Helicobacter pylori Eradication Does Not Adversely Affect the Clinical Course of Gastric Cancer: A Multicenter Study on Screening Endoscopic Examination in Japan
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
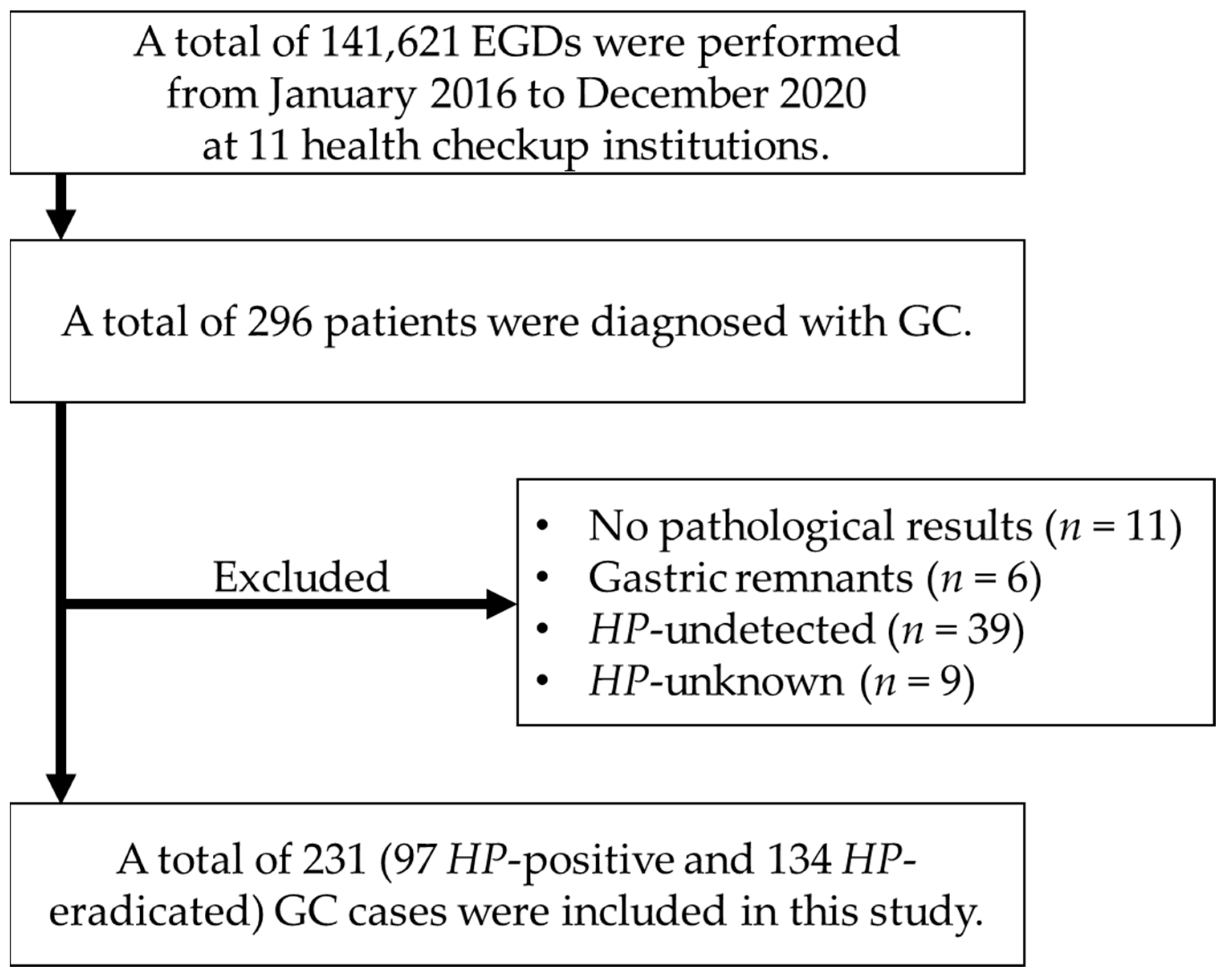
2.1. Patients
2.2. Definition of HP Infection Status of GC
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
3.1. Characteristics of Study Population before and after Propensity Score Matching
3.2. Association between HP Eradication and GCs with Deep Invasion
3.3. Estimating Factors Associated with GCs with Deep Invasion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hori, M.; Matsuda, T.; Shibata, A.; Katanoda, K.; Sobue, T.; Nishimoto, H.; Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn. J. Clin. Oncol. 2015, 45, 884–891. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M.; et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Majima, A.; Handa, O.; Naito, Y.; Dohi, O.; Okayama, T.; Yoshida, N.; Kamada, K.; Katada, K.; Uchiyama, K.; Ishikawa, T.; et al. Early-Stage Gastric Cancer Can Be Found in Improved Atrophic Mucosa over Time from Successful Helicobacter pylori Eradication. Digestion 2017, 95, 194–200. [Google Scholar] [CrossRef]
- Ami, R.; Hatta, W.; Iijima, K.; Koike, T.; Ohkata, H.; Kondo, Y.; Ara, N.; Asanuma, K.; Asano, N.; Imatani, A.; et al. Factors Associated with Metachronous Gastric Cancer Development After Endoscopic Submucosal Dissection for Early Gastric Cancer. J. Clin. Gastroenterol. 2017, 51, 494–499. [Google Scholar] [CrossRef]
- Oda, I.; Hoteya, S.; Fujishiro, M. Status of Helicobacter pylori infection and gastric mucosal atrophy in patients with gastric cancer: Analysis based on the Japan Endoscopy Database. Dig. Endosc. 2019, 31, 103. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tanaka, S.; Takata, S.; Oka, S.; Imagawa, S.; Ueda, H.; Egi, Y.; Kitadai, Y.; Yasui, W.; Yoshihara, M.; et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short-term follow-up. Aliment. Pharmacol. Ther. 2005, 21, 559–566. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, S.; Chayama, K. Characteristics and Early Diagnosis of Gastric Cancer Discovered after Helicobacter pylori Eradication. Gut Liver 2021, 15, 338–345. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hashimoto, S.; Nishikura, K.; Mizuno, K.; Takeuchi, M.; Sato, Y.; Ajioka, Y.; Aoyagi, Y. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J. Gastroenterol. 2013, 48, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sato, Y.; Terai, S. Endoscopic surveillance of gastric cancers after Helicobacter pylori eradication. World J. Gastroenterol. 2015, 21, 10553–10562. [Google Scholar] [CrossRef] [PubMed]
