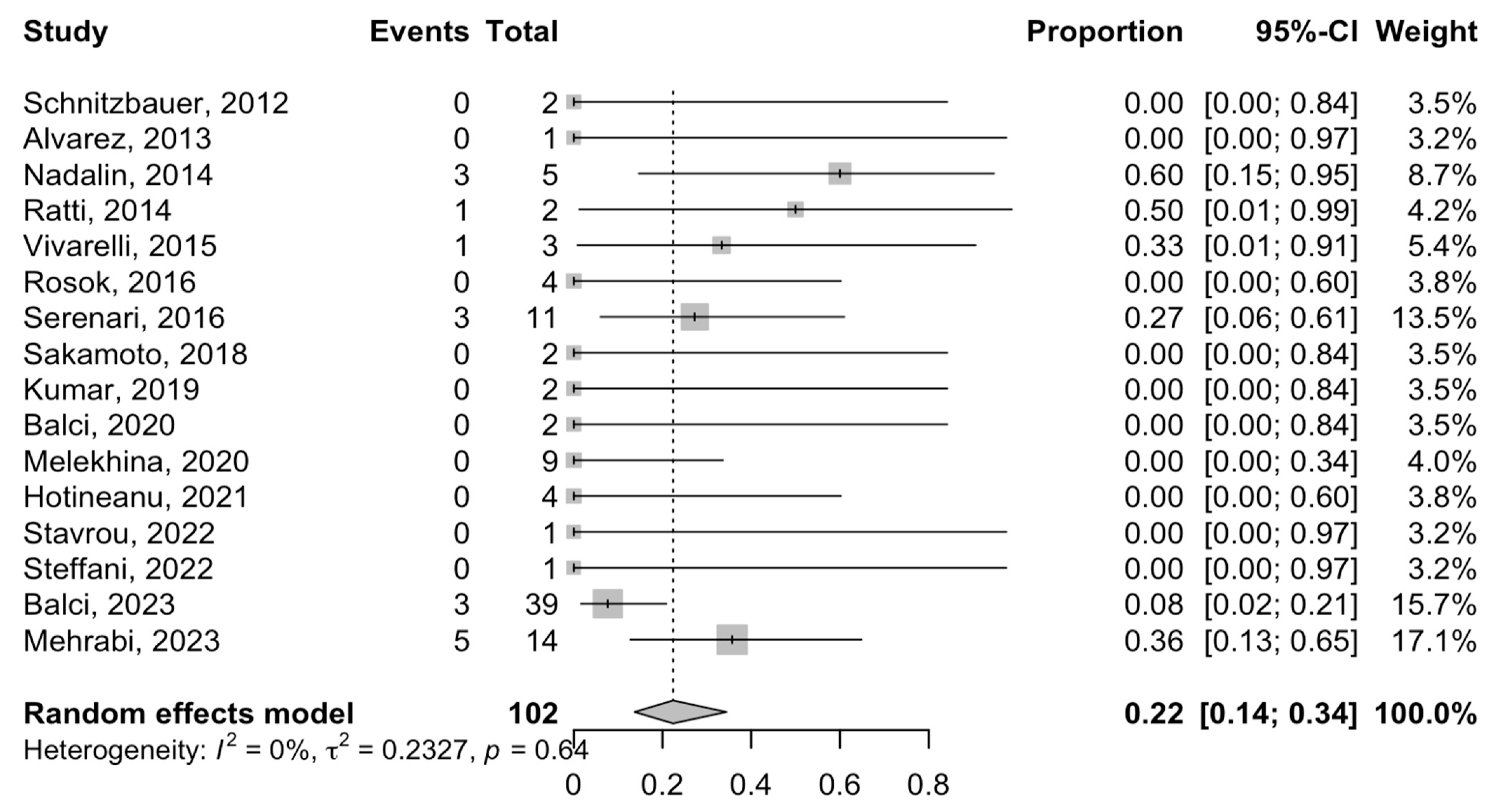
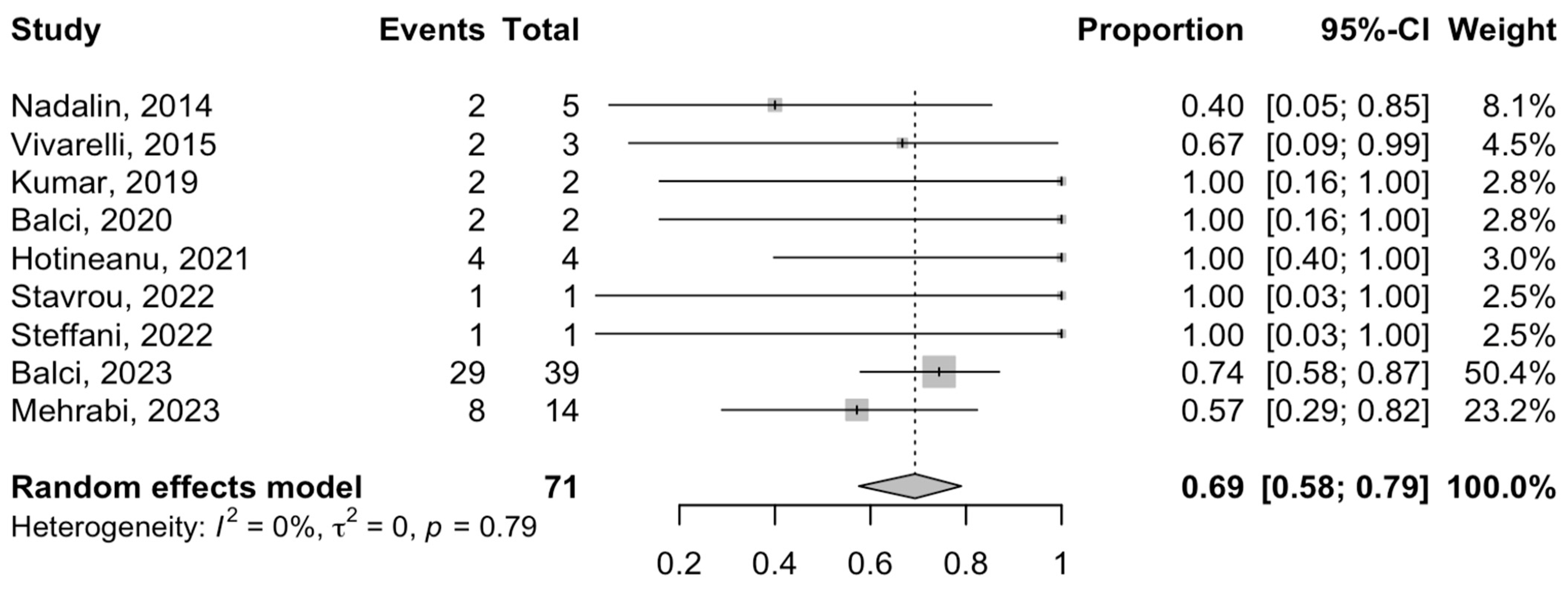
3.5. Postoperative Complications and Mortality
3.6. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cidon, E.U. Resectable Cholangiocarcinoma: Reviewing the Role of Adjuvant Strategies. Clin. Med. Insights Oncol. 2016, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tsilimigras, D.I.; Farooq, A.; Hyer, J.M.; Merath, K.; Paredes, A.Z.; Mehta, R.; Sahara, K.; Shen, F.; Pawlik, T.M. Potential survival benefit of radiofrequency ablation for small solitary intrahepatic cholangiocarcinoma in nonsurgically managed patients: A population-based analysis. J. Surg. Oncol. 2019, 120, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M. Perihilar cholangiocarcinoma: A surgeon’s viewpoint on current topics. J. Gastroenterol. 2012, 47, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Golriz, M.; Majlesara, A.; El Sakka, S.; Ashrafi, M.; Arwin, J.; Fard, N.; Raisi, H.; Edalatpour, A.; Mehrabi, A. Small for Size and Flow (SFSF) syndrome: An alternative description for posthepatectomy liver failure. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 267–275. [Google Scholar] [CrossRef]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef]
- Alvarez, F.A.; Ardiles, V.; Sanchez Claria, R.; Pekolj, J.; de Santibañes, E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): Tips and tricks. J. Gastrointest. Surg. 2013, 17, 814–821. [Google Scholar] [CrossRef]
- Schadde, E.; Ardiles, V.; Slankamenac, K.; Tschuor, C.; Sergeant, G.; Amacker, N.; Baumgart, J.; Croome, K.; Hernandez-Alejandro, R.; Lang, H.; et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: Results of a multicenter analysis. World J. Surg. 2014, 38, 1510–1519. [Google Scholar] [CrossRef]
- Olthof, P.B.; Coelen, R.J.S.; Wiggers, J.K.; Groot Koerkamp, B.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Robles Campos, R.; et al. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kalkum, E.; Klotz, R.; Seide, S.; Hüttner, F.J.; Kowalewski, K.F.; Nickel, F.; Khajeh, E.; Knebel, P.; Diener, M.K.; Probst, P. Systematic reviews in surgery-recommendations from the Study Center of the German Society of Surgery. Langenbecks Arch. Surg. 2021, 406, 1723–1731. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Belghiti, J.; Farges, O.; Ogata, S.; Sauvanet, A.; Delefosse, D.; Durand, F. The “50-50 criteria” on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann. Surg. 2005, 242, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, A.; Golriz, M.; Ramouz, A.; Khajeh, E.; Hammad, A.; Hackert, T.; Müller-Stich, B.; Strobel, O.; Al-Saegh, S.; Ghamarnejad, O.; et al. Promising Outcomes of Modified ALPPS for Staged Hepatectomy in Cholangiocarcinoma. Cancers 2023, 15, 5613. [Google Scholar] [CrossRef] [PubMed]
