Survival Outcomes in Older Women with Oestrogen-Receptor-Positive Early-Stage Breast Cancer: Primary Endocrine Therapy vs. Surgery by Comorbidity and Frailty Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
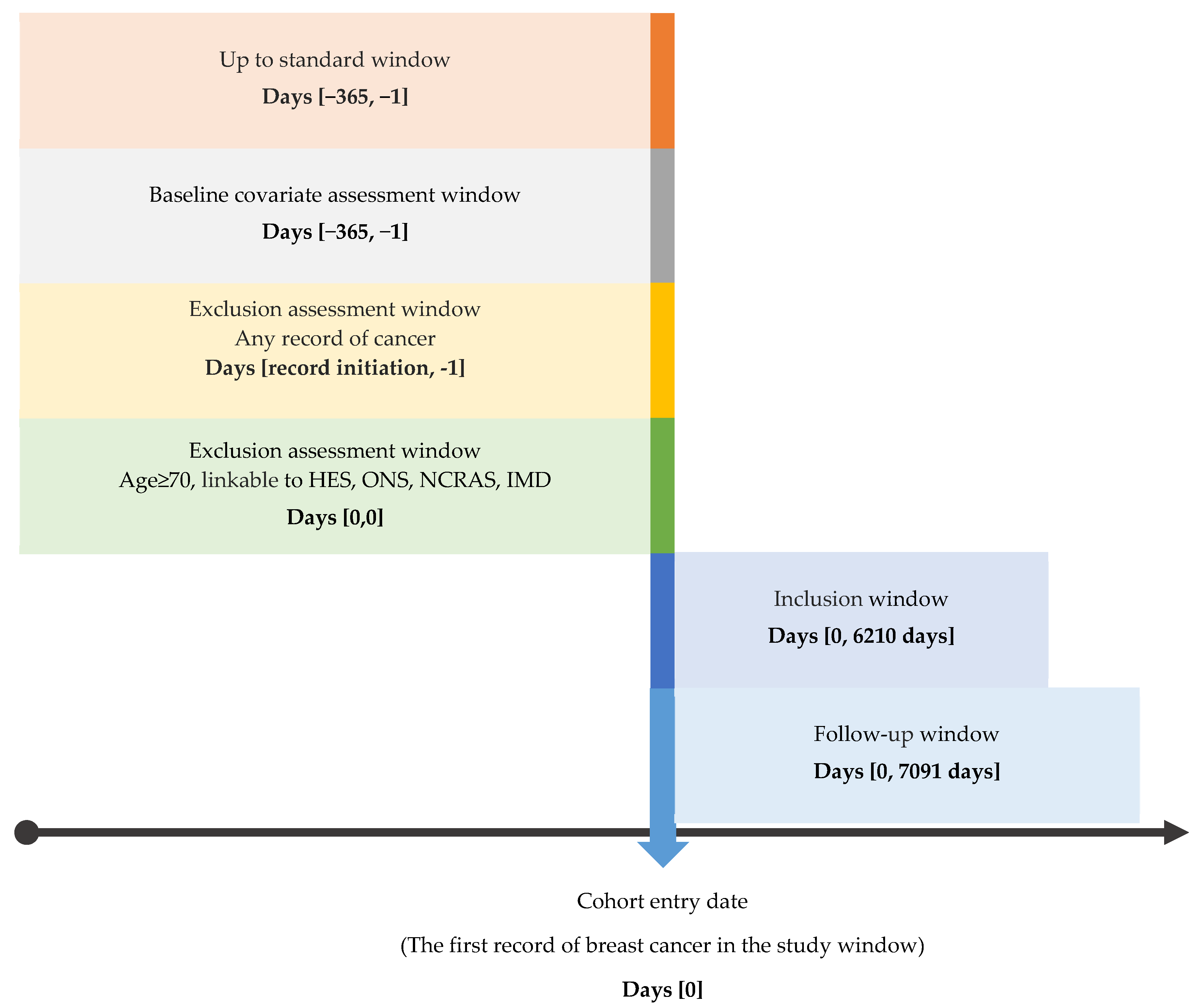