- Maehata, Y.; Nakamura, S.; Esaki, M.; Ikeda, F.; Moriyama, T.; Hida, R.; Washio, E.; Umeno, J.; Hirahashi, M.; Kitazono, T.; et al. Characteristics of Primary and Metachronous Gastric Cancers Discovered after Helicobacter pylori Eradication: A Multicenter Propensity Score-Matched Study. Gut Liver 2017, 11, 628–634. [Google Scholar] [CrossRef]
- Hata, K.; Ito, M.; Boda, T.; Kotachi, T.; Kiso, M.; Masuda, K.; Kurihara, M.; Kuroki, K.; Yorita, N.; Nagasaki, N.; et al. Gastric Cancer with Submucosal Invasion after Successful Helicobacter pylori Eradication: A Propensity Score-Matched Analysis of Patients with Annual Patient Endoscopic Survey. Digestion 2019, 99, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Motoyama, S.; Watanabe, N.; Matsuhashi, T.; Iijima, K. Chronological Changes in the Gastric Cancer Subsite in Akita, Japan: The Trends from the Data of a Hospital-Based Registration System. Tohoku J. Exp. Med. 2018, 246, 131–140. [Google Scholar] [CrossRef]
- Iijima, K.; Jin, M.; Miura, M.; Watanabe, K.; Watanabe, N.; Shimodaira, Y.; Koizumi, S.; Tobori, F.; Motoyama, S. Disturbance of Gastrointestinal Cancers Diagnoses by the COVID-19 Pandemic in a Depopulated Area of Japan: A Population-Based Study in Akita Prefecture. Tohoku J. Exp. Med. 2022, 257, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Shimodaira, Y.; Watanabe, K.; Koizumi, S.; Matsuhashi, T.; Jin, M.; Miura, M.; Onochi, K.; Yamai, K.; Fujishima, Y.; et al. A Follow-up Report on the Diagnosis of Gastrointestinal Cancer during the COVID-19 Pandemic in Akita Prefecture, Japan in 2021. Tohoku J. Exp. Med. 2023, 259, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, G.; Groene, O.; Hoare, J.; Hardwick, R.H.; Riley, S.; Crosby, T.D.; Hanna, G.B.; Cromwell, D.A. A population-based, retrospective, cohort study of esophageal cancer missed at endoscopy. Endoscopy 2014, 46, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Fukao, A. Quality assurance manual of endoscopic screening for gastric cancer in Japanese communities. Jpn. J. Clin. Oncol. 2016, 46, 1053–1061. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Tansplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Aida, K.; Yoshikawa, H.; Mochizuki, C.; Mori, A.; Muto, S.; Fukuda, T.; Otsuki, M. Clinicopathological features of gastric cancer detected by endoscopy as part of annual health checkup. J. Gastroenterol. Hepatol. 2008, 23, 632–637. [Google Scholar] [CrossRef]