- Balci, D.; Nadalin, S.; Mehrabi, A.; Alikhanov, R.; Fernandes, E.; Di Benedetto, F.; Hernandez-Alejandro, R.; Bergthor, B.; Efanov, M.; Capobianco, I.; et al. Revival of Alpps For Perihilar Cholangiocarcinoma: An international multicenter study with promising outcomes. Surgery 2023, 173, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, S.; Capoblanco, I.; Li, J.; Girotti, P.; Konigsrainer, I.; Konigsrainer, A. Indications and Limits for Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS). Lessons Learned from 15 Cases at a Single Centre. Z. Gastroenterol. 2014, 52, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ratti, F.; Cipriani, F.; Gagliano, A.; Catena, M.; Paganelli, M.; Aldrighetti, L. Defining indications to ALPPS procedure: Technical aspects and open issues. Updates Surg. 2014, 66, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Manzini, G.; Hristov, B.; Polychronidis, G.; Mokry, T.; Sommer, C.M.; Mehrabi, A.; Weitz, J.; Büchler, M.W.; Schemmer, P. Impact of Neoadjuvant Chemotherapy on Hypertrophy of the Future Liver Remnant after Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J. Am. Coll. Surg. 2015, 221, 717–728.e1. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Vincenzi, P.; Montalti, R.; Fava, G.; Tavio, M.; Coletta, M.; Vecchi, A.; Nicolini, D.; Agostini, A.; Ahmed, E.A.; et al. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS ONE 2015, 10, e0144019. [Google Scholar] [CrossRef] [PubMed]
- Rosok, B.I.; Bjornson, B.; Sparrelid, E.; Hasselgren, K.; Pomianowska, E.; Gasslander, T.; Bjornbeth, B.A.; Isaksson, B.; Sandstrom, P. Scandinavian multicenter study on the safety and feasibility of the associating liver partition and portal vein ligation for staged hepatectomy procedure. Surgery 2016, 159, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Serenari, M.; Zanello, M.; Schadde, E.; Toschi, E.; Ratti, F.; Gringeri, E.; Masetti, M.; Cillo, U.; Aldrighetti, L.; Jovine, E. Importance of primary indication and liver function between stages: Results of a multicenter Italian audit of ALPPS 2012-2014. HPB 2016, 18, 419–427. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Matsumura, M.; Yamashita, S.; Ohkura, N.; Hasegawa, K.; Kokudo, N. Partial TIPE ALPPS for Perihilar Cancer. Ann. Surg. 2018, 267, e18–e20. [Google Scholar] [CrossRef]
- Kumar, N.; Duncan, T.; O’Reilly, D.; Káposztás, Z.; Parry, C.; Rees, J.; Junnarkar, S. Partial ALPPS with a longer wait between procedures is safe and yields adequate future liver remnant hypertrophy. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 13–19. [Google Scholar] [CrossRef]
