Comparison of Liquid-Based Preparations with Conventional Smears in Thyroid Fine-Needle Aspirates: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Literature Search Strategy
2.3. Selection Criteria
2.4. Data Extraction and Risk of Bias Assessment
2.5. Statistical Analysis and Outcome Measurements
3. Results
3.1. Search and Study Selection
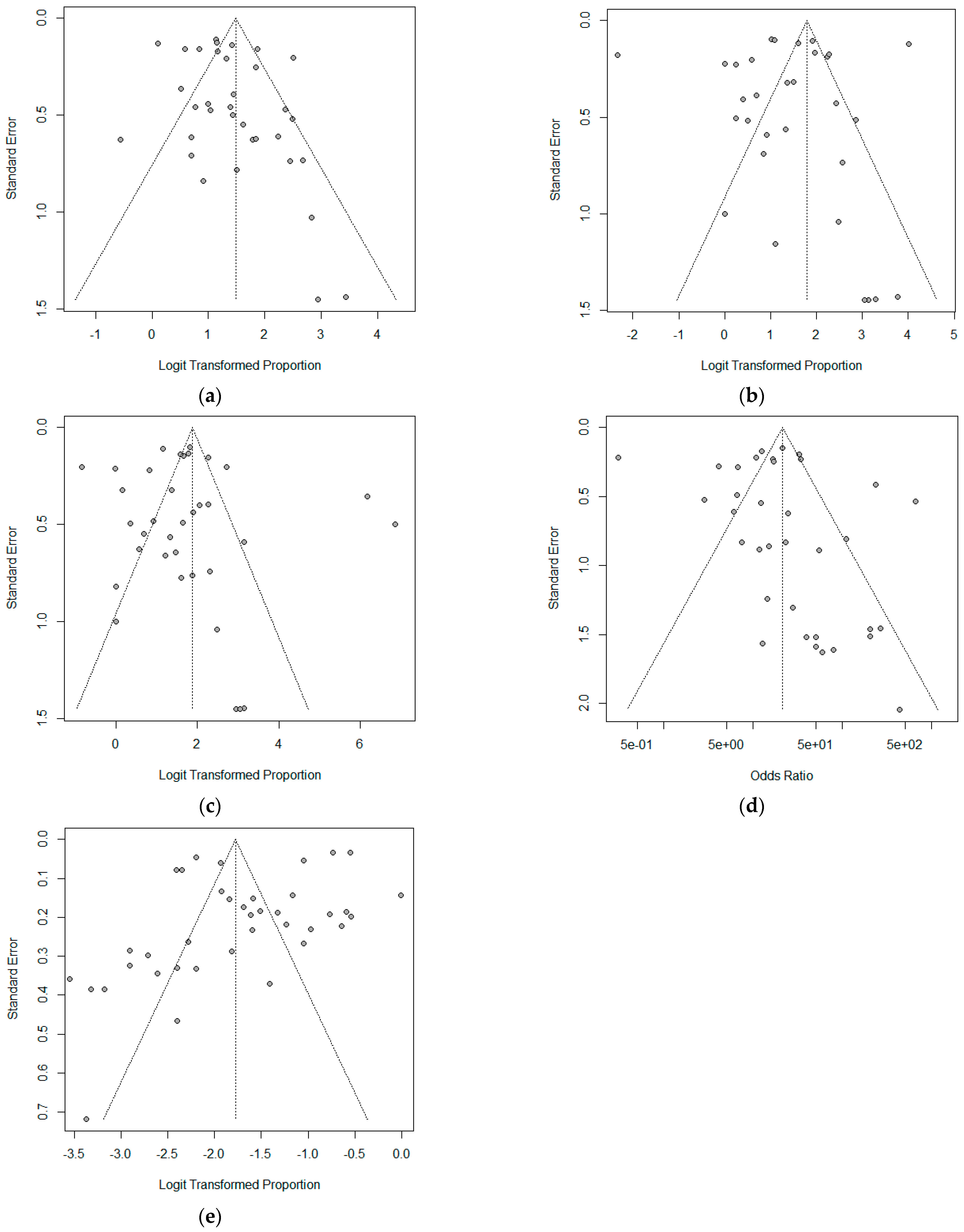
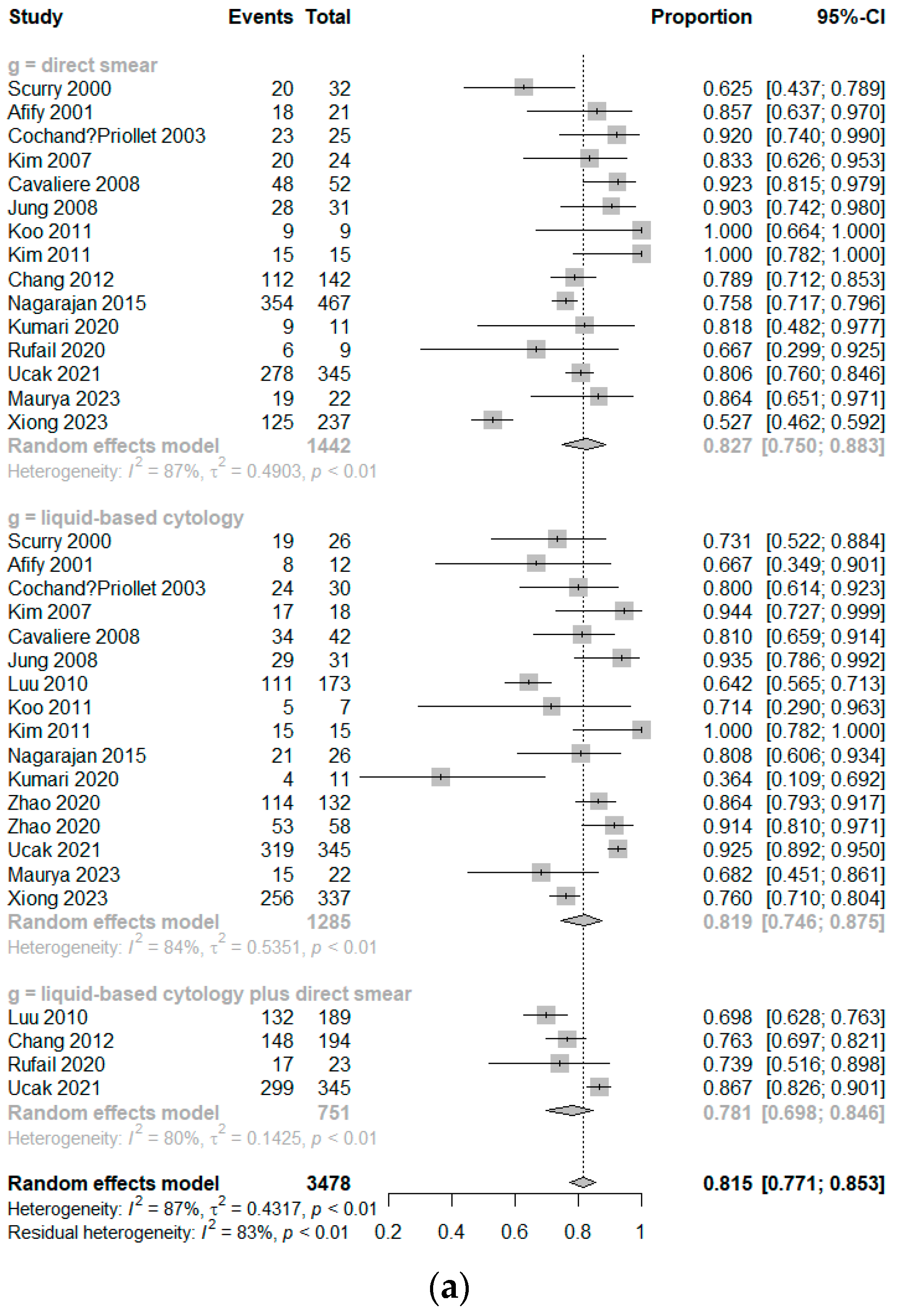
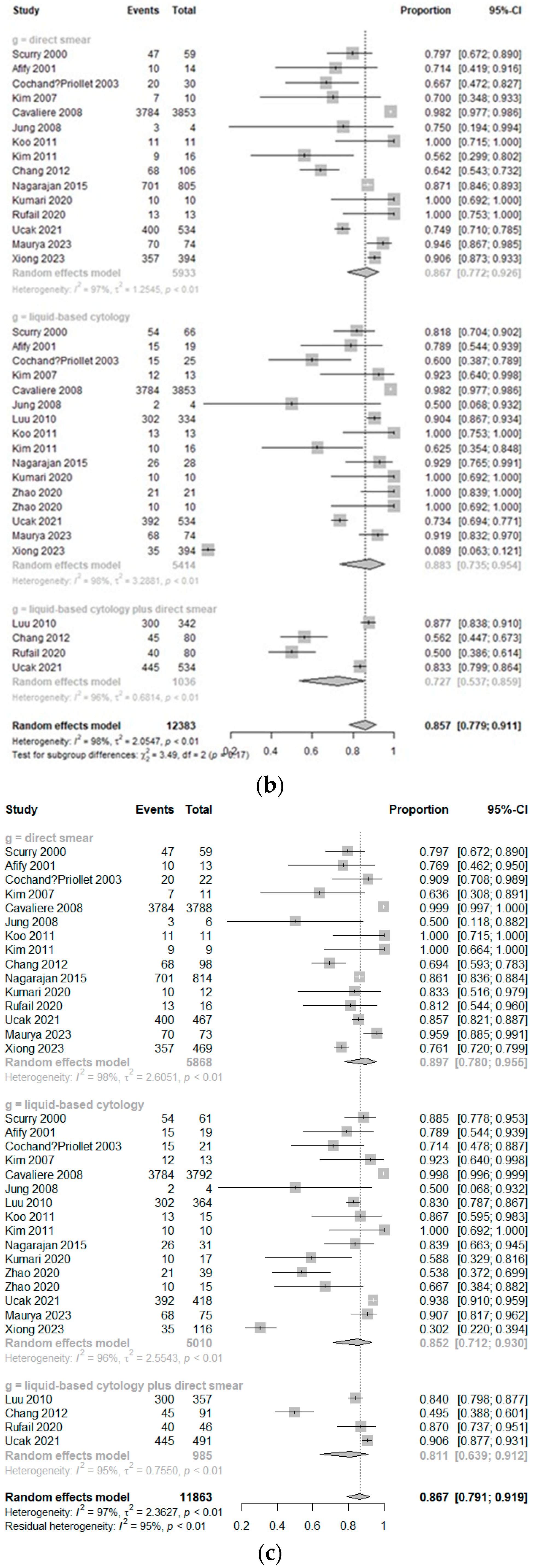
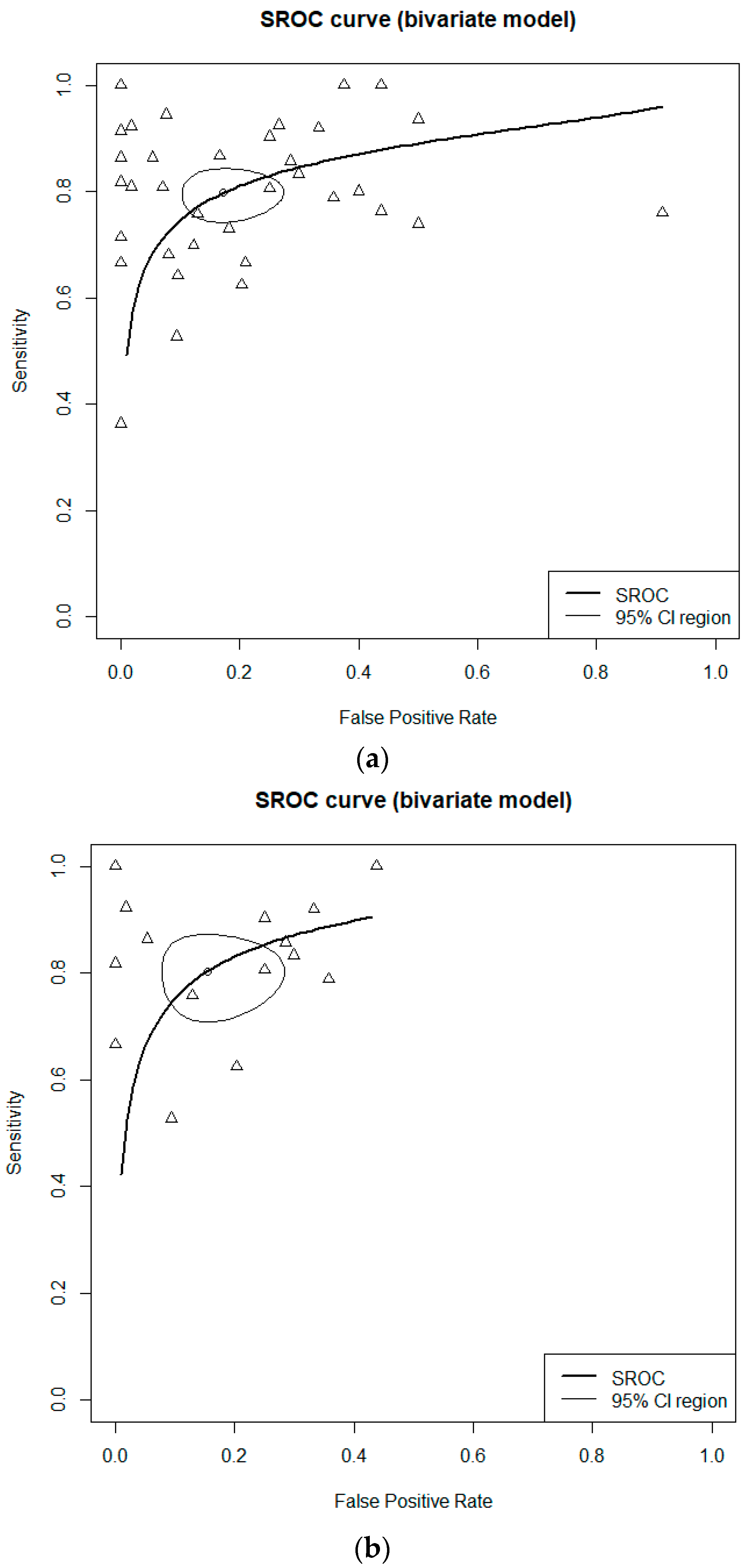
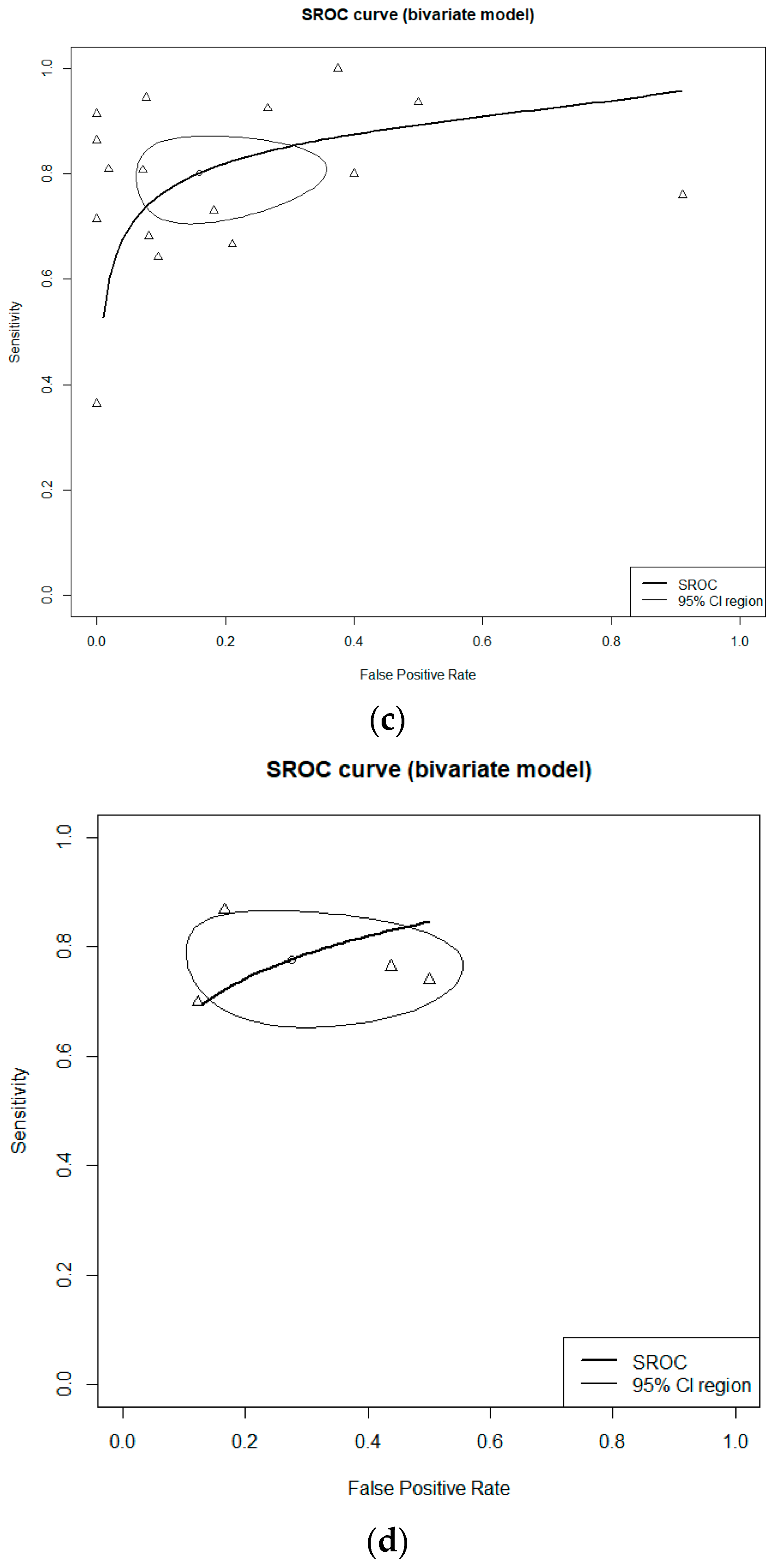
3.2. Diagnostic Accuracy
3.3. Subgroup Analysis of Diagnostic Accuracy According to the Methods of LBP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathy, K.; Misra, A.; Ghosh, J.K. Efficacy of liquid-based cytology versus conventional smears in FNA samples. J. Cytol. 2015, 32, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, H.; Zhang, P.; Li, X.; He, L.; Wang, Z.; Liu, Y. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med. Sci. Monit. 2013, 19, 49–53. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef]
- Rana, C.; Singh, K.R.; Ramakant, P.; Babu, S.; Mishra, A. Impact of cytological pitfalls in the Bethesda System of Reporting Thyroid Cytopathology, on surgical decision-making of patients with thyroid nodules: Can these pitfalls be avoided? Cytopathology 2021, 32, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, J.; Führer, D.; Luster, M.; Musholt, T.J.; Spitzweg, C.; Schott, M. Fine Needle Aspiration in the Investigation of Thyroid Nodules. Dtsch. Arztebl. Int. 2016, 113, 353–359. [Google Scholar] [CrossRef]
- Xiong, X.J.; Xiao, M.M.; Zhang, Y.X.; Liu, D.G.; Jin, M.L.; Wang, J.; Xu, H.T.; Li, Q.C.; Wu, G.P. The Accurate Interpretation and Clinical Significance of Morphological Features of Fine Needle Aspiration Cells in Papillary Thyroid Carcinoma. Anal. Cell. Pathol. 2023, 2023, 9397755. [Google Scholar] [CrossRef]
- Fadda, G.; Rossi, E.D. Liquid-based cytology in fine-needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011, 55, 389–400. [Google Scholar] [CrossRef]
- Chong, Y.; Ji, S.J.; Kang, C.S.; Lee, E.J. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr. Connect. 2017, 6, 817–829. [Google Scholar] [CrossRef]
- Maurya, M.K.; Yadav, R.; Kumar, M.; Singh, H.P.; Mishra, A.; Goel, M.M. A Comparative Analysis of Liquid-Based Cytology and Conventional Smears in Fine-Needle Aspirates of Thyroid Lesions. Cureus 2023, 15, e45353. [Google Scholar] [CrossRef]
- Nagarajan, N.; Najafian, A.; Schneider, E.B.; Zeiger, M.A.; Olson, M.T. Conventional smears versus liquid-based preparations for thyroid fine-needle aspirates: A systematic review and meta-analysis. J. Am. Soc. Cytopathol. 2015, 4, 253–260. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Trimboli, P.; Iorio, S.; Maiorino, M.I.; Longo, M.; Croce, L.; Pignatelli, M.F.; Ferrandes, S.; Cozzolino, I.; Montella, M.; et al. Repeat thyroid FNAC: Inter-observer agreement among high- and low-volume centers in Naples metropolitan area and correlation with the EU-TIRADS. Front. Endocrinol. 2022, 13, 1001728. [Google Scholar] [CrossRef]
