Dynamic Contrast-Enhanced Ultrasound in the Prediction of Advanced Hepatocellular Carcinoma Response to Systemic and Locoregional Therapies
Abstract
Simple Summary
Abstract
1. Introduction
2. Evaluating the Response of Hepatocellular Carcinoma to Treatments: A Multifaceted and Evolving Landscape
3. Dynamic Contrast-Enhanced Ultrasound in the Analysis of Advanced Hepatocellular Carcinoma Response to Systemic Treatments
4. Dynamic Contrast-Enhanced Ultrasound in the Analysis of Advanced Hepatocellular Carcinoma Response to Intraarterial Treatments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Svegliati-Baroni, G.; Ortolani, A.; Cucco, M.; Dalla Riva, G.V.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.; Di Marco, M.; Caturelli, E.; et al. Epidemiological Trends and Trajectories of MAFLD-Associated Hepatocellular Carcinoma 2002–2033: The ITA.LI.CA Database. Gut 2023, 72, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Kumar, A. Treatment Response Evaluation and Follow-up in Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2014, 4, S126–S129. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. MRECIST for HCC: Performance and Novel Refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Kudo, M.; Ikeda, M.; Ueshima, K.; Sakamoto, M.; Shiina, S.; Tateishi, R.; Nouso, K.; Hasegawa, K.; Furuse, J.; Miyayama, S.; et al. Response Evaluation Criteria in Cancer of the Liver Version 6 (Response Evaluation Criteria in Cancer of the Liver 2021 Revised Version). Hepatol. Res. 2022, 52, 329–336. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Gabriel, H.; Savsani, E.; Lyshchik, A. Contrast-Enhanced Ultrasound (CEUS) in HCC Diagnosis and Assessment of Tumor Response to Locoregional Therapies. Abdom. Radiol. 2021, 46, 3579–3595. [Google Scholar] [CrossRef]
- Kuorda, H.; Abe, T.; Fujiwara, Y.; Okamoto, T.; Yonezawa, M.; Sato, H.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Change in Arterial Tumor Perfusion Is an Early Biomarker of Lenvatinib Efficacy in Patients with Unresectable Hepatocellular Carcinoma. World J. Gastroenterol. 2019, 25, 2365–2372. [Google Scholar] [CrossRef]
- Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Lu, F.; Frehlich, L.; Wong, J.K.; Burak, K.W.; Wilson, S.R. A New Proposal for Secondary Surveillance Following Potentially Curative Therapy of HCC: Alternating MRI and CEUS. Abdom. Radiol. 2022, 47, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.; Choi, B.; Cosgrove, D.; Kudo, M.; Nolsøe, C.; Piscaglia, F.; Wilson, S.; Barr, R.; Chammas, M.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver–Update 2012. Ultraschall Med.-Eur. J. Ultrasound 2012, 34, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, L.; Wang, J.; Zhang, Y.; Guo, Q.; Li, X.; Zhang, X. Contrast-Enhanced US for Characterization of Focal Liver Lesions: A Comprehensive Meta-Analysis. Eur. Radiol. 2018, 28, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, S.; Kalra, N.; Prabhakar, N.; Dhiman, R.K.; Behera, A.; Chawla, Y.K.; Khandelwal, N. Comparison of Contrast Enhanced Ultrasound With Contrast Enhanced Computed Tomography for the Diagnosis of Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2017, 7, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Aubé, C.; Oberti, F.; Lonjon, J.; Pageaux, G.; Seror, O.; N’Kontchou, G.; Rode, A.; Radenne, S.; Cassinotto, C.; Vergniol, J.; et al. EASL and AASLD Recommendations for the Diagnosis of HCC to the Test of Daily Practice. Liver Int. 2017, 37, 1515–1525. [Google Scholar] [CrossRef]
- Dietrich, C.; Averkiou, M.; Correas, J.-M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB Introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for Quantification of Tumour Perfusion. Ultraschall Med.-Eur. J. Ultrasound 2012, 33, 344–351. [Google Scholar] [CrossRef]
- Fröhlich, E.; Muller, R.; Cui, X.-W.; Schreiber-Dietrich, D.; Dietrich, C.F. Dynamic Contrast-Enhanced Ultrasound for Quantification of Tissue Perfusion. J. Ultrasound Med. 2015, 34, 179–196. [Google Scholar] [CrossRef]
- Dietrich, C.; Dong, Y.; Froehlich, E.; Hocke, M. Dynamic Contrast-Enhanced Endoscopic Ultrasound: A Quantification Method. Endosc. Ultrasound 2017, 6, 12. [Google Scholar] [CrossRef]
- De Giorgi, U.; Aliberti, C.; Benea, G.; Conti, M.; Marangolo, M. Effect of Angiosonography to Monitor Response During Imatinib Treatment in Patients with Metastatic Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2005, 11, 6171–6176. [Google Scholar] [CrossRef]
