Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
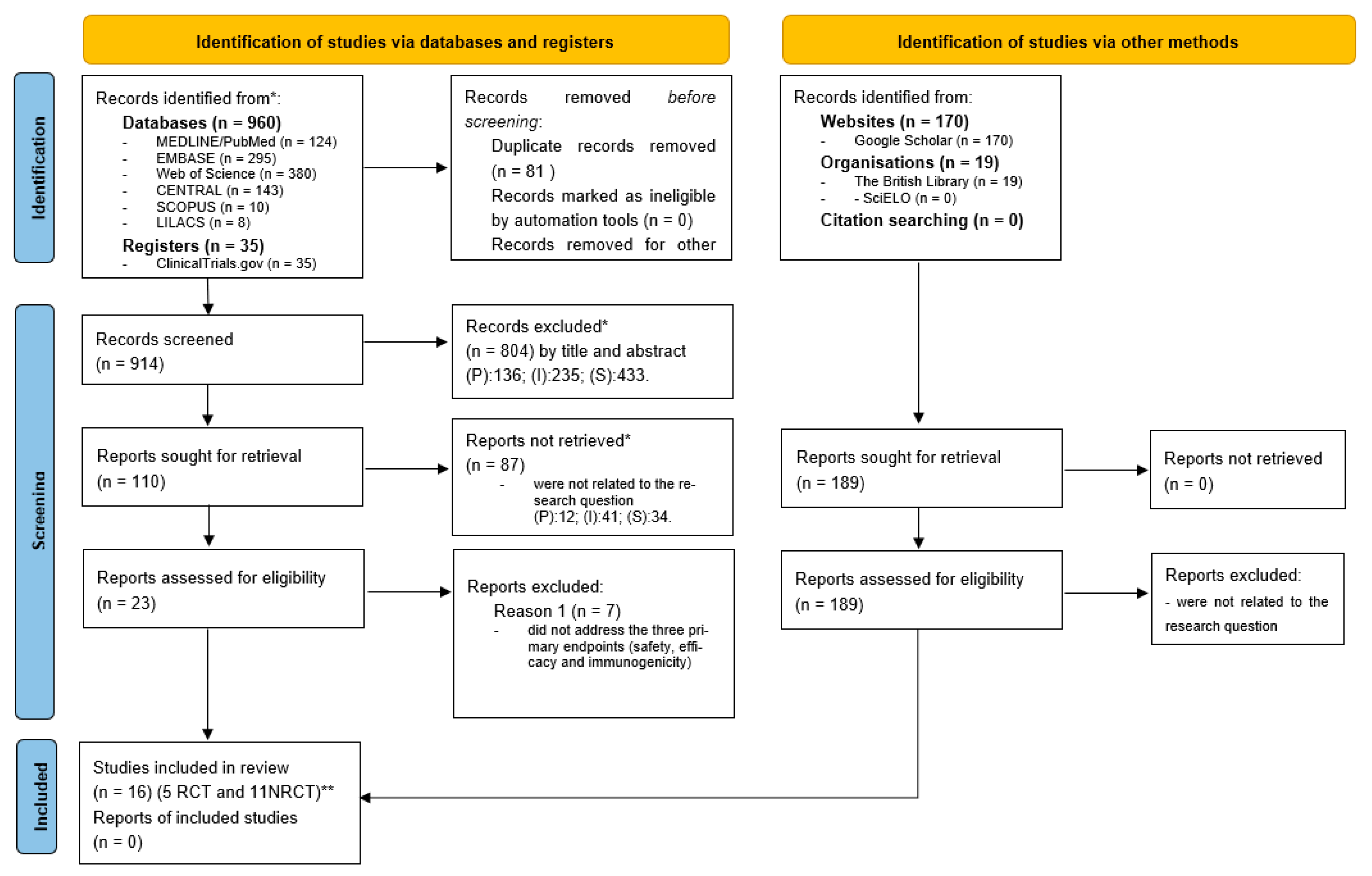
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
2.3. Role of the Funding Source
3. Results
3.1. Characteristics of the Studies
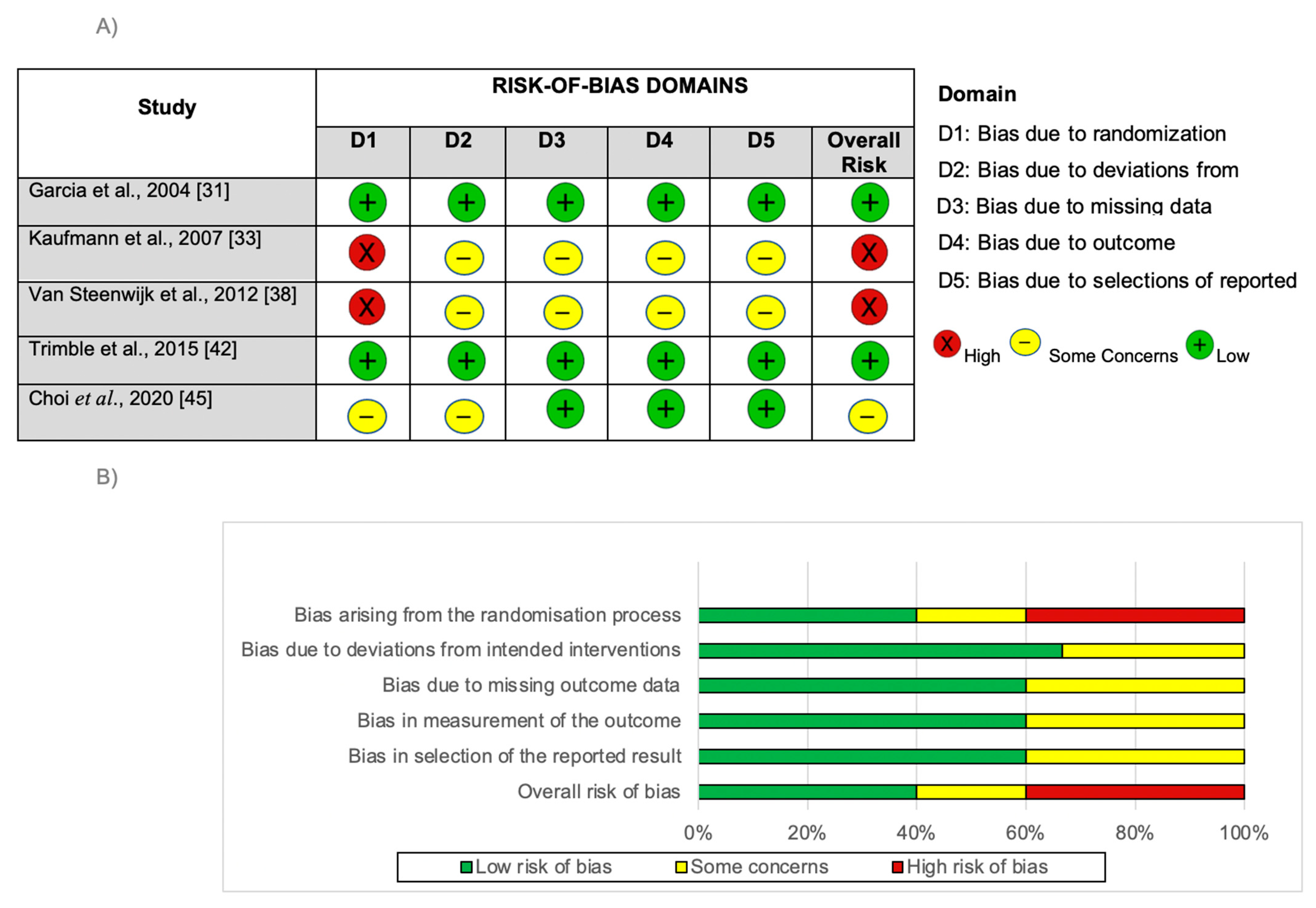
3.2. Risk of Bias
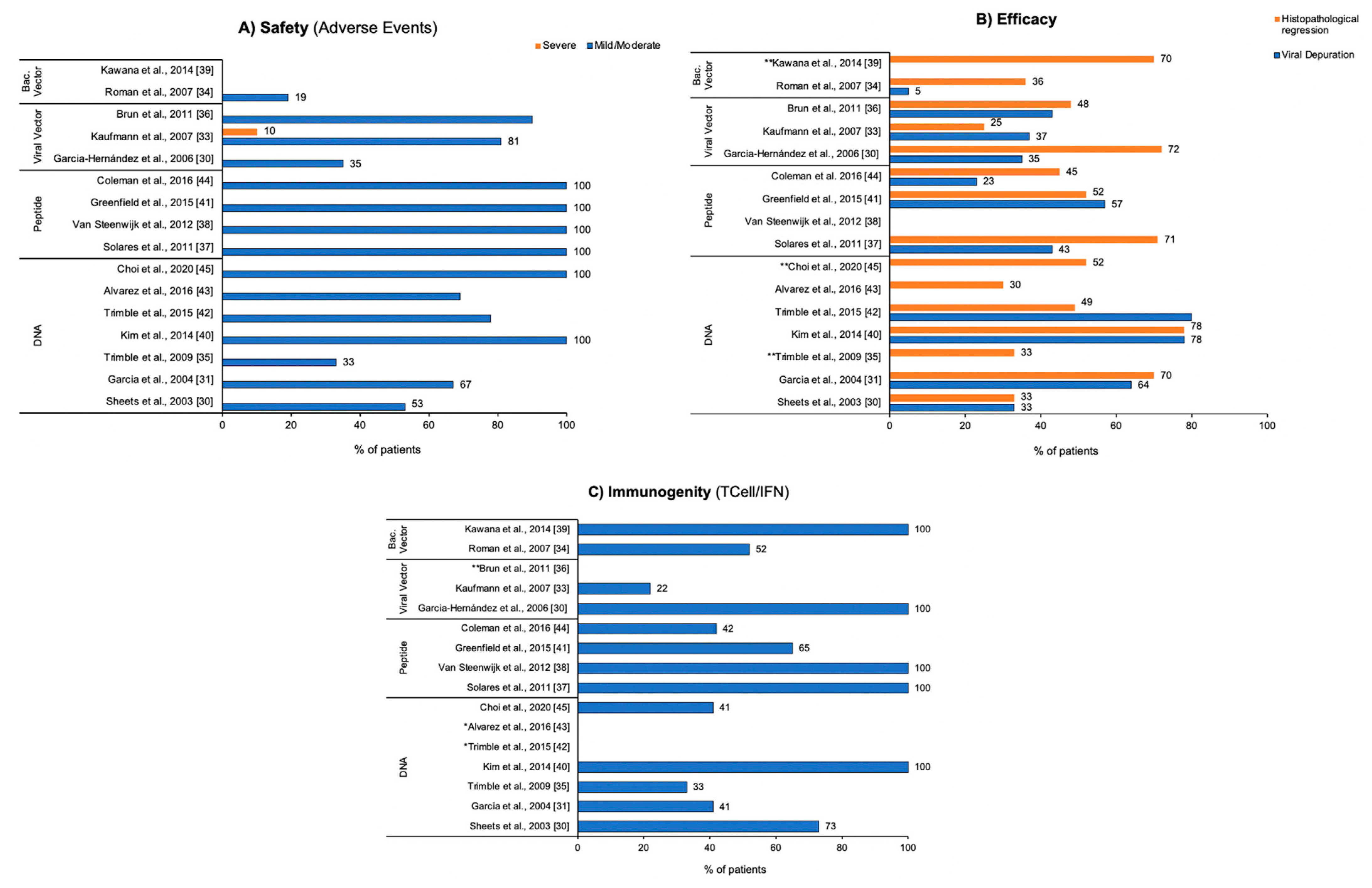
3.3. Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, F.X.; Burchell, A.N.; Schiffman, M.; Giuliano, A.R.; Sanjose, S.; Bruni, L.; Tortolero-Luna, G.; Kjaer, S.K.; Muñoz, N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008, 26, K1–K16. [Google Scholar] [CrossRef] [PubMed]
- CDC—Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/cancer/hpv/index.htm (accessed on 12 December 2018).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2021, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, C.A.; Bomfim, E.; Lopes-Júnior, L.C.; Pereira-da-Silva, G. Complementary therapies as a strategy to reduce stress and stimulate immunity of women with breast cancer. J. Evid. Based Integr. Med. 2019, 24, 2515690X19834169. [Google Scholar] [CrossRef] [PubMed]
- Vintermyr, O.K.; Iversen, O.; Thoresen, S.; Quint, W.; Molijn, A.; de Souza, S.; Rosillon, D.; Holl, K. Recurrent high-grade cervical lesion after primary conization is associated with persistent human papillomavirus infection in Norway. Gynecol. Oncol. 2014, 133, 159–166. [Google Scholar] [CrossRef]
- Mortensen, G.L.; Larsen, H.K. The quality of life of patients with genital warts: A qualitative study. BMC Public Health 2010, 10, 113. [Google Scholar] [CrossRef]
- Jardim, F.A.; Lopes-Júnior, L.C.; Nascimento, L.C.; Neves, E.T.; de Lima, R.A.G. Fertility-Related Concerns and Uncertainties in Adolescent and Young Adult Childhood Cancer Survivors. J. Adolesc. Young Adult Oncol. 2021, 10, 85–91. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Lima, R.A.G. Cancer care and interdisciplinary practice. Cad. Saude Publica 2019, 35, e00193218. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Giannouli, A.; Athanasopoulos, N.; Karountzos, V.; Vatopoulou, A.; Dimopoulos, K.; Creatsas, G. HPV infection: Immunological aspects and their utility in future therapy. Infect. Dis. Obstet. Gynecol. 2013, 2013, 540850. [Google Scholar] [CrossRef]
- Akhatova, A.; Chan, C.K.; Azizan, A.; Aimagambetova, G. The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review. Vaccines 2022, 10, 53. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Saunders: Philadelphia, PA, USA; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012, 4, 155ra138. [Google Scholar] [CrossRef] [PubMed]
- Boilesen, D.R.; Nielsen, K.N.; Holst, P.J. Novel Antigenic Targets of HPV Therapeutic Vaccines. Vaccines 2021, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Ghittoni, R.; Accardi, R.; Chiocca, S.; Tommasino, M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience 2015, 9, 526. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 21, 579. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Bomfim, E.O.; Nascimento, L.C.; Nunes, M.D.; Pereira-da-Silva, G.; Lima, R.A. Non-pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: An integrative review. Eur. J. Cancer Care 2016, 25, 921–935. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Bomfim, E.; Olson, K.; Neves, E.T.; Silveira, D.S.C.; Nunes, M.D.R.; Nascimento, L.C.; Pereira-da-Silva, G.; Lima, R.A.G. Effectiveness of hospital clowns for symptom management in paediatrics: Systematic review of randomised and non-randomised controlled trials. BMJ 2020, 16, m4290. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Urbano, I.R.; Schuab, S.I.P.C.; Pessanha, R.M.; Rosa, G.S.; Lima, R.A.G. Effectiveness of complementary therapies for the management of symptom clusters in palliative care in pediatric oncology: A systematic review. Rev. Esc. Enferm. USP 2021, 55, 03709. [Google Scholar] [CrossRef]
- Pessanha, R.M.; Schuab, S.I.P.C.; Nunes, K.Z.; Lopes-Júnior, L.C. Use of family history taking for hereditary neoplastic syndromes screening in primary health care: A systematic review protocol. PLoS ONE 2022, 17, e0271286. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Silva Junior, F.J.G.D.; Sales, J.C.E.S.; Monteiro, C.F.S.; Costa, A.P.C.; Campos, L.R.B.; Miranda, P.I.G.; Monteiro, T.A.S.; Lima, R.A.G.; Lopes-Junior, L.C. Impact of COVID-19 pandemic on mental health of young people and adults: A systematic review protocol of observational studies. BMJ Open 2020, 10, e039426. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Lima, R.A.G.; Zonta, J.B.; Sulino, M.C.; Looman, W.S.; Correa, L.M.; Okido, A.C.C. Biomarkers of stress in caregivers of children with special health care needs: A protocol for systematic review. Medicine 2022, 101, e31448. [Google Scholar] [CrossRef]