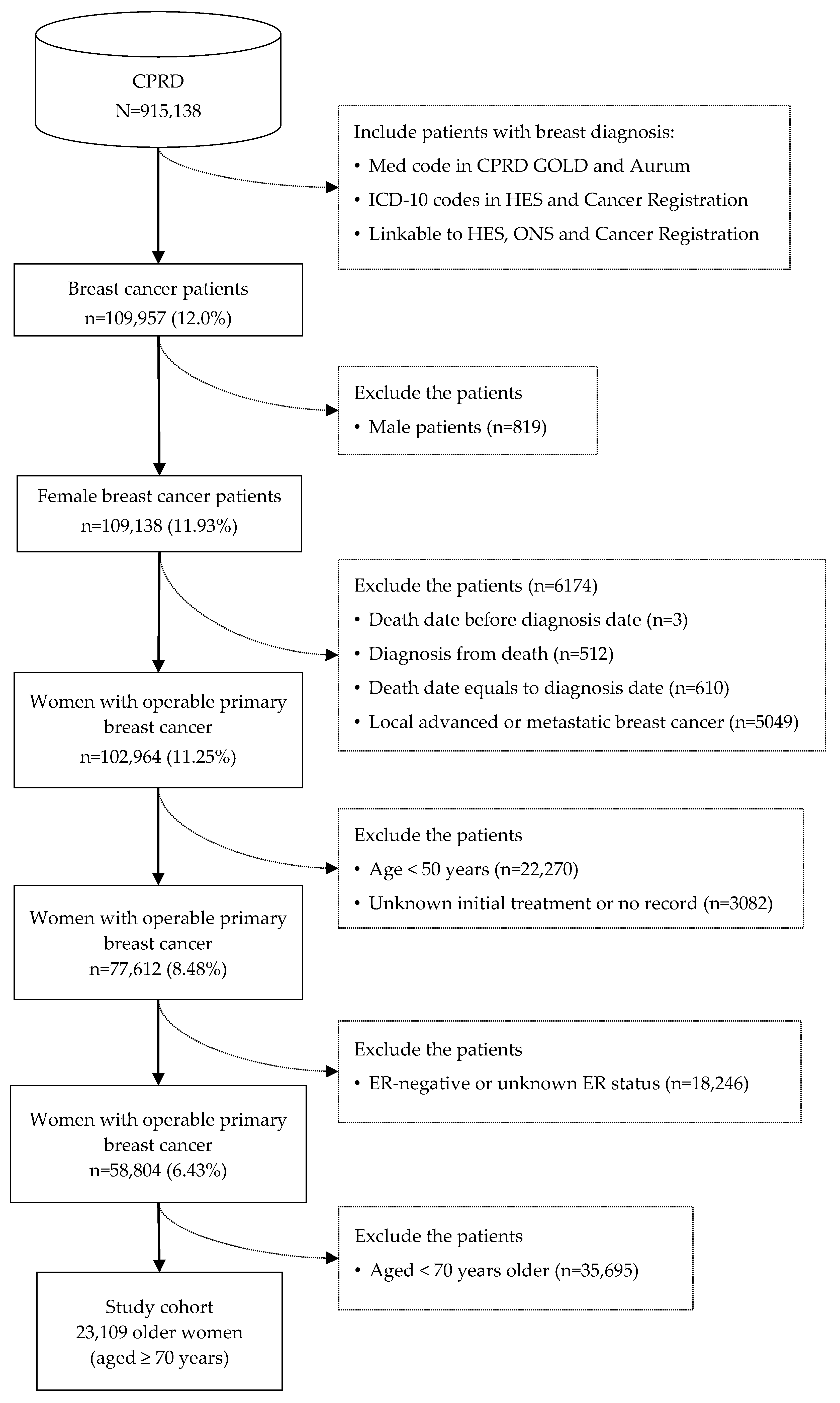
2.2. Study Population
2.3. Treatment Exposure
2.4. Outcome Measure
2.5. Covariates
2.6. Data Analysis
3. Results
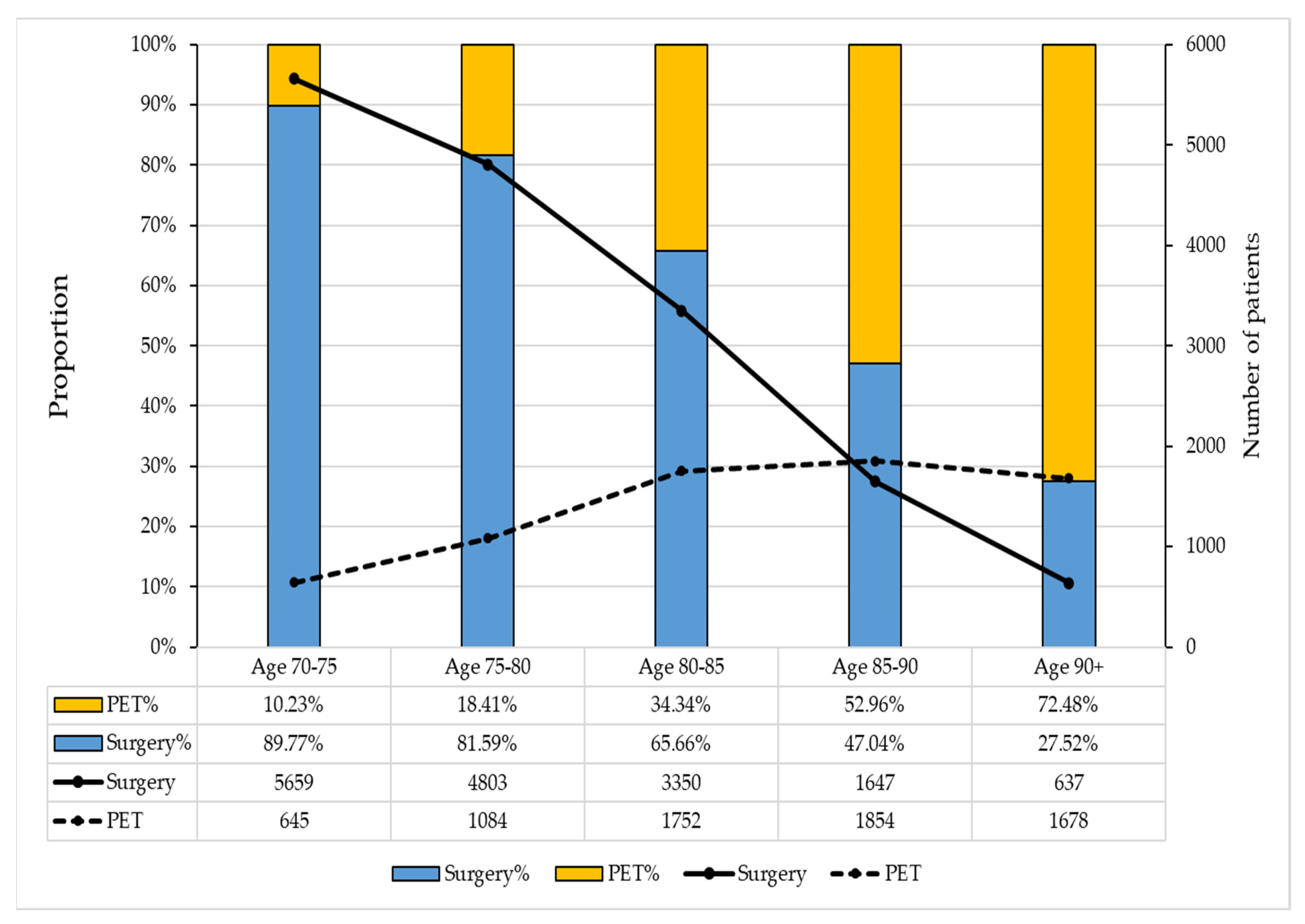
3.1. Cohort Characteristics
3.2. Overall Survival Time and Factors Associated with All-Cause Mortality
3.3. Comparative Effectiveness of PET versus Surgery by Levels of Frailty and Comorbidity
3.4. Sensitivity Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Breast Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Five (accessed on 6 April 2022).