- Balci, D.; Kirimker, E.O.; Ustuner, E.; Yilmaz, A.A.; Azap, A. Stage I-laparoscopy partial ALPPS procedure for perihilar cholangiocarcinoma. J. Surg. Oncol. 2020, 121, 1022–1026. [Google Scholar] [CrossRef]
- Melekhina, O.; Efanov, M.; Alikhanov, R.; Tsvirkun, V.; Kulezneva, Y.; Kazakov, I.; Vankovich, A.; Koroleva, A.; Khatkov, I. Percutaneous radiofrequency-assisted liver partition versus portal vein embolization before hepatectomy for perihilar cholangiocarcinoma. BJS Open 2020, 4, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chebaro, A.; Buc, E.; Durin, T.; Chiche, L.; Brustia, R.; Didier, A.; Pruvot, F.R.; Kitano, Y.; Muscari, F.; Lecolle, K.; et al. Liver Venous Deprivation (LVD) or Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS)?: A Retrospective Multicentric Study. Ann. Surg. 2021, 274, 874–880. [Google Scholar] [CrossRef]
- Hotineanu, A.; Burgoci, S.; Bortă, E. ALPPS Procedure. The New Frontier in Advanced Liver Surgery. Single Centre Experience and Literature Review. Chirurgia 2021, 116, 409–423. [Google Scholar] [CrossRef]
- Stavrou, G.A.; Kardassis, D.; Blatt, L.A.; Gharbi, A.; Donati, M. Modified ALPPS as an individual rescue treatment strategy for resection of Klatskin-tumors. Hepatobiliary Pancreat. Dis. Int. 2022, 22, 85–87. [Google Scholar] [CrossRef]
- Steffani, M.; Stöss, C.; Laschinger, M.; Assfalg, V.; Schulze, S.; Mogler, C.; Lohöfer, F.; Paprottka, P.; Hüser, N.; Friess, H.; et al. softALPPS—A novel, individual procedure for patients with advanced liver tumors. HPB 2022, 24, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.L.; Song, P.P.; Tang, W.; Cheng, N.S. An updated systematic review of the evolution of ALPPS and evaluation of its advantages and disadvantages in accordance with current evidence. Medicine 2016, 95, e3941. [Google Scholar] [CrossRef]
- Sandstrom, P.; Rosok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjornbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS Improves Resectability Compared with Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Schadde, E.; Ardiles, V.; Robles-Campos, R.; Malago, M.; Machado, M.; Hernandez-Alejandro, R.; Soubrane, O.; Schnitzbauer, A.A.; Raptis, D.; Tschuor, C.; et al. Early survival and safety of ALPPS: First report of the International ALPPS Registry. Ann. Surg. 2014, 260, 829–836, discussion 836–828. [Google Scholar] [CrossRef]
- de Santibanes, E.; Alvarez, F.A.; Ardiles, V.; Pekolj, J.; de Santibanes, M. Inverting the ALPPS paradigm by minimizing first stage impact: The Mini-ALPPS technique. Langenbecks Arch. Surg. 2016, 401, 557–563. [Google Scholar] [CrossRef]