- Rossi, E.D.; Morassi, F.; Santeusanio, G.; Zannoni, G.F.; Fadda, G. Thyroid fine needle aspiration cytology processed by ThinPrep: An additional slide decreased the number of inadequate results. Cytopathology 2010, 21, 97–102. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, I.A.; Park, S.Y.; Jung, Y.Y.; Park, S.H.; Shin, H.C. A pilot study evaluating liquid-based fine needle aspiration cytology of breast lesions: A cytomorphological comparison of SurePath® liquid-based preparations and conventional smears. Acta Cytol. 2013, 57, 391–399. [Google Scholar] [CrossRef]
- Fadda, G.; Rossi, E.D.; Raffaelli, M.; Mulè, A.; Pontecorvi, A.; Miraglia, A.; Lombardi, C.P.; Vecchio, F.M. Fine-needle aspiration biopsy of thyroid lesions processed by thin-layer cytology: One-year institutional experience with histologic correlation. Thyroid 2006, 16, 975–981. [Google Scholar] [CrossRef]
- Doyle, B.; O’Farrell, C.; Mahoney, E.; Turner, L.; Magee, D.; Gibbons, D. Liquid-based cytology improves productivity in cervical cytology screening. Cytopathology 2006, 17, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Luthra, U.K.; George, J.; Zuhairy, F.; George, S.S.; Haji, B.I. Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol. 2000, 44, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Malle, D.; Valeri, R.M.; Pazaitou-Panajiotou, K.; Kiziridou, A.; Vainas, I.; Destouni, C. Use of a thin-layer technique in thyroid fine needle aspiration. Acta Cytol. 2006, 50, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal literature search for systematic reviews in surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Stybayeva, G.; Hwang, S.H. Comparative Effectiveness of Cryotherapy and Radiofrequency Ablation for Chronic Rhinitis: A Systemic Review and Meta-analysis. Clin. Exp. Otorhinolaryngol. 2023, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Scurry, J.P.; Duggan, M.A. Thin layer compared to direct smear in thyroid fine needle aspiration. Cytopathology 2000, 11, 104–115. [Google Scholar] [CrossRef]
- Afify, A.M.; Liu, J.; Al-Khafaji, B.M. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: A comparative retrospective review. Cancer 2001, 93, 179–186. [Google Scholar] [CrossRef]
- Cochand-Priollet, B.; Prat, J.J.; Polivka, M.; Thienpont, L.; Dahan, H.; Wassef, M.; Guillausseau, P.J. Thyroid fine needle aspiration: The morphological features on ThinPrep slide preparations. Eighty cases with histological control. Cytopathology 2003, 14, 343–349. [Google Scholar] [CrossRef]
- Cavaliere, A.; Colella, R.; Puxeddu, E.; Gambelunghe, G.; Avenia, N.; d’Ajello, M.; Cartaginese, F.; Vitali, R.; Bellezza, G.; Giansanti, M.; et al. Fine needle aspiration cytology of thyroid nodules: Conventional vs thin layer technique. J. Endocrinol. Investig. 2008, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Lee, A.; Jung, E.S.; Choi, Y.J.; Jung, S.L.; Lee, K.Y. Split sample comparison of a liquid-based method and conventional smears in thyroid fine needle aspiration. Acta Cytol. 2008, 52, 313–319. [Google Scholar] [CrossRef]