- Gonçalves, C.A.; Lopes-Júnior, L.C.; Nampo, F.K.; Zilly, A.; Mayer, P.C.M.; Pereira-da-Silva, G. Safety, efficacy and immunogenicity of therapeutic vaccines in the treatment of patients with high-grade cervical intraepithelial neoplasia associated with human papillomavirus: A systematic review protocol. BMJ Open 2019, 9, e026975. [Google Scholar] [CrossRef]
- Sheets, E.E.; Urban, R.G.; Crum, C.P.; Hedley, M.L.; Politch, J.A.; Gold, M.A.; Muderspach, L.I.; Cole, G.A.; Crowley-Nowick, P.A. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am. J. Obstet. Gynecol. 2003, 188, 916–926. [Google Scholar] [CrossRef]
- Garcia, F.; Petry, K.U.; Muderspach, L.; Gold, M.A.; Braly, P.; Crum, C.P.; Magill, M.; Silverman, M.; Urban, R.G.; Hedley, M.L.; et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2004, 103, 317–326. [Google Scholar] [CrossRef]
- García-Hernández, E.; González-Sánchez, J.L.; Andrade-Manzano, A.; Contreras, M.L.; Padilla, S.; Guzmán, C.C.; Jiménez, R.; Reyes, L.; Morosoli, G.; Verde, M.L.; et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006, 13, 592. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Nieland, J.D.; Jochmus, I.; Baur, S.; Friese, K.; Gabelsberger, J.; Gieseking, F.; Gissmann, L.; Glasschröder, B.; Grubert, T.; et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int. J. Cancer 2007, 121, 2794–2800. [Google Scholar] [CrossRef]
- Roman, L.D.; Wilczynski, S.; Muderspach, L.I.; Burnett, A.F.; O’Meara, A.; Brinkman, J.A.; Kast, W.M.; Facio, G.; Felix, J.C.; Aldana, M.; et al. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2007, 106, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Peng, S.; Kos, F.; Gravitt, P.; Viscidi, R.; Sugar, E.; Pardoll, D.; Wu, T.C. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Dalstein, V.; Leveque, J.; Mathevet, P.; Raulic, P.; Baldauf, J.J.; Scholl, S.; Huynh, B.; Douvier, S.; Riethmuller, D.; et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am. J. Obstet. Gynecol. 2011, 204, e1–e169. [Google Scholar] [CrossRef] [PubMed]
- Solares, A.M.; Baladron, I.; Ramos, T.; Valenzuela, C.; Borbon, Z.; Fanjull, S.; Gonzalez, L.; Castillo, D.; Esmir, J.; Granadillo, M.; et al. Safety and immunogenicity of a human papillomavirus peptide vaccine (CIGB-228) in women with high-grade cervical intraepithelial neoplasia: First-in-human, proof-of-concept trial. ISRN Obstet. Gynecol. 2011, 2011, 292951. [Google Scholar] [CrossRef]
- Van Steenwijk, P.J.V.; Ramwadhdoebe, T.H.; Löwik, M.J.G.; van der Minne, C.E.; Berends-van der Meer, D.M.; Fathers, L.M.; Valentijn, A.R.; Oostendorp, J.; Fleuren, G.J.; Hellebrekers, B.W.; et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol. Immunother. 2012, 61, 1485–1492. [Google Scholar] [CrossRef]
- Kawana, K.; Adachi, K.; Kojima, S.; Taguchi, A.; Tomio, K.; Yamashita, A.; Nishida, H.; Nagasaka, K.; Arimoto, T.; Yokoyama, T.; et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine 2014, 32, 6233–6239. [Google Scholar] [CrossRef]
- Kim, T.J.; Jin, H.; Hur, S.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef]
- Greenfield, W.W.; Stratton, S.L.; Myrick, R.S.; Vaughn, R.; Donnalley, L.M.; Coleman, H.N.; Mercado, M.; Moerman-Herzog, A.M.; Spencer, H.J.; Andrews-Collins, N.R.; et al. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeutic vaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology 2015, 4, e1031439. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Alvarez, R.D.; Huh, W.K.; Bae, S.; Lamb, L.S., Jr.; Conner, M.G.; Boyer, J.; Wang, C.; Hung, C.F.; Sauter, E.; Paradis, M.; et al. A pilot study of pNGVL4a-CRT/E7 (detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2016, 140, 245–252. [Google Scholar] [CrossRef]
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol. Immunother. 2016, 65, 563–573. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hur, S.Y.; Kim, T.J.; Hong, S.R.; Lee, J.K.; Cho, C.H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Broeck, D.V.; Benoy, I.; Bogers, J.; Depuydt, C.; Praet, M.; Sutter, P.D.; Hoorens, A.; Hauben, E.; Poppe, W.; et al. Surveillance of effects of HPV vaccination in Belgium. Cancer Epidemiol. 2016, 41, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Lorusso, D.; Raspagliesi, F. Safety and effectiveness of Human Papillomavirus (HPV) vaccination: NCI of Milan position statement. Vaccine 2017, 35, 227–5227. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Chesson, H.W.; Curtis, C.R.; Gee, J.; Bocchini, J.A., Jr.; Unger, E.R. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1–30. [Google Scholar]