- Gall, T.M.; Sodergren, M.H.; Frampton, A.E.; Fan, R.; Spalding, D.R.; Habib, N.A.; Pai, M.; Jackson, J.E.; Tait, P.; Jiao, L.R. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann. Surg. 2015, 261, e45–e46. [Google Scholar] [CrossRef]
- Li, J.; Kantas, A.; Ittrich, H.; Koops, A.; Achilles, E.G.; Fischer, L.; Nashan, B. Avoid “All-Touch” by Hybrid ALPPS to Achieve Oncological Efficacy. Ann. Surg. 2016, 263, e6–e7. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Robles-Campos, R.; Brusadin, R.; Lopez-Conesa, A.; Navarro, A.; Arevalo-Perez, J.; Gil, P.J.; Parrilla, P. Tourniquet-ALPPS is a promising treatment for very large hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018, 9, 28267–28280. [Google Scholar] [CrossRef]
- Petrowsky, H.; Györi, G.; de Oliveira, M.; Lesurtel, M.; Clavien, P.A. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann. Surg. 2015, 261, e90–e92. [Google Scholar] [CrossRef]
- Vicente, E.; Quijano, Y.; Ielpo, B.; Fabra, I. First ALPPS procedure using a total robotic approach. Surg. Oncol. 2016, 25, 457. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Stavrou, G.A.; van Gulik, T.M.; Oldhafer, K.J. Associating liver partition and portal vein ligation for staged hepatectomy for Klatskin tumours: Hinc sunt leones! ANZ J. Surg. 2015, 85, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Linecker, M.; Stavrou, G.A.; Oldhafer, K.J.; Jenner, R.M.; Seifert, B.; Lurje, G.; Bednarsch, J.; Neumann, U.; Capobianco, I.; Nadalin, S.; et al. The ALPPS Risk Score: Avoiding Futile Use of ALPPS. Ann. Surg. 2016, 264, 763–771. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Schadde, E.; Linecker, M.; Machado, M.A.; Adam, R.; Malago, M.; Clavien, P.A.; de Santibanes, E.; Bechstein, W.O. Indicating ALPPS for Colorectal Liver Metastases: A Critical Analysis of Patients in the International ALPPS Registry. Surgery 2018, 164, 387–394. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Inagaki, F.; Omichi, K.; Ohkura, N.; Hasegawa, K.; Kokudo, N. Associating Liver Partial Partition and Transileocecal Portal Vein Embolization for Staged Hepatectomy. Ann. Surg. 2016, 264, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological, and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, F.; Baumgart, J.; Hoppe-Lotichius, M.; Schmidtmann, I.; Heinrich, S.; Lang, H. Visceral infiltration of intrahepatic cholangiocarcinoma is most prognostic after curative resection—Retrospective cohort study of 102 consecutive liver resections from a single center. Int. J. Surg. 2018, 55, 193–200. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Sun, W.L.; Ma, X.; Lu, Z.; Wu, B.Q.; Wu, H.; Wang, D.D.; Liu, Z.; Cui, P.Y. [Surgical treatment of patients with hilar cholangiocarcinoma in a single center]. Zhonghua Yi Xue Za Zhi 2019, 99, 284–287. [Google Scholar] [CrossRef]