- Luu, M.H.; Fischer, A.H.; Pisharodi, L.; Owens, C.L. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol. 2011, 119, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Lee, S.Y.; Lee, H.-C.; Park, J.-W.; Koong, S.S.; Oh, T.K.; Jeon, H.J.; Kim, E.; Lee, O.-J. CellprepPlus® Liquid-based Smear in Sono-guided Thyroid Fine Needle Aspiration: A Comparison of Conventional Method and CellprepPlus® Liquid-based Cytology. Korean J. Pathol. 2011, 45, 182. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Ko, Y.; Lim, S.; Kim, W.; Han, H.; Seol, H.; Oh, S.; Moon, W.-J.; Hwang, T. Clinical Usefulness of SurePath™ Liquid-based Cytology in Thyroid Fine Needle Aspiration: Comparison with the Conventional Smear in Diagnostic Efficacy and Applicability of BRAF Mutation Test. Korean J. Pathol. 2011, 45, 188. [Google Scholar] [CrossRef]
- Chang, H.; Lee, E.; Lee, H.; Choi, J.; Kim, A.; Kim, B.H. Comparison of diagnostic values of thyroid aspiration samples using liquid-based preparation and conventional smear: One-year experience in a single institution. Apmis 2013, 121, 139–145. [Google Scholar] [CrossRef]
- Nagarajan, N.; Schneider, E.B.; Ali, S.Z.; Zeiger, M.A.; Olson, M.T. How do liquid-based preparations of thyroid fine-needle aspiration compare with conventional smears? An analysis of 5475 specimens. Thyroid 2015, 25, 308–313. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, M. A comparison of liquid-based cytology and conventional smears in fine needle aspiration cytology of thyroid lesions: Diagnostic efficacy and pitfalls. Thyroid Res. Pract. 2020, 17, 14–18. [Google Scholar] [CrossRef]
- Rufail, M.; Jing, X.; Smola, B.; Heider, A.; Cantley, R.; Pang, J.C.; Lew, M. Comparison of Diagnostic Rates and Concordance with Subsequent Surgical Resections between Conventional Smear and ThinPrep Preparations versus ThinPrep Only in Thyroid Fine Needle Aspiration (T-FNA) Specimens. Acta Cytol. 2022, 66, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, X.; Song, C.; Wang, C. A comparative study of two liquid-based preparation methods: Membrane-based and sedimentation in fine needle aspiration cytology diagnosis in thyroid nodules. World J. Surg. Oncol. 2020, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Ucak, R.; Eryilmaz, O.T.; Ozguven, B.Y.; Uludag, M.; Kabukcuoğlu, F. Evaluation of Thyroid Fine-Needle Aspiration Biopsies according to Cytological Methods and Comparison with Histopathological Diagnosis. Şişli Etfal Hastan. Tip Bülteni 2021, 55, 93–100. [Google Scholar] [CrossRef]
- Saleh, H.A.; Hammoud, J.; Zakaria, R.; Khan, A.Z. Comparison of Thin-Prep and cell block preparation for the evaluation of Thyroid epithelial lesions on fine needle aspiration biopsy. Cytojournal 2008, 5, 3. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A.; Verma, R.; Kalra, R.; Gupta, V.; Gill, M.; Sen, R. Comparative Evaluation of Conventional Smear and Liquid Based Cytology in Diagnosis of Thyroid Lesions Using Bethesda System. J. Cytol. Histol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Mahajan, S.; Rajwanshi, A.; Srinivasan, R.; Radotra, B.; Panda, N. Should Liquid Based Cytology (LBC) be Applied to Thyroid Fine Needle Aspiration Cytology Samples: Comparative Analysis of Conventional and LBC Smears. J. Cytol. 2021, 38, 198. [Google Scholar] [CrossRef]