- Petrosky, E.; Bocchini Jr, J.A.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300. [Google Scholar]
- Van Poelgeest, M.I.E.; Welters, M.J.P.; van Esch, E.M.G.; Stynenbosch, L.F.; Kerpershoek, G.; van Persijn van Meerten, E.L.; van den Hende, M.; Löwik, M.J.; Berends-van der Meer, D.M.; Fathers, L.M.; et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef]
- Van Poelgeest, M.I.E.; Welters, M.J.P.; Vermeij, R.; Stynenbosch, L.F.; Loof, N.M.; Berends-van der Meer, D.M.; Löwik, M.J.; Hamming, I.L.; van Esch, E.M.; Hellebrekers, B.W.; et al. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: Lesion clearance is related to the strength of the T-cell response. Clin. Cancer Res. 2016, 22, 2342–2350. [Google Scholar] [CrossRef]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Lowik, M.J.; Berends-van der Meer, D.M.; Vloon, A.P.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- Rosales, R.; López-Contreras, M.; Rosales, C.J.; Magallanes-Molina, J.R.; Gonzalez-Vergara, R.; Arroyo-Cazarez, J.M.; Ricardez-Arenas, A.; Del Follo-Valencia, A.; Padilla-Arriaga, S.; Guerrero, M.V.; et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum. Gene Ther. 2014, 25, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Daayana, S.; Elkord, E.; Winters, U.; Pawlita, M.; Roden, R.; Stern, P.L.; Kitchener, H.C. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer 2020, 102, 1129. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.R.; Kloor, M.; Prigge, E.S.; Sauer, M.; Al-Batran, S.E.; Kaufmann, A.M.; Schneider, A.; et al. A phase 1/2a study to test the safety and immunogenicity of a p16INK4a peptide vaccine in patients with advanced human papillomavirus-associated cancers. Cancer 2016, 122, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.B.; Hills, N.; Shiboski, S.; Powell, K.; Jay, N.; Hanson, E.; Miller, S.; Clayton, L.; Farhat, S.; Broering, J.; et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001, 285, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Chandravati. Human Papillomavirus-mediated cervical cancer awareness and Gardasil vaccination: A pilot survey among North Indian women. J. Community Health 2013, 38, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of Toll-like receptors [TLR] 2 (-196 to -174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef]
- Pandey, S.; Mittal, B.; Srivastava, M.; Singh, S.; Srivastava, K.; Lal, P.; Mittal, R.D. Evaluation of Toll-like receptors 3 (c.1377C/T) and 9 (G2848A) gene polymorphisms in cervical cancer susceptibility. Mol. Biol. Rep. 2011, 38, 4715–4721. [Google Scholar] [CrossRef]
- Nieto, K.; Gissmann, L.; Schädlich, L. Human papillomavirus-specific immune therapy: Failure and hope. Antivir. Ther. 2010, 15, 951. [Google Scholar] [CrossRef]
- Chuang, C.; Hoory, T.; Monie, A.; Wu, A.; Wang, M.C.; Hung, C.F. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+ CD25+ T regulatory cells. Vaccine 2009, 27, 684–689. [Google Scholar] [CrossRef]
- Karaki, S.; Anson, M.; Tran, T.; Giusti, D.; Blanc, C.; Oudard, S.; Tartour, E. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines 2016, 4, 37. [Google Scholar] [CrossRef]
- Cordeiro, M.N.; Lima, R.C.P.; Paolini, F.; Melo, A.R.D.S.; Campos, A.P.F.; Venuti, A.; De Freitas, A.C. Current research into novel therapeutic vaccines against cervical cancer. Expert. Rev. Anticancer. Ther. 2018, 18, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.M.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 9, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.S.M.; Fotouhi, F.; Mehrbod, P.; Keshavarz, M.; Alikhani, M.Y.; Ghaemi, A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb. Pathog. 2020, 147, 104438. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.S.M.; Noor, T.; Munim, Z.H.; Alikhani, M.Y.; Ghaemi, A. A bibliometric review of oncolytic virus research as a novel approach for cancer therapy. Virol. J. 2021, 18, 98. [Google Scholar] [CrossRef]
- Nejad, A.S.M.; Fotouhi, F.; Mehrbod, P.; Alikhani, M.Y. Antitumor immunity enhancement through Newcastle viral oncolysate in mice model: A promising method to treat tumors. Saudi J. Biol. Sci. 2021, 28, 5833–5840. [Google Scholar] [CrossRef]