- Masic, I.; Miokovic, M.; Muhamedagic, B. Evidence based medicine—New approaches and challenges. Acta Inform. Medica 2008, 16, 219–225. [Google Scholar] [CrossRef]
- Shiffman, R.N.; Wright, A. Evidence-based clinical decision support. Yearb. Med. Inform. 2013, 8, 120–127. [Google Scholar]
- Tang, S.W.; Hurria, A.; Morgan, D.A.; Cheung, K.L. Is surgery always indicated in older women with breast cancer? Minerva Chir. 2010, 65, 555–568. [Google Scholar]
- Burton, M.; Kilner, K.; Wyld, L.; Lifford, K.J.; Gordon, F.; Allison, A.; Reed, M.; Collins, K.A. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psycho Oncol. 2017, 26, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2018; p. 61. [Google Scholar]
- World Health Organisation. World Cancer Report; World Health Organisation: Geneva, Switzerland, 2014; p. 632. [Google Scholar]
- Gannon, M.M.; Medina, J.; Horgan, K.; Dodwell, D. National Audit Breast Cancer in Older Patients 2022; Royal College of Surgeons of England: London, UK, 2022; p. 94. [Google Scholar]
- Chan, K.S.; Chong, M.T.H.; Chia, C.L.K.; Cheung, K.L. Revisiting primary endocrine therapy versus surgery in older women with breast cancer: Meta-analysis. Br. J. Surg. 2023, 110, 420–431. [Google Scholar] [CrossRef]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.J.; Cheung, K.L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet. Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef]
- Reed, M.W.; Wyld, L.; Ellis, P.; Bliss, J.; Leonard, R.; ACTION and ESTEeM Trial Management Groups. Breast cancer in older women: Trials and tribulations. Clin. Oncol. 2009, 21, 99–102. [Google Scholar] [CrossRef]
- Hutchins, L.F.; Unger, J.M.; Crowley, J.J.; Coltman, C.A., Jr.; Albain, K.S. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N. Engl. J. Med. 1999, 341, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Greenberg, D.; Wishart, G.C.; Pharoah, P. Patient and tumour characteristics, management, and age-specific survival in women with breast cancer in the East of England. Br. J. Cancer 2011, 104, 564–570. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Liefers, G.; De Craen, A.; Kuppen, P.; Van De Water, W.; Portielje, J.; Van Der Geest, L.; Janssen-Heijnen, M.; Dekkers, O.; Van De Velde, C.J.B.; et al. Breast cancer in elderly compared to younger patients in the Netherlands: Stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res. Treat. 2010, 124, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, K.; Moran, A.; Howell, A.; Bundred, N.; Campbell, M.; Todd, C. Older women with operable breast cancer are less likely to have surgery. Br. J. Surg. 2007, 94, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, K.; Sowerbutts, A.; Bundred, N.; Pilling, M.; Degner, L.; Stockton, C.; Todd, C. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br. J. Cancer 2014, 110, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, K.; Todd, C.; Moran, A.; Howell, A.; Bundred, N.; Campbell, M. Non-standard management of breast cancer increases with age in the UK: A population based cohort of women > or = 65 years. Br. J. Cancer 2007, 96, 1197–1203. [Google Scholar] [CrossRef][Green Version]
- Van de Water, W.; Markopoulos, C.; van de Velde, C.J.; Seynaeve, C.; Hasenburg, A.; Rea, D.; Putter, H.; Nortier, J.W.; de Craen, A.J.; Hille, E.T.J.J. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor–positive breast cancer. JAMA 2012, 307, 590–597. [Google Scholar] [CrossRef]
- Ebell, M.H.; Bentivegna, M.; Hulme, C. Cancer-Specific Mortality, All-Cause Mortality, and Overdiagnosis in Lung Cancer Screening Trials: A Meta-Analysis. Ann. Fam. Med 2020, 18, 545–552. [Google Scholar] [CrossRef]
- Herrett, E.; Gallagher, A.M.; Bhaskaran, K.; Forbes, H.; Mathur, R.; van Staa, T.; Smeeth, L. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol. 2015, 44, 827–836. [Google Scholar] [CrossRef]
- Wolf, A.; Dedman, D.; Campbell, J.; Booth, H.; Lunn, D.; Chapman, J.; Myles, P. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int. J. Epidemiol. 2019, 48, 1740–1740g. [Google Scholar] [CrossRef]
- Henson, K.E.; Elliss-Brookes, L.; Coupland, V.H.; Payne, E.; Vernon, S.; Rous, B.; Rashbass, J. Data Resource Profile: National Cancer Registration Dataset in England. Int. J. Epidemiol. 2019, 49, 16–16h. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Wijlaars, L.; Zylbersztejn, A.; Cromwell, D.; Hardelid, P. Data Resource Profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int. J. Epidemiol. 2017, 46, 1093–1093i. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Improving Outcomes: A Strategy for Cancer; Public Health England: London, UK, 2011; p. 101. [Google Scholar]
- The Office for National Statistics. User Guide to Mortality Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/userguidetomortalitystatisticsjuly2017 (accessed on 14 August 2022).