- Asaka, M. A new approach for elimination of gastric cancer deaths in Japan. Int. J. Cancer 2013, 132, 1272–1276. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Chen, F.; Tao, T.; Hu, Z.; Wang, J.; You, J.; Wong, B.C.Y.; Chen, J.; Ye, W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology 2022, 163, 154–162.e3. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Heo, J.; Lee, H.S.; Cho, C.M.; Jeon, S.W. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment. Pharmacol. Ther. 2014, 39, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Koike, T.; Asonuma, S.; Okata, H.; Uno, K.; Oikawa, T.; Iwai, W.; Yonechi, M.; Fukushi, D.; Kayaba, S.; et al. Smoking history and severe atrophic gastritis assessed by pepsinogen are risk factors for the prevalence of synchronous gastric cancers in patients with gastric endoscopic submucosal dissection: A multicenter prospective cohort study. J. Gastroenterol. 2023, 58, 433–443. [Google Scholar] [CrossRef]
- Oka, K.; Iwai, N.; Okuda, T.; Hara, T.; Inada, Y.; Tsuji, T.; Komaki, T.; Sakagami, J.; Naito, Y.; Kagawa, K.; et al. Clinical Features of False-Negative Early Gastric Cancers: A Retrospective Study of Endoscopic Submucosal Dissection Cases. Gastroenterol. Res. Pract. 2021, 2021, 6635704. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kato, M.; Takahashi, M.; Haneda, M.; Shinada, K.; Nishida, U.; Yoshida, T.; Sonoda, N.; Ono, S.; Nakagawa, M.; et al. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter 2011, 16, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, L.; Tsilegeridis-Legeris, T.; Lam, S. Clinical and Endoscopic Characteristics Associated with Post-Endoscopy Upper Gastrointestinal Cancers: A Systematic Review and Meta-analysis. Gastroenterology 2022, 162, 1123–1135. [Google Scholar] [CrossRef]
- Yasuda, T.; Dohi, O.; Yamada, S.; Ishida, T.; Iwai, N.; Hongo, H.; Terasaki, K.; Tanaka, M.; Yamada, N.; Kamada, K.; et al. Risk and prognostic factors of invasive gastric cancer detection during surveillance endoscopy: Multi-institutional cross-sectional study. Dig. Endosc. 2023, 35, 592–602. [Google Scholar] [CrossRef]
- Argueta, E.A.; Moss, S.F. The prevention of gastric cancer by Helicobacter pylori eradication. Curr. Opin. Gastroenterol. 2021, 37, 625–630. [Google Scholar] [CrossRef]
- Shin, W.S.; Xie, F.; Chen, B.; Yu, P.; Yu, J.; To, K.F.; Kang, W. Updated Epidemiology of Gastric Cancer in Asia: Decreased Incidence but Still a Big Challenge. Cancers 2023, 15, 2639. [Google Scholar] [CrossRef]