| Acronym PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| P—Population | Patients with high-grade CIN (grade 2/3 associated with HPV) | Patients with associated immunosuppression |
| I—Intervention | Patients receiving therapeutic vaccines for the treatment of high-grade CIN 2/3 associated with HPV | Use of prophylactic vaccines for the treatment of CIN 2/3 associated with HPV or other neoplasms |
| C—Control | Patients who received placebo or patients serving as their own control | |
| O—Outcomes | Safety, efficacy, and immunogenicity of therapeutic vaccines used in patients with high-grade CIN 2/3 associated with HPV | Studies that do not report at the same time the three endpoints (safety, efficacy, and immunogenicity) |
| S—Study Design | RCT or NRCT | Reviews, theses, dissertations, expert opinions, editorials, protocols, clinical guidelines, and conference proceedings |
| Citation/Design Country | Study Characteristics | Conclusion | |||||
|---|---|---|---|---|---|---|---|
| Sample | Vaccine Type/Immunogen | Adverse Events (AEs) | Virological Response | Histopathological Regression/Lesion Size | Immune Response | ||
| Sheets et al. 2003 [30] NRCT USA | N = 15 Age: 19–44 Groups: Vaccine: 15 Placebo: 0 |
| AEs present in 53% of patients, the most common reaction were erythema, discomfort or other mild or moderate reactions. | Samples from responder patients were negative for HPV after vaccine treatment. | 33% of patients had complete histological responses and complete response to ZYC101 in lesion size. | 73% of patients had significant HPV-specific T cell response after vaccination and 87%, when considering the follow-up period. | The vaccine is associated with complete histological response with decrease in lesion size in 33% of patients, immune response in 73%, and no serious adverse events. |
| Garcia et al. 2004 [31] RCT USA | N = 161 Age: not reported Groups: Vaccine: 111 Placebo: 50 |
| The most common adverse events were related to the injection site, classified as mild to moderate with no major systemic side effects reported | ZYC101a induced clearance to HPV in subjects with HPV-16 or HPV-18 as well as subjects with other HPV types had higher clearance rates than matched patients who received placebo (64% versus 22% and 73% versus 25%, respectively) | The resolution of CIN 2/3 in the subgroup of women younger than 25 years was a statistically higher disease resolution rate for subjects treated with ZYC101a compared with placebo (70% versus 23%, respectively). The proportion of subjects within each treatment group without colposcopically visible lesions increased slowly but consistently, from 0% at baseline to 35–40% at the time of LEEP. The patients <25 years tended to have smaller lesions | Increased HPV-specific T cell response in patients <25 years was found in 12 patients (37%), and in patients ≥25 years, this percentage was 45%. | The vaccine was shown to be safe and well tolerated in all patients. The data found in the study support the continued clinical development of ZYC101a for the treatment of CIN 2/3 in women <25 y.o. |
| Garcia-Hernández et al., 2006 [32] NRCT Mexico | N = 54 Age: average 35 years old Groups: Vaccine: 34 Conization: 20 |
| Only a few moderate events were observed, the most frequent being headache, flu symptoms, fever, chills, moderate abdominal pain, and joint pain. | DNA viral load was significantly reduced in patients treated with MVA E2. Twelve of thirty-four patients efficiently eliminated all the HPV DNA. In 5 patients, the viral load decreased by 95%. In the other patients, the viral load was reduced between 15 and 50%. None of the 20 patients in the control group treated by conization eliminated HPV. Conization cleared the lesions in 80%, but the patients did not clear HPV. | Three weeks after the end of treatment, 56.25% of the patients with CIN 3 and two with CIN 2 were free of lesions. In 11 patients, the lesion was reduced to 50% of its original size. In 2 other patients, the CIN 3 lesion was reduced to CIN 2 and in another, the CIN 3 lesion was reduced to CIN 1. In addition, through colposcopy, 55.8% of the patients did not show the presence of HPV infection and the lesion was diagnosed as having been reduced by 100%. In 32.44% of patients, the lesion was reduced by up to 60%. | All patients developed antibodies against the MVA E2 vaccine and developed a specific cytotoxic response against papilloma-transformed cells. All patients treated with MVA E2 developed cytotoxic T lymphocytes (CTL) directed against tumor cells and the presence of CLT was correlated with lesion clearance | The vaccine can be considered safe and is a very promising candidate for the treatment of cervical lesions induced by high-grade CIN 3 HPV. The treatment leads to the elimination of the lesion as well as the elimination of viral DNA, leaving patients with better protection against future recurrences due to HPV reinfection. |