- Shaikh, R.A.; Siahpush, M.; Singh, G.K.; Tibbits, M. Socioeconomic status, smoking, alcohol use, physical activity, and dietary behavior as determinants of obesity and body mass index in the United States: Findings from the National Health Interview Survey. Int. J. MCH AIDS 2015, 4, 22. [Google Scholar] [CrossRef]
- Larsen, S.B.; Kroman, N.; Ibfelt, E.H.; Christensen, J.; Tjonneland, A.; Dalton, S.O. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol. 2015, 54, 780–788. [Google Scholar] [CrossRef]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—A systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Crooks, C.J.; West, J.; Card, T.R. A comparison of the recording of comorbidity in primary and secondary care by using the Charlson Index to predict short-term and long-term survival in a routine linked data cohort. BMJ Open 2016, 5, e007974. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ruth, C.; Brownell, M.; Isbister, J.; MacWilliam, L.; Gammon, H.; Singal, D.; Boreskewich, E. Long-Term Outcomes of Manitoba’s Insight Mentoring Program: A Comparative Statistical Analysis; Manitoba Centre for Health Policy: Winnipeg, Canada, 2015. [Google Scholar]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.J.; Westreich, D.; Wiesen, C.; Sturmer, T.; Brookhart, M.A.; Davidian, M. Doubly robust estimation of causal effects. Am. J. Epidemiol. 2011, 173, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Cleves, M.; Gould, W.; Gould, W.; Gutierrez, R.; Marchenko, Y. An Introduction to Survival Analysis Using Stata; Stata Press: College Station, TX, USA, 2008. [Google Scholar]
- Zhang, X.; Zhang, M.-J.; Fine, J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat. Med. 2011, 30, 1933–1951. [Google Scholar] [CrossRef]
- Wyld, L.; Reed, M.W.R.; Morgan, J.; Collins, K.; Ward, S.; Holmes, G.R.; Bradburn, M.; Walters, S.; Burton, M.; Herbert, E.; et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur. J. Cancer 2021, 142, 48–62. [Google Scholar] [CrossRef]
- Wang, Y.; Gavan, S.; Steinke, D.; Cheung, K.L.; Chen, L. Health and Economic Loss of Primary Endocrine Therapy to Older Women with Operable Early-stage Breast Cancer—A Cost-effectiveness and Value of Implementation Analysis. In Proceedings of the 33rd Prescribing and Research in Medicines Management, Manchester, UK, 10 June 2022; p. 31. [Google Scholar]
- Burton, M.; Collins, K.A.; Lifford, K.J.; Brain, K.; Wyld, L.; Caldon, L.; Gath, J.; Revell, D.; Reed, M.W. The information and decision support needs of older women (>75 yrs) facing treatment choices for breast cancer: A qualitative study. Psycho-Oncology 2015, 24, 878–884. [Google Scholar] [CrossRef]
- Gannon, M.; Medina, J.; Miller, K.; Cromwell, D. National Audit of Breast Cancer in Older Patients 2020 Annual Report; The Royal College of Surgeons of England: London, UK, 2020; p. 74. [Google Scholar]