- Januszewicz, W.; Turkot, M.H.; Malfertheiner, P.; Regula, J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers 2023, 15, 664. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Imada, T.; Okuno, T.; Yoshida, T.; Yokota, K.; Oguma, K.; Kita, M.; Okada, H.; et al. Reinfection rate of Helicobacter pylori after eradication treatment: A long-term prospective study in Japan. J. Gastroenterol. 2012, 47, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, A.; Kosunen, T.U.; Puolakkainen, P.; Sipponen, P.; Harkonen, M.; Laxen, F.; Virtamo, J.; Haapiainen, R.; Rautelin, H. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic gastritis. APMIS 2003, 111, 619–624. [Google Scholar] [CrossRef] [PubMed]
| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| HP-Eradicated (n = 134) | HP-Positive (n = 97) | p Value | ASD | HP-Eradicated (n = 81) | HP-Positive (n = 81) | p Value | ASD | |
| Male sex, n (%) | 115 (85.8) | 84 (86.6) | 1.00 | 0.02 | 72 (88.9) | 71 (87.7) | 1.00 | 0.04 |
| Age, years, median (IQR) | 65 (61–71) | 64 (59–70) | 0.31 | 0.13 | 64 (59–71) | 66 (60–70) | 0.94 | 0.03 |
| Metachronous cancer, n (%) | 11 (8.2) | 7 (7.2) | 1.00 | 0.04 | 6 (7.4) | 6 (7.4) | 1.00 | <0.01 |
| Smoking status, n (%) | ||||||||
| Current smoker | 35 (26.1) | 28 (28.9) | 0.84 | 0.08 | 22 (27.2) | 24 (29.6) | 0.89 | 0.07 |
| Past smoker | 27 (20.1) | 17 (17.5) | 16 (19.8) | 14 (17.3) | ||||
| Never smoker | 67 (50.0) | 48 (49.5) | 43 (53.1) | 43 (53.1) | ||||
| Unknown | 5 (3.7) | 4 (4.1) | 0 (0.0) | 0 (0.0) | ||||
| Drinking status, n (%) | ||||||||
| Current drinker | 92 (68.7) | 68 (70.1) | 1.00 | 0.04 | 60 (74.1) | 61 (75.3) | 1.00 | 0.07 |
| Past drinker | 5 (3.7) | 3 (3.1) | 3 (3.7) | 2 (2.5) | ||||
| Never drinker | 30 (22.4) | 21 (21.6) | 18 (22.2) | 18 (22.2) | ||||
| Unknown | 7 (5.2) | 5 (5.2) | 0 (0.0) | 0 (0.0) | ||||
| Longitudinal location, n (%) | ||||||||
| Upper third | 28 (20.9) | 15 (15.5) | 0.31 | 0.14 | 16 (19.8) | 14 (17.3) | 0.84 | 0.06 |
| Middle or lower third | 106 (79.1) | 82 (84.5) | 65 (80.2) | 67 (82.7) | ||||
| Macroscopic type, n (%) | ||||||||
| Elevated type | 22 (16.4) | 24 (24.7) | 0.13 | 0.21 | 10 (12.3) | 21 (25.9) | 0.05 | 0.35 |
| Depressed type | 112 (83.6) | 73 (75.3) | 71 (87.7) | 60 (74.1) | ||||
| Tumor size, n (%) | ||||||||
| <20 mm | 90 (67.2) | 55 (56.7) | 0.13 | 0.22 | 59 (72.8) | 43 (53.1) | 0.01 | 0.42 |
| ≥20 mm | 44 (32.8) | 42 (43.3) | 22 (27.2) | 38 (46.9) | ||||
| Histological type, n (%) | ||||||||
| Differentiated type | 112 (83.6) | 83 (85.6) | 0.72 | 0.06 | 71 (87.7) | 70 (86.4) | 1.00 | 0.04 |
| Undifferentiated type | 22 (16.4) | 14 (14.4) | 10 (12.3) | 11 (13.6) | ||||
| Interval between previous and diagnostic endoscopic examination, n (%) | ||||||||
| ≤1 year | 78 (58.2) | 30 (30.9) | <0.01 | 0.73 | 45 (55.6) | 25 (30.9) | <0.01 | 0.75 |