- Alam, M.Q.; Pandey, P.; Ralli, M.; Singh Chauhan, J.P.; Aggarwal, R.; Chaturvedi, V.; Kapoor, A.; Trivedi, K.; Agarwal, S. Comparative analysis of cytomorphology of thyroid lesion on conventional cytology versus liquid-based cytology and categorize the lesions according to The Bethesda System for Reporting Thyroid Cytopathology. J. Cancer Res. Ther. 2022, 18, S259–S266. [Google Scholar] [CrossRef]
- Sayer, A. Comparison of Conventional Smear and Liquid-Based Cytology in Adequacy of Thyroid Fine-Needle Aspiration Biopsies without an Accompanying Cytopathologist. Med. Bull. Sisli Etfal Hosp. 2022, 56, 353. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.K.; Chae, S.W.; Lee, K.B.; Han, E.M.; Kang, S.H.; Sohn, J.H. The Usefulness of SurePath Liquid-Based Smear in Sono-Guided Thyroid Fine Needle Aspiration; a Comparison of a Conventional Smear and SurePath Liquid-Based Cytology. Korean J. Cytopathol. 2007, 18, 143–152. [Google Scholar]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Stamataki, M.; Anninos, D.; Brountzos, E.; Georgoulakis, J.; Panayiotides, J.; Christoni, Z.; Peros, G.; Karakitsos, P. The role of liquid-based cytology in the investigation of thyroid lesions. Cytopathology 2008, 19, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ardito, G.; Rossi, E.D.; Revelli, L.; Moschella, F.; Giustozzi, E.; Fadda, G.; Marzola, M.C.; Rubello, D. The role of fine-needle aspiration performed with liquid-based cytology in the surgical management of thyroid lesions. In Vivo 2010, 24, 333–337. [Google Scholar] [PubMed]
- Geers, C.; Bourgain, C. Liquid-based FNAC of the thyroid: A 4-year survey with SurePath. Cancer Cytopathol. 2011, 119, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Paul, A.K.; Kim, N.; Baek, J.H.; Choi, Y.J.; Ha, E.J.; Lee, K.D.; Lee, H.S.; Shin, D.; Kim, N. Computer-aided diagnosis for classifying benign versus malignant thyroid nodules based on ultrasound images: A comparison with radiologist-based assessments. Med. Phys. 2016, 43, 554. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, B.; Karabağ, A.; Kasap, H.A.; Çivi Çetin, K.; Bal, C.; Şimşek, G. Diagnostic Performance Comparison of Liquid-Based Preparation Methods in Thyroid FNAs. J. Cytol. 2023, 40, 184–191. [Google Scholar] [CrossRef]
- Biscotti, C.V.; Hollow, J.A.; Toddy, S.M.; Easley, K.A. ThinPrep versus conventional smear cytologic preparations in the analysis of thyroid fine-needle aspiration specimens. Am. J. Clin. Pathol. 1995, 104, 150–153. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Kwon, H.J.; Song, M.K.; Kwak, J.Y. Combined use of conventional smear and liquid-based preparation versus conventional smear for thyroid fine-needle aspiration. Endocrine 2016, 53, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Raffaelli, M.; Zannoni, G.F.; Pontecorvi, A.; Mulè, A.; Callà, C.; Lombardi, C.P.; Fadda, G. Diagnostic efficacy of conventional as compared to liquid-based cytology in thyroid lesions: Evaluation of 10,360 fine needle aspiration cytology cases. Acta Cytol. 