Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Animals and Tissue Cell Samples
2.2. The PDX Model Construction Method for Immune Reconstruction
2.3. Treatment Plan and Grouping
2.4. Flow Cytometry Analysis
2.5. Immunofluorescence
2.6. Statistical Methods
3. Results
3.1. Combination Radiation and Anti-PD-1 Therapy Induces a Systemic Anti-Tumor Response
3.1.1. Changes in Body Weight of Mice in Each Group
3.1.2. Changes in Tumor Size in Each Group
3.2. Irradiation and Anti-PD-1 Therapy Enhance Anti-Tumor T Cell Immunity
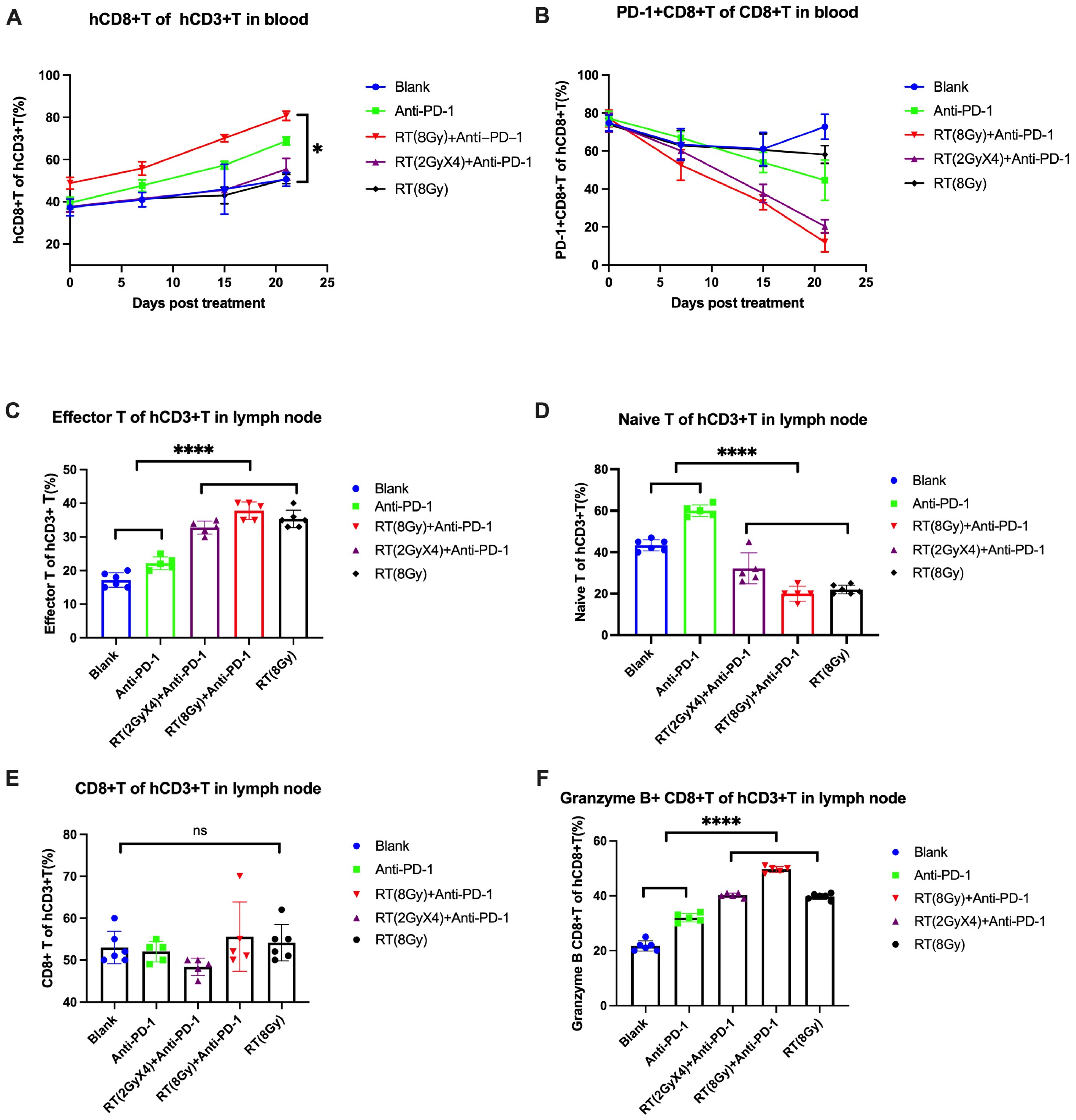
3.2.1. Level and Function of T Cells in Peripheral Blood
3.2.2. Level and Function of T Cells in Lymph Nodes
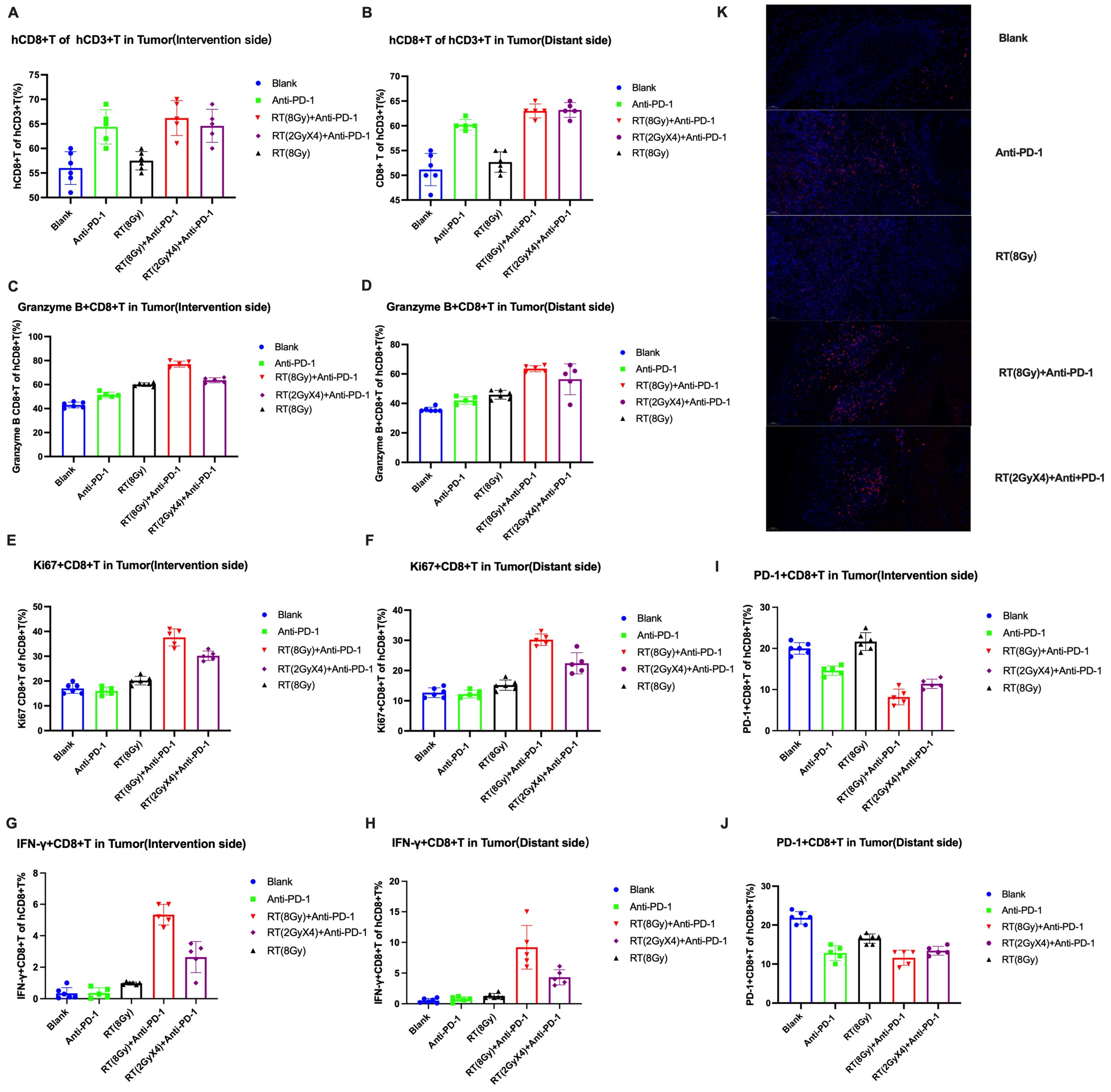
3.2.3. Changes in T Cell Levels and Functions in Tumor Tissue
Local Infiltration of Functional T-Cell Tumors
Differential Analysis of CD8+T Cells under Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, G.; Guo, W.; Chen, X.; Huang, Z. Prognosis of endoscopic surgery and traditional open resection in mucosal melanoma of the nasal cavity and paranasal sinus. Melanoma Res. 2019, 29, 47–52. [Google Scholar] [CrossRef]
- Ganti, A.; Raman, A.; Shay, A.; Kuhar, H.N.; Auger, S.R.; Patel, T.; Kuan, E.C.; Diaz, A.Z.; Batra, P.S.; Tajudeen, B.A. Treatment modalities in sinonasal mucosal melanoma: A national cancer database analysis. Laryngoscope 2019, 130, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and signifificance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEY-NOTE-001,002,006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers 2023, 15, 3013. [Google Scholar] [CrossRef] [PubMed]