| >1 year, ≤2 years | 28 (20.9) | 15 (15.5) | 21 (25.9) | 14 (17.3) | ||||
| >2 years or never | 28 (20.9) | 52 (53.6) | 15 (18.5) | 42 (51.9) | ||||
| Depth of tumor invasion, n (%) | ||||||||
| T1a | 96 (71.6) | 72 (74.2) | 0.96 | 0.08 | 60 (74.1) | 56 (69.1) | 0.82 | 0.16 |
| T1b1 | 9 (6.7) | 5 (5.2) | 5 (6.2) | 5 (6.2) | ||||
| T1b2 | 20 (14.9) | 13 (13.4) | 12 (14.8) | 13 (16.0) | ||||
| T2 or deeper | 9 (6.7) | 7 (7.2) | 4 (4.9) | 7 (8.6) | ||||
| Adjusted OR a | 95% CI | p Value | ||
|---|---|---|---|---|
| Longitudinal location | Upper third | 2.01 | 0.78–5.13 | 0.15 |
| Middle or lower third | reference | |||
| Macroscopic type | Depressed type | 2.64 | 0.77–9.08 | 0.12 |
| Elevated type | reference | |||
| Tumor size | ≥20 mm | 5.13 | 2.30–11.40 | <0.01 |
| Histological type | Undifferentiated type | 3.23 | 1.11–9.37 | 0.03 |
| Differentiated type | reference | |||
| Interval between previous and diagnostic endoscopic examination | ≤1 year | reference | ||
| >1 year, ≤2 years | 1.32 | 0.45–3.90 | 0.62 | |
| >2 years or never | 1.76 | 0.68–4.58 | 0.25 | |
| HP infection status | HP-eradicated | 1.16 | 0.48–2.81 | 0.74 |
| HP-positive | reference |
| Adjusted OR a | 95% CI | p Value | ||
|---|---|---|---|---|
| Longitudinal location | Upper third | 3.16 | 1.10–9.05 | 0.03 |
| Middle or lower third | reference | |||
| Macroscopic type | Depressed type | 11.10 | 1.30–94.00 | 0.03 |
| Elevated type | reference | |||
| Tumor size | ≥20 mm | 7.15 | 2.81–18.20 | <0.01 |
| Histological type | Undifferentiated type | 2.98 | 0.96–9.31 | 0.06 |
| Differentiated type | reference | |||
| Interval between previous and diagnostic endoscopic examination | ≤1 year | reference | ||
| >1 year, ≤2 years | 2.55 | 0.72–9.08 | 0.15 | |
| >2 years or never | 2.71 | 0.88–8.33 | 0.08 | |
| HP infection status | HP-eradicated | 1.16 | 0.42–3.19 | 0.78 |
| HP-positive | reference |
| Adjusted OR a | 95% CI | p Value | ||
|---|---|---|---|---|
| Sex | Male | 1.58 | 0.54–4.63 | 0.40 |
| Age | 0.98 | 0.94–1.03 | 0.43 | |
| Metachronous cancer | 0.19 | 0.02–1.60 | 0.13 | |
| Longitudinal location | Upper third | 2.36 | 1.06–5.25 | 0.04 |
| Middle or lower third | reference | |||
| Macroscopic type | Depressed type | 1.27 | 0.49–3.29 | 0.63 |
| Elevated type | reference | |||
| Tumor size | ≥20 mm | 5.88 | 2.95–11.70 | <0.01 |
| Histological type | Undifferentiated type | 3.35 | 1.43–7.86 | <0.01 |
| Differentiated type | reference | |||
| Interval between previous and diagnostic endoscopic examination | ≤1 year | reference | ||
| >1 year, ≤2 years | 1.16 | 0.44–3.05 | 0.77 | |
| >2 years or never | 1.81 | 0.81–4.07 | 0.15 | |