- Schadde, E.; Raptis, D.A.; Schnitzbauer, A.A.; Ardiles, V.; Tschuor, C.; Lesurtel, M.; Abdalla, E.K.; Hernandez-Alejandro, R.; Jovine, E.; Machado, M.; et al. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann. Surg. 2015, 262, 780–786. [Google Scholar] [CrossRef]
- Matsuo, K.; Hiroshima, Y.; Yamazaki, K.; Kasahara, K.; Kikuchi, Y.; Kawaguchi, D.; Murakami, T.; Ishida, Y.; Tanaka, K. Immaturity of Bile Canalicular-Ductule Networks in the Future Liver Remnant While Associating Liver Partition and Portal Vein Occlusion for Staged Hepatectomy (ALPPS). Ann. Surg. Oncol. 2017, 24, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Lesurtel, M.; Melloul, E.; Limani, P.; Tschuor, C.; Graf, R.; Humar, B.; Clavien, P.A. ALPPS: From human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann. Surg. 2014, 260, 839–846, discussion 846–837. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; D’Amico, F.E.; Furlanetto, A.; Perin, L.; Gringeri, E. Robotic hepatectomy and biliary reconstruction for perihilar cholangiocarcinoma: A pioneer western case series. Updates Surg. 2021, 73, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Gumbs, A.A.; Jarufe, N.; Gayet, B. Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg. Endosc. 2013, 27, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.G.; Brice, G. Why Artificial Intelligence Surgery (AIS) is better than current Robotic-Assisted Surgery (RAS). Artif. Intell. Surg. 2022, 2, 207–212. [Google Scholar] [CrossRef]
- Giannone, F.; Felli, E.; Cherkaoui, Z.; Mascagni, P.; Pessaux, P. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers 2021, 13, 6268. [Google Scholar] [CrossRef]
- Schneider, C.; Allam, M.; Stoyanov, D.; Hawkes, D.J.; Gurusamy, K.; Davidson, B.R. Performance of image guided navigation in laparoscopic liver surgery—A systematic review. Surg. Oncol. 2021, 38, 101637. [Google Scholar] [CrossRef]


| Author, Year | Country | Study Period | Sample Size | Mean Age | Indication | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRLM | HCC | ihCC | phCC | GBC | Metastasis | Other | |||||
| Schnitzbauer 2012 [7] | Germany | 2007–2011 | 25 | 63 | - | 3 | 2 | 2 | 1 | 16 | 1 |
| Alvarez 2013 [10] | Argentina | 2011–2012 | 15 | 54 | 10 | 1 | - | 1 | - | 3 | - |
| Nadalin 2014 [22] | Germany | 2010–2013 | 15 | 67 | 5 | 1 | 4 | 5 | - | - | - |