- Martinov, T.; Fife, B.T. Fractionated radiotherapy combined with PD-1 pathway blockade promotes CD8 T cell-mediated tumor clearance for the treatment of advanced malignancies. Ann. Transl. Med. 2016, 4, 82. [Google Scholar] [PubMed]
- Yin, G.; Guo, W.; Liu, H.; Huang, Z.; Chen, X. Efficacy of radiotherapy combined with immune checkpoint inhibitors in patients with melanoma: A systemic review and meta-analysis. Melanoma Res. 2022, 32, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Krombach, J.; Hennel, R.; Brix, N.; Orth, M.; Schoetz, U.; Ernst, A.; Schuster, J.; Zuchtriegel, G.; Reichel, C.A.; Bierschenk, S.; et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 2018, 8, e1523097. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Y.; Guo, Z.; Si, T.; Xing, W.; Yu, W.; Wang, Y. Cryoablation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Oncotarget 2018, 10, 4180–4191. [Google Scholar] [CrossRef]
- Watanabe, T.; Firat, E.; Scholber, J.; Gaedicke, S.; Heinrich, C.; Luo, R.; Ehrat, N.; Multhoff, G.; Schmitt-Graeff, A.; Grosu, A.L.; et al. Deep abscopal response to radiotherapy and anti-PD-1 in an oligometastatic melanoma patient with unfavorable pretreatment immune signature. Cancer Immunol. Immunother. 2020, 69, 1823–1832. [Google Scholar] [CrossRef]