| HP infection status | HP-eradicated | 1.63 | 0.77–3.44 | 0.20 |
| HP-positive | reference |
| Adjusted OR a | 95% CI | p Value | ||
|---|---|---|---|---|
| Sex | Male | 4.75 | 1.07–21.20 | 0.04 |
| Age | 0.97 | 0.92–1.02 | 0.29 | |
| Metachronous cancer | 0.31 | 0.03–2.77 | 0.29 | |
| Longitudinal location | Upper third | 2.70 | 1.12–6.47 | 0.03 |
| Middle or lower third | reference | |||
| Macroscopic type | Depressed type | 1.80 | 0.57–5.71 | 0.32 |
| Elevated type | reference | |||
| Tumor size | ≥20 mm | 7.92 | 3.56–17.60 | <0.01 |
| Histological type | Undifferentiated type | 3.71 | 1.48–9.33 | <0.01 |
| Differentiated type | reference | |||
| Interval between previous and diagnostic endoscopic examination | ≤1 year | reference | ||
| >1 year, ≤2 years | 1.84 | 0.63–5.36 | 0.27 | |
| >2 years or never | 2.76 | 1.09–6.96 | 0.03 | |
| HP infection status | HP-eradicated | 1.74 | 0.75–4.03 | 0.20 |
| HP-positive | reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Watanabe, K.; Fukuda, S.; Yoshida, T.; Dohmen, T.; Fujiwara, J.; Matsuyama, M.; Fujimori, S.; Funaoka, M.; Shirayama, K.; et al. Helicobacter pylori Eradication Does Not Adversely Affect the Clinical Course of Gastric Cancer: A Multicenter Study on Screening Endoscopic Examination in Japan. Cancers 2024, 16, 733. https://doi.org/10.3390/cancers16040733
Takahashi S, Watanabe K, Fukuda S, Yoshida T, Dohmen T, Fujiwara J, Matsuyama M, Fujimori S, Funaoka M, Shirayama K, et al. Helicobacter pylori Eradication Does Not Adversely Affect the Clinical Course of Gastric Cancer: A Multicenter Study on Screening Endoscopic Examination in Japan. Cancers. 2024; 16(4):733. https://doi.org/10.3390/cancers16040733
Chicago/Turabian StyleTakahashi, So, Kenta Watanabe, Sho Fukuda, Tatsuki Yoshida, Takahiro Dohmen, Junichi Fujiwara, Mari Matsuyama, Shusei Fujimori, Masato Funaoka, Kodai Shirayama, and et al. 2024. "Helicobacter pylori Eradication Does Not Adversely Affect the Clinical Course of Gastric Cancer: A Multicenter Study on Screening Endoscopic Examination in Japan" Cancers 16, no. 4: 733. https://doi.org/10.3390/cancers16040733
APA StyleTakahashi, S., Watanabe, K., Fukuda, S., Yoshida, T., Dohmen, T., Fujiwara, J., Matsuyama, M., Fujimori, S., Funaoka, M., Shirayama, K., Horikawa, Y., Fushimi, S., Uchikoshi, S., Onochi, K., Okubo, R., Hoshino, T., Horii, T., Kuramitsu, T., Sakaki, K., ... Iijima, K. (2024). Helicobacter pylori Eradication Does Not Adversely Affect the Clinical Course of Gastric Cancer: A Multicenter Study on Screening Endoscopic Examination in Japan. Cancers, 16(4), 733. https://doi.org/10.3390/cancers16040733