- Hille, U.; Soergel, P.; Länger, F.; Schippert, C.; Makowski, L.; Hillemanns, P. Aromatase Inhibitors as Solely Treatment in Postmenopausal Breast Cancer Patients. Breast J. 2012, 18, 145–150. [Google Scholar] [CrossRef]
- Morgan, J.; Richards, P.; Ward, S.; Francis, M.; Lawrence, G.; Collins, K.; Reed, M.; Wyld, L. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br. J. Surg. 2015, 102, 1056–1063. [Google Scholar] [CrossRef]
- Quiroga-García, V.; Cirauqui-Cirauqui, B.; Bugés-Sánchez, C.; Luna-Tomás, M.Á.; Castellà-Fernández, E.M.; Mariscal-Martínez, A.; Margelí-Vila, M. Primary Hormone Therapy in Elderly Women with Hormone-Sensitive Locoregional Breast Cancer: Endocrine Therapy Alone Is a Reasonable Alternative in Selected Patients. Breast Care 2015, 10, 179–183. [Google Scholar] [CrossRef]
- Wink, C.J.; Woensdregt, K.; Nieuwenhuijzen, G.A.P.; van der Sangen, M.J.C.; Hutschemaekers, S.; Roukema, J.A.; Tjan-Heijnen, V.C.G.; Voogd, A.C. Hormone Treatment without Surgery for Patients Aged 75 Years or Older with Operable Breast Cancer. Ann. Surg. Oncol. 2012, 19, 1185–1191. [Google Scholar] [CrossRef][Green Version]
- De Glas, N.A.; Kiderlen, M.; de Craen, A.J.M.; Hamaker, M.E.; Portielje, J.E.A.; van de Velde, C.J.H.; Liefers, G.J.; Bastiaannet, E. Assessing treatment effects in older breast cancer patients: Systematic review of observational research methods. Cancer Treat. Rev. 2015, 41, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Carter, P.; Kar, S.; Vithayathil, M.; Mason, A.M.; Michaëlsson, K.; Burgess, S. Smoking, alcohol consumption, and cancer: A mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 2020, 17, e1003178. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.; Stewart, A.L.; Carey, S. Physical functioning: Definitions, measurement, and expectations. Adv. Ren. Replace. Ther. 1999, 6, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.R.; Ward, S.E.; Brennan, A.; Bradburn, M.; Morgan, J.L.; Reed, M.W.R.; Richards, P.; Rafia, R.; Wyld, L.; Age Gap Trial Steering, G. Cost-Effectiveness Modeling of Surgery Plus Adjuvant Endocrine Therapy Versus Primary Endocrine Therapy Alone in UK Women Aged 70 and Over With Early Breast Cancer. Value Health 2021, 24, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.G.M.; Bastiaannet, E.; Kiderlen, M.; Hilling, D.E.; Boelens, P.G.; Walsh, P.M.; van Eycken, E.; Siesling, S.; Broggio, J.; Wyld, L.; et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: A population-based cohort study from the EURECCA Breast Cancer Group. Br. J. Cancer 2018, 119, 121–129. [Google Scholar] [CrossRef] [PubMed]

| Source | Dataset | Available Duration | Population |
|---|---|---|---|
| National Cancer Registration and Analysis Services (Set 18) | Cancer Registry | January 1990 to December 2016 | England |
| Systemic Anti-Cancer Treatment | January 2014 to September 2017 | England | |
| National Radiotherapy Dataset | April 2012 to September 2017 | England | |