- Newton, J.M.; Hanoteau, A.; Liu, H.C.; Gaspero, A.; Parikh, F.; Gartrell-Corrado, R.D.; Hart, T.D.; Laoui, D.; Van Ginderachter, J.A.; Dharmaraj, N.; et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J. Immunother. Cancer 2019, 7, 216. [Google Scholar] [CrossRef]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef]
- Shen, Y.; Peng, L.; Zou, Q.; Zhao, Y.; Ma, D. Phenotype, function and clinical significance of peripheral blood CD8+PD-1+ T cels in patients with gastric cancer. Chongqing Med. 2020, 49, 3531–3535. [Google Scholar]
- Zhou, L.; Ren, F.; Zhang, G.; Lu, X. Relationship between the expression of PD-1 on CD8+ T cell and the clinical pathological parameters in non-small cell lung cancer. Chin. Clin. Oncol. 2015, 20, 625–628. [Google Scholar]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, L.W.; McNeilly, C.; Xiang, C.; Kang, K.; Diaz-Montero, C.M.; Yu, J.S.; Gastman, B.R. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology 2018, 8, e1507669. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Nomura, M.; Ohashi, S.; Hiratsuka, T.; Nakai, Y.; Saito, T.; Kondo, Y.; Fukuyama, K.; Kikuchi, O.; Yamada, A.; et al. Experimental model for the irradiation-mediated abscopal effect and factors influencing this effect. Am. J. Cancer Res. 2020, 10, 440–453. [Google Scholar] [PubMed]
- Maity, A.; Mick, R.; Huang, A.C.; George, S.M.; Farwell, M.D.; Lukens, J.N.; Berman, A.T.; Mitchell, T.C.; Bauml, J.; Schuchter, L.M.; et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br. J. Cancer 2018, 119, 1200–1207. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune- mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379. [Google Scholar] [CrossRef] [PubMed]