| Kaufmann et al. 2007 [33] RCT Germany | N = 39 Age: 20–38 Groups: Vaccine: 26 Placebo: 13 |
| Patients reported mild-to-moderate adverse events at the injection site, such as pain, induration, and itching. Reported systemic reactions were symptoms of flu and fatigue. Most of all AEs were fully recovered by the end of the study. The second AE most associated with treatment was headache. | After 48 weeks of treatment, six patients (37%) in the HPV 16 L1E7 vaccine group were HPV16 DNA negative, whereas only 1 of the placebo patients (14%) became HPV 16 DNA negative. | Histological regression to CIN 1 or normal was observed in 39% (9/23) of patients who received the vaccine and in only 25% (3/12) of patients in the placebo group. No statistically significant differences were found between the treatment and control groups with a reduction in lesion size greater than or equal to 50% | None of the patients in the placebo group had increased antibody titers, in the vaccine group a significant increase in L1-specific antibodies was observed. Measurement of isotypes showed induction of IgG (all patients), IgM (low dose 7/12; high dose 12/12) and IgA (low dose 11/12; high dose 10/12). T cell response after vaccination against E7 antigen (5 of 23 patients) was observed. | The vaccine had a very good safety profile, with only minor adverse events attributable to immunization, suggesting that it is safe and well tolerated. Antibodies with high titers against HPV 16 L1 and cellular immune responses were observed, and a trend of clinical efficacy highlighting the potentially therapeutic characteristic of this tested strategy |
| Roman et al. 2007 [34] NRCT USA | N = 21 Age: average 26 years old Groups: Vaccine: 21 Placebo: 0 |
| No grade III or IV toxicities were observed. There were four women who had Grade 2 injection site reactions which were of short duration (lasting less than a week). | Viral clearance occurred in only 1 woman. HPV clearance was not associated with lesion regression or immune response. | Seven of the twenty women (35%) evaluated for response had complete regression of their intraepithelial neoplasia at the time of LLETZ, one (5%) regressed to CIN I, eleven (55%) had a stable disease, and one (5%) progressed due to a worsening injury. Of the 17 women who completed 1 year follow-up after LLETZ, 13 (77%) remained without evidence of recurrent CIN at their last follow-up, and 4 of 13 women (31%) were PCR negative for HPV at the end of the study. | 52% of patients had evidence of an immune response to at least one peptide, suggesting that the vaccine was immunogenic in women with high-grade CIN and HPV infection. | Vaccine was considered safe and well tolerated. The HPV clearance appeared to be limited and generated modest levels of immunity and clinical response in patients with high-grade CIN. Although, the small number of patients evaluated, and the known spontaneous regression rate of CIN preclude any definitive conclusions as to the usefulness of the vaccine that has been tested |
| Trimble et al. 2009 [35] NRCT USA | N = 15 Age: 18–50 Groups: Vaccine: 15 Placebo: 0 |
| Most adverse events were mild with transient discomfort at the injection site. Systemic symptoms after vaccination were also reported by 5 of 15 subjects. | Not reported | Complete histological regression occurred in 3/9 (33%) patients in the highest dose cohort (3 mg) on week 15. Although the difference is not significant, it is slightly greater than would be expected in a control cohort (25%). | Vaccination did not elicit antibody responses. Measurable titers at study entry of anti-E6 IgG antibody in 3/15 (20%) and anti-E7 IgG antibody in 2/15 (13.3%) were noted. E7 titers were not increased after vaccination with E7DNA synthesis in any dose cohort. | The vaccine was safe and well tolerated. |
| Brun et al. 2011 [36] NRCT France | N = 10 Age: 25–44 Groups: Vaccine: 10 Placebo: 0 |