- Zheng, S.-G. Role of Contrast-Enhanced Ultrasound in Follow-up Assessment after Ablation for Hepatocellular Carcinoma. World J. Gastroenterol. 2013, 19, 855. [Google Scholar] [CrossRef]
- Ainora, M.E.; Iezzi, R.; Ponziani, F.R.; Garcovich, M.; Di Stasio, E.; Riccardi, L.; Annicchiarico, B.E.; Abbate, V.; De Gaetano, A.M.; Siciliano, M.; et al. Contrast-Enhanced Ultrasound in the Short-Term Evaluation of Hepatocellular Carcinoma after Locoregional Treatment. Dig. Dis. 2020, 38, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Bai, Y.; Xie, X.; Feng, Y.; Yang, Y.; Zhu, Q. RECIST 1.1 versus MRECIST for Assessment of Tumour Response to Molecular Targeted Therapies and Disease Outcomes in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e052294. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Koscielny, S.; Chami, L.; Chebil, M.; Benatsou, B.; Roche, A.; Ducreux, M.; Malka, D.; Boige, V. Advanced Hepatocellular Carcinoma: Early Evaluation of Response to Bevacizumab Therapy at Dynamic Contrast-Enhanced US with Quantification—Preliminary Results. Radiology 2011, 258, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.M.; Al Zahrani, H.; Jang, H.J.; Menezes, R.; Hudson, J.; Burns, P.; McNamara, M.G.; Kandel, S.; Khalili, K.; Knox, J.; et al. Detection of Early Tumor Response to Axitinib in Advanced Hepatocellular Carcinoma by Dynamic Contrast Enhanced Ultrasound. Ultrasound Med. Biol. 2016, 42, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Frampas, E.; Lassau, N.; Zappa, M.; Vullierme, M.-P.; Koscielny, S.; Vilgrain, V. Advanced Hepatocellular Carcinoma: Early Evaluation of Response to Targeted Therapy and Prognostic Value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary Results. Eur. J. Radiol. 2013, 82, e205–e211. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Bonastre, J.; Kind, M.; Vilgrain, V.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A.; Labbe-Devilliers, C.; et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: The French multicenter support for innovative and expensive techniques study. Investig. Radiol. 2014, 49, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Chami, L.; Koscielny, S.; Chebil, M.; Massard, C.; Benatsou, B.; Bidault, S.; Cioffi, A.; Blay, J.-Y.; Le Cesne, A. Quantitative Functional Imaging by Dynamic Contrast Enhanced Ultrasonography (DCE-US) in GIST Patients Treated with Masatinib. Investig. New Drugs 2012, 30, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Bailey, C.; Atri, M.; Stanisz, G.; Milot, L.; Williams, R.; Kiss, A.; Burns, P.N.; Bjarnason, G.A. The Prognostic and Predictive Value of Vascular Response Parameters Measured by Dynamic Contrast-Enhanced-CT, -MRI and -US in Patients with Metastatic Renal Cell Carcinoma Receiving Sunitinib. Eur. Radiol. 2018, 28, 2281–2290. [Google Scholar] [CrossRef]
- Lassau, N.; Koscielny, S.; Albiges, L.; Chami, L.; Benatsou, B.; Chebil, M.; Roche, A.; Escudier, B.J. Metastatic Renal Cell Carcinoma Treated with Sunitinib: Early Evaluation of Treatment Response Using Dynamic Contrast-Enhanced Ultrasonography. Clin. Cancer Res. 2010, 16, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Lamuraglia, M.; Escudier, B.; Chami, L.; Schwartz, B.; Leclère, J.; Roche, A.; Lassau, N. To Predict Progression-Free Survival and Overall Survival in Metastatic Renal Cancer Treated with Sorafenib: Pilot Study Using Dynamic Contrast-Enhanced Doppler Ultrasound. Eur. J. Cancer 2006, 42, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, X.; Chen, L.; Wang, Z.; Shi, Y.; Mao, H.; Dai, G.; Yu, X. Anti-Angiogenic Therapy with Contrast-Enhanced Ultrasound in Colorectal Cancer Patients with Liver Metastasis. Medicine 2017, 96, e6731. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Coiffier, B.; Kind, M.; Vilgrain, V.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A.; Labbe-Devilliers, C.; et al. Selection of an Early Biomarker for Vascular Normalization Using Dynamic Contrast-Enhanced Ultrasonography to Predict Outcomes of Metastatic Patients Treated with Bevacizumab. Ann. Oncol. 2016, 27, 1922–1928. [Google Scholar] [CrossRef]