| Ratti 2014 [23] | Italy | 2012 | 8 | 58 | 5 | - | - | 2 | 1 | - | - |
| Vivarelli 2015 [25] | Italy | 2013–2014 | 9 | 60 | 4 | 1 | 1 | 3 | - | - | - |
| Kremer 2016 [24] | Germany | 2011–2014 | 19 | 57 | 11 | - | 2 | 5 | 1 | - | - |
| Rosok 2016 [26] | Norway, Sweden | 2012–2015 | 36 | 67 | 25 | 4 | - | 4 | - | - | 3 |
| Serenari 2016 [27] | Italy | 2012–2014 | 50 | 62 | - | 8 | 8 | 11 | 1 | 22 | - |
| Sakamoto 2018 [28] | Japan | 2015–2017 | 3 | 67 | - | - | - | 2 | - | - | 1 |
| Kumar 2019 [29] | Singapore | 2014–2019 | 8 | 61 | 6 | - | - | 2 | - | - | - |
| Balci 2020 [30] | Turkey | 2012–2019 | 2 | 53 | - | - | - | 2 | - | - | - |
| Melekhina 2020 [31] | Russia | 2013–2018 | 11 | 58 | - | - | - | 11 | - | - | - |
| Chebaro 2021 [32] | France | 2011–2020 | 85 | 62 | 73 | 1 | 3 | 3 | 2 | - | 3 |
| Hotineanu 2021 [33] | Moldova | 2018–2020 | 18 | 62 | 7 | 6 | - | 4 | - | 1 | - |
| Stavrou 2022 [34] | Germany | 2018 | 2 | 67 | - | - | - | 1 | - | - | 1 |
| Steffani 2022 [35] | Germany | 2019 | 4 | NA | - | 1 | - | 1 | - | - | 2 |
| Balci 2023 [21] | Turkey (multicentric) | 2010–2020 | 39 | 60.5 | - | - | - | 39 | - | - | - |
| Mehrabi 2023 [20] | Germany | 2011–2021 | 21 (30 ^) | 64.1 | - | - | 7 | 14 | - | - | - |
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Score (Quality) |
|---|---|---|---|---|---|---|---|---|---|
| Schnitzbauer 2012 [7] | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 8 (intermediate) |
| Alvarez 2013 [10] | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 8 (intermediate) |
| Nadalin 2014 [22] | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 8 (intermediate) |
| Ratti 2014 [23] | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 7 (low) |
| Vivarelli 2015 [25] | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 8 (intermediate) |
| Kremer 2016 [24] | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 0 | 11 (intermediate) |
| Rosok 2016 [26] | 2 | 1 | 0 | 2 | 0 | 2 | 2 | 0 | 9 (intermediate) |
| Serenari 2016 [27] | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 7 (low) |
| Sakamoto 2018 [28] | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 5 (low) |
| Kumar 2019 [29] | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 7 (low) |
| Balci 2020 [30] | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 7 (low) |
| Melekhina 2020 [31] | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 (intermediate) |
| Chebaro 2021 [32] | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 (intermediate) |
| Hotineanu 2021 [33] | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 9 (intermediate) |