- Liniker, E.; Menzies, A.M.; Kong, B.Y.; Cooper, A.; Ramanujam, S.; Lo, S.; Kefford, R.F.; Fogarty, G.B.; Guminski, A.; Wang, T.W.; et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016, 5, e1214788. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Stallworth, D.G.; Kim, Y.; Johnstone, P.A.; Harrison, L.B.; Caudell, J.J.; Yu, H.H.; Etame, A.B.; Weber, J.S.; Gibney, G.T. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann. Oncol. 2016, 27, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, E.; Baghad, B.; Fort, M.; Aouidad, I.; Roger, A.; Beauchet, A.; Otmezguine, Y.; Blom, A.; Longvert, C.; Boru, B.; et al. Efficacy of late concurrent hypofractionated radiotherapy in advanced melanoma patients failing anti-PD-1 monotherapy. Int. J. Cancer 2020, 147, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
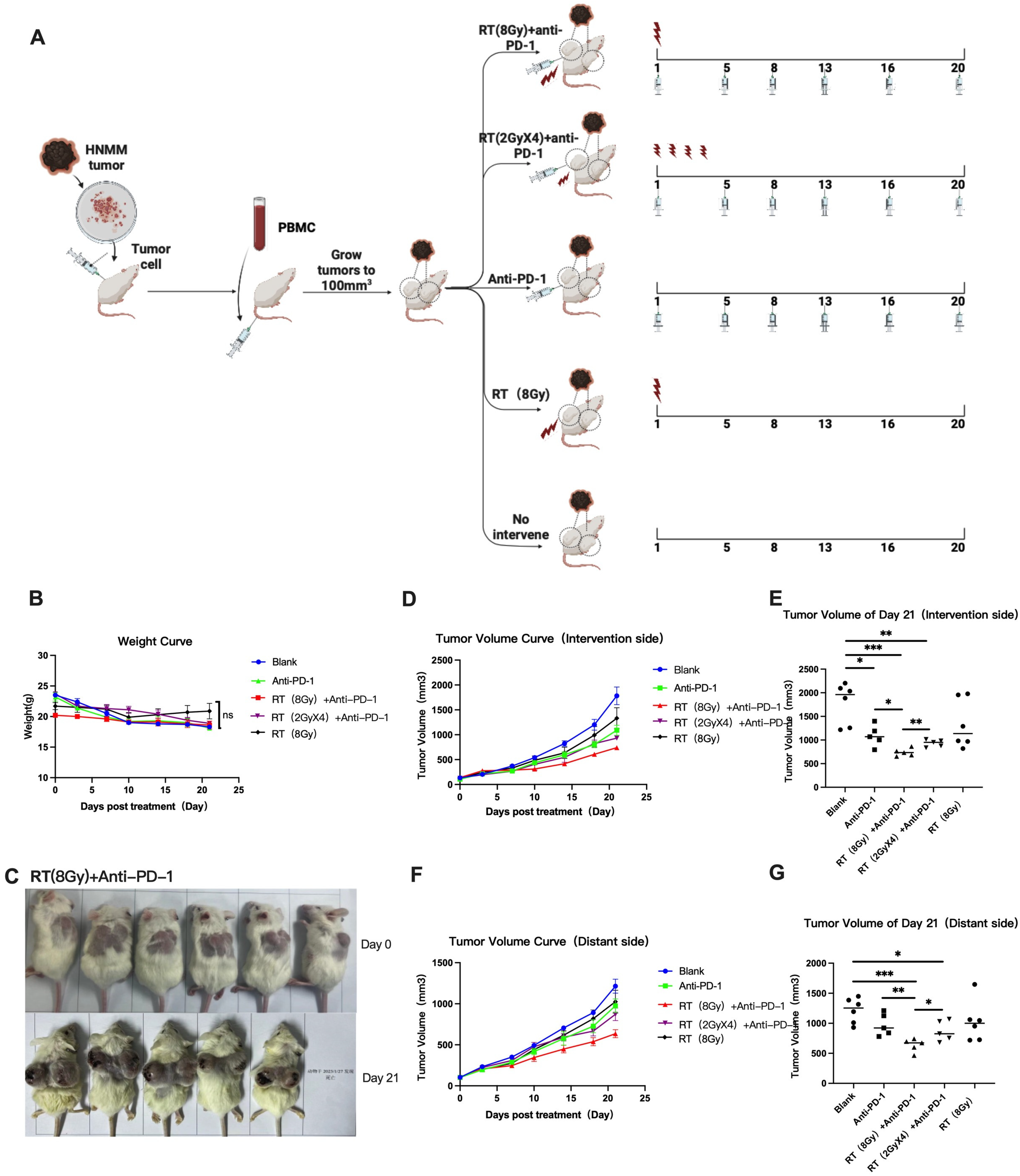
| Treatment Group | Treatment Plan |
|---|---|
| Anti PD-1 | PD-1 inhibitor of 10 mg/kg for 2–3 weeks, BIW |
| RT (8 Gy)+anti PD-1 | 8 Gy single-dose irradiation on one side for 1 time + PD-1 inhibitor of 10 mg/kg for 2–3 weeks, BIW |
| RT (2 GyX4)+anti PD-1 | 2 Gy single-dose irradiation on one side for 4 times + PD-1 inhibitor of 10 mg/kg for 2–3 weeks, BIW |
| RT (8 Gy) | 8 Gy single-dose irradiation on one side for 1 time + same dose of physiological saline at the same time as PD-1 inhibitor |