2009, 53, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.R.; Sidawy, M.K.; Ferfelli, M.; Tabbara, S.O.; Bronner, N.A.; Brosky, K.R.; Sherman, M.E. Utility of thin-layer preparations in thyroid fine-needle aspiration: Diagnostic accuracy, cytomorphology, and optimal sample preparation. Cancer 1998, 84, 17–25. [Google Scholar] [CrossRef]
- O’Malley, M.E.; Weir, M.M.; Hahn, P.F.; Misdraji, J.; Wood, B.J.; Mueller, P.R. US-guided fine-needle aspiration biopsy of thyroid nodules: Adequacy of cytologic material and procedure time with and without immediate cytologic analysis. Radiology 2002, 222, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Baek, K.H.; Kim, J.Y.; Kim, T.J.; Lee, E.J.; Kang, C.S. Comparison of EASYPREP(®) and SurePath(®) in thyroid fine-needle aspiration. Diagn. Cytopathol. 2016, 44, 283–290. [Google Scholar] [CrossRef]
- Sharma, R.; Zaheer, S.; Ahluwalia, C. Diagnostic utility of conventional and liquid-based cytology in the management of thyroid lesions; an institutional experience. Cytojournal 2022, 19, 36. [Google Scholar] [CrossRef]
- Szporn, A.H.; Yuan, S.; Wu, M.; Burstein, D.E. Cellular swirls in fine needle aspirates of papillary thyroid carcinoma: A new diagnostic criterion. Mod. Pathol. 2006, 19, 1470–1473. [Google Scholar] [CrossRef]
- Xiao, M.M.; Zhao, Y.B.; Liu, D.G.; Qiu, X.S.; Wang, E.H.; Wu, G.P. The Morphological Analysis of Cells in the Bronchoscopic Brushing and TBNA of Patients with Lung Adenocarcinoma. Cell Transplant. 2020, 29, 963689720923599. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Lombardi, C.P.; Pontecorvi, A.; Vellone, V.G.; Zannoni, G.F.; Larocca, L.M.; Fadda, G. BRAF (V600E) mutation analysis on liquid-based cytology-processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013, 121, 291–297. [Google Scholar] [CrossRef]
- Davey, E.; Barratt, A.; Irwig, L.; Chan, S.F.; Macaskill, P.; Mannes, P.; Saville, A.M. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet 2006, 367, 122–132. [Google Scholar] [CrossRef] [PubMed]


| Study | Design | Total Number of Patients (n) | Age of Patients (Years, Median (Range) or Mean ± SD) | Sex (F/M) | Nationality | Comparison | Inadequate Smear (n/N) | Reference Test |
|---|---|---|---|---|---|---|---|---|
| Scurry 2000 [22] | Retrospective | 327 | 48 (19–84) | 189/138 | Canada | Direct smear | 40/109 | Surgery confirmed |
| Scurry 2000 [22] | Retrospective | 327 | 48 (19–84) | 189/138 | Canada | Thin-Prep liquid-based preparation | 31/90 | Surgery confirmed |
| Afify 2001 [23] | Retrospective | 209 | NA | NA | USA | Thin-Prep liquid-based preparation | 26/95 | Surgery confirmed |
| Afify 2001 [23] | Retrospective | 209 | NA | NA | USA | Thin-Prep liquid-based preparation | 26/95 | Surgery confirmed |
| Afify 2001 [23] | Retrospective | 209 | NA | NA | USA | Direct smear | 9/46 | Surgery confirmed |