| Stavrou 2022 [34] | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 7 (low) |
| Steffani 2022 [35] | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 8 (intermediate) |
| Balci 2023 [21] | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 (intermediate) |
| Mehrabi 2023 [20] | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 (intermediate) |
| Author, Year | phCC (%) | Type of ALPPS | Type of Resection | BDA (n) | BDA (Stage I or II) |
|---|---|---|---|---|---|
| Schnitzbauer 2012 [7] | 2 (8) | Classic | Right trisectionectomy | Yes | 2nd |
| Alvarez 2013 [10] | 1 (6.7) | Classic | Right trisectionectomy | Yes (1) | 1st |
| Nadalin 2014 [22] | 5 (33.3) | Classic | Right trisectionectomy | Yes (5) | 2nd |
| Ratti 2014 [23] | 2 (25) | Classic | Right trisectionectomy | Yes (2) | 1st |
| Vivarelli 2015 [25] | 3 (33.3) | Classic | NA | Yes (3) | 1st |
| Kremer 2016 [24] | 5 (26.3) | Classic | Right trisectionectomy | Yes (5) | NA |
| Rosok 2016 [26] | 4 (11.1) | Classic | NA | No | NA |
| Serenari 2016 [27] | 11 (22) | Classic | Right trisectionectomy | No | NA |
| Sakamoto 2018 [28] | 2 (66.7) | Partial TIPE ALPPS | 1 right hepatectomy, 1 left trisectionectomy | Yes (2) | NA |
| Kumar 2019 [29] | 2 (25) | Partial | Right trisectionectomy | No | NA |
| Balci 2020 [30] | 2 (100) | Laparoscopic Partial | Right trisectionectomy | Yes (2) | 2nd |
| Melekhina 2020 [31] | 9 (100) | PRALPPS | NA | Yes (9) | 2nd |
| Chebaro 2021 [32] | 3 (3.5) | Classic | NA | NA | NA |
| Hotineanu 2021 [33] | 4 (22) | Classic/Anterior/Partial | Right trisectionectomy | Yes (4) | 1st |
| Stavrou 2022 [34] | 1 (50) | Partial | Right trisectionectomy | Yes (1) | 1st |
| Steffani 2022 [35] | 1 (25) | Soft-ALPPS | NA | NA | NA |
| Balci 2023 [21] | 39 (100) | Classic/Modified | 12 right hepatectomy, 27 left trisectionectomy | Yes | 1st (8 patients) 2nd (31 patients) |
| Mehrabi 2023 [20] | 14 (67) | Classic/Modified | 4 right hepatectomy, 10 left trisectionectomy | Yes | 2nd |
| Author, Year | phCC (%) | R0 n (%) | Major Morbidity n (%) | PHLF n (%) | Mortality n (%) | 1-yr DFS n (%) | 1-yr OS n (%) | |
|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | |||||||
| Schnitzbauer 2012 [7] | 2 (8) | 2 (100) | NA | NA | 0 (0) | 0 (0) | NA | NA |
| Alvarez 2013 [10] | 1 (6.7) | 1 (100) | 0 (0) | 0 (0) | 0 (0) a | 0 (0) | NA | NA |
| Nadalin 2014 [22] | 5 (33.3) | 4 (80) | NA | NA | NA | 3 (60) | 2 (40) | 2 (40) |
| Ratti 2014 [23] | 2 (25) | 2 (100) | 1 (50) | 2 (100) | NA | 1 (50) | NA | NA |
| Vivarelli 2015 [25] | 3 (33.3) | 3 (100) | NA | 1 (33) | 1 (33) b | 1 (33) | NA | 2 (67) |
| Kremer 2016 [24] | 5 (26.3) | 5 (100) | NA | NA | NA | NA | 3 (60) | NA |