| Blank | No intervention; without irradiation + same dose of physiological saline at the same time as PD-1 inhibitor |
| Treatment Group | TGI (%) | |||
|---|---|---|---|---|
| Day 10 | Day 14 | Day 18 | Day 21 | |
| Anti PD-1 | 20.0% | 29.5% | 35.1% | 40.4% |
| RT (8 Gy)+anti PD-1 | 58.1% | 59.8% | 56.2% | 63.5% |
| RT (2 GyX4)+anti PD-1 | 31.8% | 39.3% | 35.0% | 51.2% |
| RT (8 Gy) | 11.3% | 25.9% | 18.8% | 26.7% |
| Treatment Group | TGI (%) | |||
|---|---|---|---|---|
| Day 10 | Day 14 | Day 18 | Day 21 | |
| Anti PD-1 | 19.3% | 21.3% | 20.8% | 21.4% |
| RT (8 Gy)+anti PD-1 | 37.8% | 42.6% | 44.8% | 52.0% |
| RT (2 GyX4)+anti PD-1 | 4.4% | 19.0% | 28.6% | 30.9% |
| RT (8 Gy) | 13.0% | 14.7% | 9.4% | 16.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Guo, W.; Chen, X.; Zhang, Y.; Huang, Z. Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma. Cancers 2024, 16, 675. https://doi.org/10.3390/cancers16030675
Yin G, Guo W, Chen X, Zhang Y, Huang Z. Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma. Cancers. 2024; 16(3):675. https://doi.org/10.3390/cancers16030675
Chicago/Turabian StyleYin, Gaofei, Wei Guo, Xiaohong Chen, Yang Zhang, and Zhigang Huang. 2024. "Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma" Cancers 16, no. 3: 675. https://doi.org/10.3390/cancers16030675
APA StyleYin, G., Guo, W., Chen, X., Zhang, Y., & Huang, Z. (2024). Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma. Cancers, 16(3), 675. https://doi.org/10.3390/cancers16030675