| Afify 2001 [23] | Retrospective | 209 | NA | NA | USA | Direct smear | 9/46 | Surgery confirmed |
| Cochand-Priollet 2003 [24] | Case control | 50 | 46.9 (24–70) | 36/14 | France | Direct smear | 10/120 | Surgery confirmed |
| Cochand-Priollet 2003 [24] | Case control | 50 | 46.9 (24–70) | 36/14 | France | Thin-Prep liquid-based preparation | 27/120 | Surgery confirmed |
| Malle 2006 [17] | Retrospective | 459 | NA | NA | Greece | Direct smear | 8/285 | Only inadequate smear checked for this study |
| Malle 2006 [17] | Retrospective | 459 | NA | NA | Greece | Thin-Prep liquid-based preparation | 7/174 | Only inadequate smear checked for this study |
| Kim 2007 [41] | Prospective | 172 | NA | NA | Korea | SurePathTM liquid-based cytology | 16/172 | Surgery confirmed |
| Kim 2007 [41] | Prospective | 172 | NA | NA | Korea | Direct smear | 36/172 | Surgery confirmed |
| Stamataki 2007 [45] | Retrospective | 157 | NA | NA | Greece | Thin-Prep liquid-based preparation | Surgery confirmed | |
| Cavaliere 2008 [25] | Prospective | 3875 | NA | NA | Italy | Thin-Prep liquid-based preparation | 1252/3875 | Surgery confirmed |
| Cavaliere 2008 [25] | Prospective | 3875 | NA | NA | Italy | Direct smear | 1416/3875 | Surgery confirmed |
| Jung 2008 [26] | Prospective | 193 | 48.9 (20–81) | 164/29 | Korea | Direct smear | 12/193 | Surgery confirmed |
| Jung 2008 [26] | Prospective | 193 | 48.9 (20–81) | 164/29 | Korea | SurePathTM liquid-based cytology | 10/193 | Surgery confirmed |
| Saleh 2008 [36] | Retrospective | 126 | NA | NA | USA | Direct smear | 45/126 | Only inadequate smear checked for this study |
| Saleh 2008 [36] | Retrospective | 126 | NA | NA | USA | Thin-Prep liquid-based preparation | 40/126 | Only inadequate smear checked for this study |
| Ardito 2010 [46] | Retrospective | 353 | 45.8 (13–82) | 267/86 | Italy | Thin-Prep liquid-based preparation + Direct smear | Surgery confirmed | |
| Geers 2010 [47] | Retrospective | 178 | NA | NA | Belgium | SurePath liquid-based preparation | Surgery confirmed | |
| Luu 2010 [27] | Prospective | 4101 | NA | NA | USA | Thin-Prep liquid-based preparation + Direct smear | 174/2000 | Surgery confirmed |
| Luu 2010 [27] | Prospective | 4101 | NA | NA | USA | Thin-Prep liquid-based preparation | 173/2102 | Surgery confirmed |
| Koo 2011 [28] | Prospective | 30 | NA | NA | Korea | CellprepPlus liquid-based preparation | 96/193 | Surgery confirmed |
| Koo 2011 [28] | Prospective | 30 | NA | NA | Korea | Direct smear | 32/193 | Surgery confirmed |
| Kim 2011 [29] | Prospective | 30 | NA | NA | Korea | Direct smear | Surgery confirmed | |
| Kim 2011 [29] | Prospective | 30 | NA | NA | Korea | SurePath liquid-based preparation | Surgery confirmed | |
| Chang 2012 [30] | Prospective | 4290 | 50 | 3662/628 | Korea | Direct smear | 458/1767 | Surgery confirmed |
| Chang 2012 [30] | Prospective | 4290 | 50 | 3662/628 | Korea | Thin-Prep liquid-based preparation + Direct smear | 318/2523 | Surgery confirmed |