| Clinical Practice Research Datalink (Set 19) | CPRD Gold | 1987 to June 2020 (Set 19) | UK |
| CPRD Aurum | 1995 to June 2020 (Set 19) | England | |
| Hospital Episode Statistics (Set 18) | Admitted Patient Care | April 1997 to June 2019 (Set 18) | England |
| Outpatient | April 2003 to June 2019 (Set 18) | England | |
| Office for National Statistics (Set 18) | Death registration | January 1998 to May 2019 | England |
| Index of Multiple Deprivation | April 2007 to November 2015 | England |
| Factor | Surgery (n = 16,096) | PET (n = 7013) | p Value * | SMD ** | |
|---|---|---|---|---|---|
| Age | Median (IQR) | 77 (73–82) | 85 (80–89) | <0.001 | - |
| Mean (SD) | 77.8 (5.9) | 84.3 (6.8) | <0.001 | - | |
| Follow-up (year) | Median (IQR) | 6.49 (3.72–10.16) | 2.77 (1.15–5.10) | <0.001 | - |
| Mean (SD) | 7.23 (4.41) | 3.73 (3.51) | <0.001 | - | |
| CCI | Median (IQR) | 2 (2–3) | 3 (2–4) | <0.001 | - |
| Low (0–2) | 80.4% | 70.2% | <0.001 | 0.002 | |
| Intermediate (3–4) | 16.0% | 21.8% | <0.001 | 0.002 | |
| High (≥5) | 3.6% | 8.0% | <0.001 | −0.008 | |
| HFRS | Median (IQR) | 2.9 (1.5–5.4) | 5.5 (2.3–11.2) | <0.001 | - |
| Non-frail (0–5) | 51.2% | 12.9% | <0.001 | 0.013 | |
| Pre-frail (6–15) | 9.1% | 21.2% | <0.001 | −0.012 | |
| Frail (≥15) | 1.5% | 9.2% | <0.001 | −0.014 | |
| Missing | 38.2% | 56.7% | <0.001 | 0.007 | |
| IMD | Median (IQR) | 5 (2–7) | 5 (3–8) | 0.129 | - |
| 1–2 | 20.6% | 18.7% | 0.001 | 0.051 | |
| 3–4 | 18.4% | 17.6% | 0.182 | 0.014 | |
| 5–6 | 16.0% | 16.3% | 0.482 | −0.006 | |
| 7–8 | 7.8% | 7.9% | 0.681 | −0.019 | |
| 9–10 | 10.9% | 14.5% | <0.001 | −0.042 | |
| Missing | 26.5% | 25.0% | 0.006 | −0.059 |
| Factors | Surgery (n = 16,096) | PET (n = 7013) | |
|---|---|---|---|
| Tumour Grade | G1 | 2380 (14.79%) | 588 (8.38%) |
| G2 | 7895 (49.05%) | 1918 (27.35%) | |
| G3 | 3224 (20.03%) | 476 (6.79%) | |
| GX | 1033 (6.42%) | 1921 (27.39%) | |
| Missing | 1564 (9.72%) | 2110 (30.09%) | |
| NPI | Median (IQR) | 3.6 (3.24–4.5) | 3.3 (2.4–3.6) |
| I | 73 (0.45%) | 5 (0.07%) | |
| II | 434 (2.70%) | 0 (0.00%) | |
| III | 1039 (6.46%) | 7 (0.10%) | |
| IV | 2057 (12.78%) | 9 (0.13%) | |
| V | 543 (3.37%) | 1 (0.01%) | |
| Missing | 11,950 (74.24%) | 6991 (99.69%) | |
| HER-2 status | Positive | 553 (3.44%) | 132 (1.88%) |
| Negative | 3970 (24.66%) | 945 (13.47%) | |
| Unknown | 11,573 (71.90%) | 5936 (84.64%) | |
| HER-2 and ER/PR | Positive | 553 (3.43%) | 132 (1.88%) |
| Hazard Ratio (95%CI) | p Value | |
|---|---|---|
| Age (raw) | 1.1 (1.1, 1.1) | <0.001 |
| Age (time-varying) | 1.0 (1.0, 1.0) | 0.108 |
| Treatment of PET (reference: surgery) | 1.9 (1.8, 2.0) | <0.001 |
| Frailty (reference: non-frail) | ||
| Pre-frail level | 1.4 (1.3, 1.5) | <0.001 |
| Frail level | 2 (1.9, 2.2) | <0.001 |
| CCI (reference: low level) | ||
| Intermediate level | 1.3 (1.3, 1.4) | <0.001 |
| High level | 1.8 (1.7, 1.9) | <0.001 |
| IMD (reference: IMD decile 1–2) | ||
| 3–4 | 1.0 (1.0, 1.1) | <0.001 |