| Rosok 2016 [26] | 4 (11.1) | 3 (75) | NA | NA | NA | 0 (0) | NA | NA |
| Serenari 2016 [27] | 11 (22) | 11 (100) | NA | NA | NA | 3 (27) | NA | NA |
| Sakamoto 2018 [28] | 2 (66.7) | NA | 0 (0) | 1 (50) | 0 (0) | 0 (0) | NA | NA |
| Kumar 2019 [29] | 2 (25) | NA | 1 (50) | 1 (50) | 0 (0) | 0 (0) | NA | 2 (100) |
| Balci 2020 [30] | 2 (100) | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 2 (100) |
| Melekhina 2020 [31] | 9 (100) | 8 (89) | 3 (27) | 6 (67) | 1 (11) a | 0 (0) | NA | NA |
| Chebaro 2021 [32] | 3 (3.5) | NA | NA | NA | NA | NA | NA | NA |
| Hotineanu 2021 [33] | 4 (22) | 4 (100) | NA | NA | NA | 0 (0) | 4 (100) | 4 (100) |
| Stavrou 2022 [34] | 1 (50) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Steffani 2022 [35] | 1 (25) | 1 (100) | 0 (0) | 0 (0) | 0 (0) a | 0 (0) | 0 (0) | 0 (0) |
| Balci 2023 [21] | 39 (100) | 32 (82) | 9 (23) | 12 (31) | 11 (28) a | 3 (7.6) | 30 (36) | 29 (36) |
| Mehrabi 2023 [20] | 14 (67) | 10 (71) | 1 (7.1) | 8 (57) | 2 (14.3) a | 5 (36) | 6 (43) | 8 (57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golriz, M.; Ramouz, A.; Hammad, A.; Aminizadeh, E.; Sabetkish, N.; Khajeh, E.; Ghamarnejad, O.; Carvalho, C.; Rio-Tinto, H.; Chang, D.-H.; et al. Promising Results of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Perihilar Cholangiocarcinoma in a Systematic Review and Single-Arm Meta-Analysis. Cancers 2024, 16, 771. https://doi.org/10.3390/cancers16040771
Golriz M, Ramouz A, Hammad A, Aminizadeh E, Sabetkish N, Khajeh E, Ghamarnejad O, Carvalho C, Rio-Tinto H, Chang D-H, et al. Promising Results of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Perihilar Cholangiocarcinoma in a Systematic Review and Single-Arm Meta-Analysis. Cancers. 2024; 16(4):771. https://doi.org/10.3390/cancers16040771
Chicago/Turabian StyleGolriz, Mohammad, Ali Ramouz, Ahmed Hammad, Ehsan Aminizadeh, Nastaran Sabetkish, Elias Khajeh, Omid Ghamarnejad, Carlos Carvalho, Hugo Rio-Tinto, De-Hua Chang, and et al. 2024. "Promising Results of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Perihilar Cholangiocarcinoma in a Systematic Review and Single-Arm Meta-Analysis" Cancers 16, no. 4: 771. https://doi.org/10.3390/cancers16040771
APA StyleGolriz, M., Ramouz, A., Hammad, A., Aminizadeh, E., Sabetkish, N., Khajeh, E., Ghamarnejad, O., Carvalho, C., Rio-Tinto, H., Chang, D.-H., Joao, A. A., Goncalves, G., & Mehrabi, A. (2024). Promising Results of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Perihilar Cholangiocarcinoma in a Systematic Review and Single-Arm Meta-Analysis. Cancers, 16(4), 771. https://doi.org/10.3390/cancers16040771