| Nagarajan 2015 [31] | Retrospective | 1407 | 50 (7–84) | NA | USA | Direct smear | 516/5169 | Surgery confirmed |
| Nagarajan 2015 [31] | Retrospective | 1407 | 50 (7–84) | NA | USA | Liquid-based preparation (not specified) | 52/306 | Surgery confirmed |
| Chang 2016 [48] | Retrospective | 30 | NA | NA | Korea | EASYPREPV liquid-based preparation | 21/253 | Surgery confirmed |
| Chang 2016 [48] | Retrospective | 30 | NA | NA | Korea | SurePath liquid-based preparation | 15/253 | Surgery confirmed |
| Gupta 2018 [37] | Prospective | 60 | NA | NA | India | Thin-Prep liquid-based preparation | 2/60 | Surgery confirmed |
| Gupta 2018 [37] | Prospective | 60 | NA | NA | India | Direct smear | 5/60 | Surgery confirmed |
| Kumari 2020 [32] | Prospective | 100 | NA | NA | India | Direct smear | 10/100 | Surgery confirmed |
| Kumari 2020 [32] | Prospective | 100 | NA | NA | India | SurePath liquid-based preparation | 14/100 | Surgery confirmed |
| Rufail 2020 [33] | Retrospective | 584 | NA | NA | USA | Direct smear | 19/73 | Surgery confirmed |
| Rufail 2020 [33] | Retrospective | 584 | NA | NA | USA | Thin-Prep liquid-based preparation + Direct smear | 65/511 | Surgery confirmed |
| Zhao 2020 [34] | Retrospective | 221 | 20–76 | 178/43 | China | Thin-Prep liquid-based preparation | Surgery confirmed | |
| Zhao 2020 [34] | Retrospective | 221 | 20–76 | 178/43 | China | SurePath liquid-based preparation | Surgery confirmed | |
| Mahajan 2021 [38] | Prospective case–control | 200 | 21–72 | 170/30 | India | Direct smear | 7/200 | Surgery confirmed |
| Mahajan 2021 [38] | Prospective case–control | 200 | 21–72 | 170/30 | India | SurePath liquid-based preparation | 36/200 | Surgery confirmed |
| Ucak 2021 [35] | Retrospective | 879 | 46.7 (18–82) | 700/179 | Turkey | Liquid-based preparation (not specified) | Surgery confirmed | |
| Ucak 2021 [35] | Retrospective | 879 | 46.7 (18–82) | 700/179 | Turkey | Direct smear | Surgery confirmed | |
| Ucak 2021 [35] | Retrospective | 879 | 46.7 (18–82) | 700/179 | Turkey | Liquid-based preparation (not specified) + Direct smear | Surgery confirmed | |
| Alam 2022 [39] | Prospective | 131 | 33.15 ± 12.38 | NA | India | SurePath liquid-based preparation | 22/131 | Surgery confirmed |
| Alam 2022 [39] | Prospective | 131 | 33.15 ± 12.38 | NA | India | Direct smear | 9/131 | Surgery confirmed |
| Sayer 2022 [40] | Retrospective | 572 | 54.3 ± 10.16 | 446/126 | Turkey | Direct smear | 63/266 | Surgery confirmed |
| Sayer 2022 [40] | Retrospective | 572 | 54.3 ± 10.16 | 446/126 | Turkey | SurePath liquid-based preparation | 49/359 | Surgery confirmed |
| Erdoğan 2023 [49] | Retrospective | 4855 | 41–60 | 4069/786 | Turkey | SurePath liquid-based preparation | 324/2898 | Surgery confirmed |
| Erdoğan 2023 [49] | Retrospective | 4855 | 41–60 | 4069/786 | Turkey | Cytospin liquid-based cytology | 250/957 | Surgery confirmed |
| Maurya 2023 [9] | Prospective, observational | 250 | 12–72 | 224/26 | India | SurePath liquid-based preparation | 39/250 | Surgery confirmed |
| Maurya 2023 [9] | Prospective, observational | 250 | 12–72 | 224/26 | India | Direct smear | 13/250 | Surgery confirmed |