| 90% of patients reported some local and systemic adverse event. Intensity ranged from mild to moderate, with no episodes of grade 3 local reaction | Nine of twenty-one patients showed improvement in their HPV 16-associated infection. HPV 16 mRNA clearance was associated with cytological and colposcopy regression in 7 of 10 responders. Of the 10 respondents, 8 did not have HPV 16 DNA. | 48% (10 of 21) of patients responded to clinical treatment within the 6 months. They showed no or small changes in colposcopy and cytological diagnosis showed low-grade lesions or less, and 8 of them did not undergo surgery. The median times to disappearance of high-grade lesion, for HPV 16 E6 and E7 clearance, and HR-HPV DNA clearance were 13.5; 13.3, and 26 weeks, respectively. | At baseline, all patients had E7 antibody responses and 3 (19%) had E6 antibody responses. After treatment with TG4001, no patient developed or improved an antibody response to E6 or E7 as assessed by this method | The vaccine was safe and well tolerated. The results obtained in the trials of this study provided promising results for the development and further study of the TG4001 vaccine for the treatment of cervical intraepithelial neoplasia (CIN 2/3). |
| Solares et al. 2011 [37] NRCT Cuba | N = 7 Age: 24–43 Groups: Vaccine: 7 Placebo: 0 |
| No toxicity beyond grade 3 was observed in the experiment. All patients reported local pain at the vaccination site and 6 patients reported a burning sensation. | HPV was eliminated in three of the five patients with complete response. | The colposcopic response was evidenced in 6 of the 7 patients (85.7%), 4 (57.1%) complete and 2 (28.6%) partials. Histological analyzes indicated that 57.1% of patients (4/7) had complete regression, while 14.3% (1/7) had a decreased histological grade. | Cellular immune response was observed in all patients after vaccination. | Vaccination with CIGB-228 is safe and well tolerated. Moreover, resulted in lesion regression and HPV clearance. vaccination is capable of inducing IFN-N-associated T-cell responses in women with high-grade CIN. |
| Van Steenwijk et al. 2012 [38] RCT Netherlands | N = 10 Age: not reported Groups: Vaccine: 5 Placebo: 5 |
| All 5 patients in the vaccination group experienced adverse reactions that were mainly flu-like symptoms and injection site reactions. There were dropouts associated with side effects. Study ended prematurely. | In most patients, there was no change in the viral status | In most patients there was no change in histopathological status. There was no clearance of HPV at the time of excision. | A strong IFN-associated T cell-specific response to HPV was detected in all vaccinated patients. Vaccination of patients with HSIL resulted in increased immunity to HPV 16-specific T cells. At the time of HFS treatment, HPV 16-specific IFN-γ production was found in 3/5 vaccinated patients. Three of the 4 who received placebo remained unresponsive to HPV 16 E6/E7. | The study was stopped prematurely. Suggested the development of future studies focused on the development of a better tolerated formulation. No conclusions can be drawn about vaccine-enhanced T-cell infiltration into the lesion. Overall, the study shows that the vaccine may increase the number of circulating IFN-γ-producing HPV 16-specific T cells in patients with high-grade lesions. |
| Kawana et al. 2014 [39] NRCT Japan | N = 10 Age: unreported Groups: Vaccine: 10 Placebo: 0 |
| No patient had serious side effects induced by vaccine. No patient was withdrawn from the study due to adverse event. | Not reported | Combining the patients from Steps 1 and 2 who received four capsules/day, 7 of 10 patients (70%) had a histopathological regression to CIN2 on week 9, and 1 patient had a negative pathology grade for CIN2 on week 12. Of the 13 patients who received four–six capsules/day, 9 patients (69%) with a pathological grade lower than CIN2 did not require additional surgical treatment and were followed up cytologically. The histopathological regression for CIN2 in response to a GLBL101c regimen of four capsules/day was 80%. | Oral administration of GLBL101c predominantly induces E7-CMI from the mucosa towards the cervical epithelium | Oral administration with GLBL101c can be considered safe and well tolerated. Oral administration of E7-expressing Lactobacillus-based vaccine can induce E7-specific mucosal immunity in uterine cervical lesions. The vaccine was able to induce mucosal E7-CMI, but had no systemic response |
| Kim et al. 2014 [40] NRCT Korea | N = 9 Age: 23–44 groups Vaccine: 9 Placebo: 0 |