| 5–6 | 1.0 (1.0, 1.1) | <0.001 |
| 7–8 | 1.1 (1.0, 1.2) | <0.001 |
| 9–10 | 1.2 (1.1, 1.3) | <0.001 |
| No observations | 1.2 (1.1, 1.3) | <0.001 |
| Group | All-Cause Mortality | Breast Cancer-Specific Mortality | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Level of frailty | ||||
| Non-frail (HFRS: 0 to 5) (n = 19,327) | 2.1 (2.0, 2.2) | <0.0001 | 3.0 (2.8, 3.2) | <0.0001 |
| Pre-frail (HFRS: 6 to 15) (n = 2903) | 1.9 (1.7, 2.1) | <0.0001 | 2.1 (1.7, 2.5) | <0.0001 |
| Frail (HFRS ≥ 15) (n = 879) | 1.2 (1.1, 1.5) | 0.006 | 1.2 (0.9, 1.9) | 0.251 |
| Level of comorbidity | ||||
| Low level of CCI (n = 17,863) | 2.1 (2.0, 2.2) | <0.0001 | 3.0 (2.8, 3.2) | <0.0001 |
| Intermediate level of CCI (n = 4101) | 1.9 (1.7, 2.1) | <0.0001 | 2.2 (1.9, 2.6) | <0.0001 |
| High level of CCI (n = 1145) | 1.4 (1.2, 1.7) | <0.0001 | 1.5 (1.1, 2.1) | 0.015 |
| Group | Model 1 | Model 2 | ||
|---|---|---|---|---|
| SHR (95% CI) | p Value | SHR (95% CI) | p Value | |
| Level of frailty | ||||
| Non-frail (0–5) | 2.2 (2.1, 2.3) | <0.0001 | 1.2 (1.1, 1.3) | <0.0001 |
| Pre-frail (6–15) | 1.9 (1.7, 2.1) | <0.0001 | 1.4 (1.2, 1.5) | <0.0001 |
| Frail (≥15) | 1.2 (1.0, 1.4) | 0.082 | 1.1 (0.9, 1.4) | 0.261 |
| Level of comorbidity | ||||
| Low level of CCI | 2.2 (2.1, 2.3) | <0.0001 | 1.2 (1.1, 1.2) | <0.0001 |
| Intermediate level of CCI | 1.9 (1.7, 2.1) | <0.0001 | 1.4 (1.2, 1.5) | <0.0001 |
| High level of CCI | 1.4 (1.2, 1.6) | <0.0001 | 1.1 (0.9, 1.4) | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Steinke, D.; Gavan, S.P.; Chen, T.-C.; Carr, M.J.; Ashcroft, D.M.; Cheung, K.-L.; Chen, L.-C. Survival Outcomes in Older Women with Oestrogen-Receptor-Positive Early-Stage Breast Cancer: Primary Endocrine Therapy vs. Surgery by Comorbidity and Frailty Levels. Cancers 2024, 16, 749. https://doi.org/10.3390/cancers16040749
Wang Y, Steinke D, Gavan SP, Chen T-C, Carr MJ, Ashcroft DM, Cheung K-L, Chen L-C. Survival Outcomes in Older Women with Oestrogen-Receptor-Positive Early-Stage Breast Cancer: Primary Endocrine Therapy vs. Surgery by Comorbidity and Frailty Levels. Cancers. 2024; 16(4):749. https://doi.org/10.3390/cancers16040749
Chicago/Turabian StyleWang, Yubo, Douglas Steinke, Sean P. Gavan, Teng-Chou Chen, Matthew J. Carr, Darren M. Ashcroft, Kwok-Leung Cheung, and Li-Chia Chen. 2024. "Survival Outcomes in Older Women with Oestrogen-Receptor-Positive Early-Stage Breast Cancer: Primary Endocrine Therapy vs. Surgery by Comorbidity and Frailty Levels" Cancers 16, no. 4: 749. https://doi.org/10.3390/cancers16040749
APA StyleWang, Y., Steinke, D., Gavan, S. P., Chen, T.-C., Carr, M. J., Ashcroft, D. M., Cheung, K.-L., & Chen, L.-C. (2024). Survival Outcomes in Older Women with Oestrogen-Receptor-Positive Early-Stage Breast Cancer: Primary Endocrine Therapy vs. Surgery by Comorbidity and Frailty Levels. Cancers, 16(4), 749. https://doi.org/10.3390/cancers16040749