| Xiong 2023 [6] | Retrospective | 337 | 21–71 | 266/71 | China | SurePath liquid-based preparation | Surgery confirmed | |
| Xiong 2023 [6] | Retrospective | 337 | 21–71 | 266/71 | China | Direct smear | Surgery confirmed |
| Study | Selection a | Comparability b | Exposure c | The Newcastle–Ottawa Scale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | ||
| Scurry 2000 [22] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Afify 2001 [23] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Cochand-Priollet 2003 [24] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Malle 2006 [17] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Kim 2007 [41] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Cavaliere 2008 [25] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Jung 2008 [26] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Saleh 2008 [36] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | 6 |
| Luu 2010 [27] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Koo 2011 [28] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Kim 2011 [29] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | 6 |
| Chang 2012 [30] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Nagarajan 2015 [31] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Gupta 2018 [37] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Kumari 2020 [32] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Rufail 2020 [33] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Zhao 2020 [34] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Ucak 2021 [35] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Mahajan 2021 [38] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Alam 2022 [39] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7 |
| Sayer 2022 [40] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Maurya 2023 [9] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Xiong 2023 [6] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.J.; Lee, H.W.; Stybayeva, G.; Hwang, S.H. Comparison of Liquid-Based Preparations with Conventional Smears in Thyroid Fine-Needle Aspirates: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 751. https://doi.org/10.3390/cancers16040751
Kang YJ, Lee HW, Stybayeva G, Hwang SH. Comparison of Liquid-Based Preparations with Conventional Smears in Thyroid Fine-Needle Aspirates: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(4):751. https://doi.org/10.3390/cancers16040751
Chicago/Turabian StyleKang, Yun Jin, Hyeon Woo Lee, Gulnaz Stybayeva, and Se Hwan Hwang. 2024. "Comparison of Liquid-Based Preparations with Conventional Smears in Thyroid Fine-Needle Aspirates: A Systematic Review and Meta-Analysis" Cancers 16, no. 4: 751. https://doi.org/10.3390/cancers16040751
APA StyleKang, Y. J., Lee, H. W., Stybayeva, G., & Hwang, S. H. (2024). Comparison of Liquid-Based Preparations with Conventional Smears in Thyroid Fine-Needle Aspirates: A Systematic Review and Meta-Analysis. Cancers, 16(4), 751. https://doi.org/10.3390/cancers16040751