| AEs were considered mild (grade 1) and all patients fully recovered within three days of vaccination. | Until week 36 (VF2), 7 patients eliminated the virus that had been found at the beginning of treatment (HPV 16 and/or 18) and also had lesion regression, resulting in a perfect correlation between clinical and virological responses. | Eight weeks after the last vaccination (VF1), 6 of the 9 patients were free of lesions. GX-188E vaccination led to a clinically and virologically significant complete response rate of 78%. Viral clearance (4 of 9 patients) and cytological regression (3 of 9 patients) were already apparent on week 12 and most complete responders (6 of 7) cleared cervical lesions by week 20 after vaccination | The vaccine induced a significant E6/E7-specific IFN-γ-producing T cell response in all 9 patients with CIN3. the antibody titers to E6 were not induced in any dose cohort patients after vaccination. Three of the 9 patients generated weak anti-E7 antibody responses following vaccination with antibody titers ranging from 1:8 to 1:256. | The administration of GX-188E, being considered safe and well-tolerated. The vaccination in patients with CIN 3 substantially increased both HPV-specific CD8 T cell. Although the (n = 9) is too small to reach a definitive conclusion |
| Greenfield et al. 2015 [41] NRCT USA | N = 24 Age: 22–42 Groups: Vaccine: 24 Placebo: 0 |
| The most common AEs reported were immediate responses related to the injection site with no signs of toxicity. | At least one HPV type present at entry became undetectable in 13 of 23 (57%) patients. Per dose, rates were 83%, 50%, 50% and 40%, with the highest undetectability at the lowest dose | The best histological response was seen at the 50 µg dose with a regression rate of 83% (n = 6), and the overall rate was 52% (n = 23). CIN 2/3 was no longer present in 9 of 23 (39%) patients who completed the study (complete responders), and CIN 2/3 lesions measured ≤0.2 mm2 in 3 (13%) patients (partial responders). Five of the 12 patients with no visible lesions after vaccination were histological nonresponses with persistent CIN 2/3. | Th1 cells were significantly increased after four vaccinations. New CD3 T cell responses and at least one region of the E6 protein were detected in 15 of 23 patients (65%), with the increase in responses after vaccination being statistically significant in 10 patients (43%). The best CD3 T cell response rates to E6 were at doses of 50 and 250 µg (83%). | The PepCan vaccine is safe, no signs of vaccine-related toxicity were identified. As the number of subjects in each dose group was small (n = 6), this study was not designed to show significant differences. The systemic level of Th1 cells increased significantly, suggesting that Candida, who induces interleukin-12 (IL-12) in vitro, may have an effect on Th1 promotion. |
| Trimble et al. 2015 [42] RCT USA | N = 167 Age: 24–41 Groups: Vaccine: 125 Placebo: 42 |
| Injection site reactions occurred in most patients, however, only erythema showed a statistically significant difference between the vaccine group and the placebo group. Four patients discontinued dosing due to an adverse event. No related serious adverse events were reported | Concomitant analysis of histopathological regression with viral clearance as per protocol: 40.2% (VGX-3100 group) and 14.3% (placebo). Viral clearance was more associated with patients who received VGX-3100 (80%) than in the placebo group (50%) | Histopathological regression according to the protocol: 49.5% (VGX-3100 group) and 30.6% (placebo). | VGX-3100 induced significantly increased frequencies of activated, antigen-specific CD8+ T cells identified by cell surface expression of CD137, which also expressed perforins compared to placebo. Humoral responses were also greater in patients in the VGX-3100 group compared to those in the placebo group. | Treatment with VGX-3100 was well tolerated. The trial showed that the administration of the DNA vaccine encoded with viral proteins can trigger adaptive immune responses that have a therapeutic effect on cervical lesions. These findings suggest that VGX-3100 offers a non-surgical option for the treatment of 2/3 CIN that could change the approach to treating this very common disease |
| Alvarez et al. 2016 [43] NRCT USA | N = 32 Age: 20–44 Groups: Vaccine: 32 Placebo: 0 | DNA vaccine (pNGVL4a-CRT/E7 (detox)
| 69% of patients experienced vaccine-related adverse events. The events were more related to the injection site and were not greater than grade 1 events. No serious vaccine-related adverse events were observed. | No differences were found between pre- and post-vaccination viral loads in any of the treatment cohorts | Histological regression for CIN 1 or less occurred in 8 of 27 (30%) patients who received all vaccinations. Persistent 2/3 CIN was observed in 19 (70%) patients. | Immune responses to E7 were minimal, and not significantly different from responses to HPV 16 E6, which was not included in pNGVL4a-CRT-E7. | The vaccine was well tolerated. An increase in the specific immune response to HPV was noted. Although a local CD8+ T cell response appeared to be more robust with intralesional vaccination, none of the vaccination routes were immunogenic. |
| Coleman et al. 2016 [44] NRCT USA | N = 34 Age: Not reported Groups: Vaccine: 34 Placebo: 0 | Peptide vaccine (Pepcan)
| No dose-limiting toxicities were observed. The most common adverse events were mild to moderate at the injection site. | Three of the 13 women in whom HPV 16 was detected early became undetectable after vaccination and was persistent in nine patients. | Histological regression rates were 50% at the 50 μg doses (7 of 14) and 100 μg (3 of 6), 33% at the 250 μg dose and 40% at the 500 μg dose, 45% in total (14 of 31). | The immunological profile revealed an increase in type 1 helper T cells after vaccinations. | The Pepcan vaccine proved to be safe and demonstrated a decrease in HPV 16 viral load as well as histological regression. |
| Choi et al. 2020 [45] RCT South Korea | N = 71 Age: 19–50 Groups: Vaccine: 64 Placebo: 0 | DNA vaccine (GX-188E) HPV 16 AND 18—E6/E7 | AE (occurring in 94.4% and 100.0% in the 1 and 4 mg GX-188E groups, respectively). None serious AEs were related to the DNA vaccine. | Not reported | Histopathologic regression occurred in 35 (67%) of the 52 patients. 77% of the patients with histologic regression showed HPV clearance. | IFN-γ ELISpot responses ≥3-fold over baseline indicated the drug was efficacious. | GX-188E was well tolerated by all the patients. |
| * Domains ROBINS-I | Overall Judgment ROBINS-I ** | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Confounding Bias | Participant Selection Bias | Classification of Intervention Bias | Bias Due to Intervention Deviations | Incomplete Data Bias | Outcome Measurement Bias | Selective Outcome Reporting Bias | |
| Sheets et al. 2003 [30] | Moderate | Serious | Low | Low | Low | Low | Moderate | Serious |
| Garcia-Hernández et al. 2006 [32] | Moderate | Serious | Low | Serious | Low | Low | Low | Serious |
| Roman et al. 2007 [34] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Trimble et al. 2009 [35] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Brun et al. 2011 [36] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Solares et al. 2011 [37] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Kawana t al. 2014 [39] | Moderate | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Kim et al. 2014 [40] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Greenfield et al. 2015 [41] | Moderate | Serious | Low | Low | Moderate | Low | Moderate | Serious |
| Alvarez et al. 2016 [43] | Moderate | Moderate | Low | Low | Moderate | Low | Moderate | Moderate |
| Coleman et al. 2016 [44] | Moderate | Serious | Low | Low | Moderate | Low | Moderate | Serious |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, C.A.; Pereira-da-Silva, G.; Silveira, R.C.C.P.; Mayer, P.C.M.; Zilly, A.; Lopes-Júnior, L.C. Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review. Cancers 2024, 16, 672. https://doi.org/10.3390/cancers16030672
Gonçalves CA, Pereira-da-Silva G, Silveira RCCP, Mayer PCM, Zilly A, Lopes-Júnior LC. Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review. Cancers. 2024; 16(3):672. https://doi.org/10.3390/cancers16030672
Chicago/Turabian StyleGonçalves, Caroline Amélia, Gabriela Pereira-da-Silva, Renata Cristina Campos Pereira Silveira, Paulo César Morales Mayer, Adriana Zilly, and Luís Carlos Lopes-Júnior. 2024. "Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review" Cancers 16, no. 3: 672. https://doi.org/10.3390/cancers16030672
APA StyleGonçalves, C. A., Pereira-da-Silva, G., Silveira, R. C. C. P., Mayer, P. C. M., Zilly, A., & Lopes-Júnior, L. C. (2024). Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review. Cancers, 16(3), 672. https://doi.org/10.3390/cancers16030672