- Zocco, M.A.; Garcovich, M.; Lupascu, A.; Di Stasio, E.; Roccarina, D.; Annicchiarico, B.E.; Riccardi, L.; Ainora, M.E.; Ponziani, F.; Caracciolo, G.; et al. Early Prediction of Response to Sorafenib in Patients with Advanced Hepatocellular Carcinoma: The Role of Dynamic Contrast Enhanced Ultrasound. J. Hepatol. 2013, 59, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.M.; Minhaj, A.A.; Maldonado, K.L.; Kingsley, C.V.; Cortes, A.C.; Taghavi, H.; Polak, U.; Mitchell, J.M.; Ensor, J.E.; Bankson, J.A.; et al. Comparison of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and Contrast-Enhanced Ultrasound for Evaluation of the Effects of Sorafenib in a Rat Model of Hepatocellular Carcinoma. Magn. Reson. Imaging 2019, 57, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Waldner, M.J.; Goertz, R.S.; Strobel, D. Quantification of Dynamic Contrast-Enhanced Ultrasound in HCC: Prediction of Response to a New Combination Therapy of Sorafenib and Panobinostat in Advanced Hepatocellular Carcinoma. Case Rep. 2012, 2012, bcr2012007576. [Google Scholar] [CrossRef]
- Shiozawa, K. Evaluation of Sorafenib for Hepatocellular Carcinoma by Contrast-Enhanced Ultrasonography: A Pilot Study. World J. Gastroenterol. 2012, 18, 5753. [Google Scholar] [CrossRef]
- Lassau, N.; Chami, L.; Chebil, M.; Benatsou, B.; Bidault, S.; Girard, E.; Abboud, G.; Roche, A. Dynamic Contrast-Enhanced Ultrasonography (DCE-US) and Anti-Angiogenic Treatments. Discov Med. 2011, 11, 18–24. [Google Scholar]
- Egger, C.; Goertz, R.; Strobel, D.; Lell, M.; Neurath, M.; Knieling, F.; Scharf, M. Dynamic Contrast-Enhanced Ultrasound (DCE-US) for Easy and Rapid Evaluation of Hepatocellular Carcinoma Compared to Dynamic Contrast-Enhanced Computed Tomography (DCE-CT)—A Pilot Study. Ultraschall Med.-Eur. J. Ultrasound 2012, 33, 587–592. [Google Scholar] [CrossRef]
- Cao, J.; Dong, Y.; Fan, P.; Mao, F.; Wang, W. Feasibility of Dynamic Three-Dimensional Contrast-Enhanced Ultrasound in Focal Liver Lesions: Image Quality Evaluation and Correlation of Quantification with Two-Dimensional Contrast-Enhanced Ultrasound. Clin. Hemorheol. Microcirc. 2019, 72, 305–316. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, Z. A Review on Real-Time 3D Ultrasound Imaging Technology. BioMed Res. Int. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Nam, K.; Stanczak, M.; Lyshchik, A.; Machado, P.; Kono, Y.; Forsberg, F.; Shaw, C.M.; Eisenbrey, J.R. Evaluation of Hepatocellular Carcinoma Transarterial Chemoembolization Using Quantitative Analysis of 2D and 3D Real-Time Contrast Enhanced Ultrasound. Biomed. Phys. Eng. Express 2018, 4, 035039. [Google Scholar] [CrossRef]
- Knieling, F.; Waldner, M.; Goertz, R.; Zopf, S.; Wildner, D.; Neurath, M.; Bernatik, T.; Strobel, D. Early Response to Anti-Tumoral Treatment in Hepatocellular Carcinoma—Can Quantitative Contrast-Enhanced Ultrasound Predict Outcome? Ultraschall Med.-Eur. J. Ultrasound 2012, 34, 38–46. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Zhang, J.-B.; Fan, P.-L.; Xiong, Y.-Q.; Zhuang, P.-Y.; Zhang, W.; Xu, H.-X.; Gao, D.-M.; Kong, L.-Q.; Wang, L.; et al. Antiangiogenic Effects of Pazopanib in Xenograft Hepatocellular Carcinoma Models: Evaluation by Quantitative Contrast-Enhanced Ultrasonography. BMC Cancer 2011, 11, 28. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Saito, K.; Rognin, N.; Kamiyama, N.; Furuichi, Y.; Imai, Y. Hepatocellular Carcinoma Treated with Sorafenib: Early Detection of Treatment Response and Major Adverse Events by Contrast-Enhanced US. Liver Int. 2013, 33, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Yamashita, K.; Osawa, L.; Komiyama, Y.; Nakakuki, N.; Muraoka, M.; Suzuki, Y.; Sato, M.; Takano, S.; Fu-kasawa, M.; et al. Prediction of Therapeutic Response Using Contrast-Enhanced Ultrasound in Japanese Patients Treated with Atezolizumab and Bevacizumab for Unresectable Hepato-cellular Carcinoma. Oncology 2023, 101, 173–184. [Google Scholar] [CrossRef]
- Spârchez, Z.; Mocan, T.; Radu, P.; Anton, O.; Bolog, N. Contrast Enhanced Ultrasonography in Assessing the Treatment Response to Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Med. Ultrason. 2016, 18, 96. [Google Scholar] [CrossRef][Green Version]
- Uller, W.; Wiggermann, P.; Gössmann, H.; Klebl, F.; Salzberger, B.; Stroszczynski, C.; Jung, E.M. Evaluation of the Microcirculation of Hepatocellular Carcinomas Using Contrast-Enhanced Ultrasound with Intraarterial and Intravenous Contrast Application during Transarterial Chemoembolization with Drug-Eluting Beads (DEB-TACE): Preliminary Data. Clin. Hemorheol. Microcirc. 2011, 49, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Moschouris, H.; Malagari, K.; Papadaki, M.G.; Kornezos, I.; Stamatiou, K.; Anagnostopoulos, A.; Chatzimichael, K.; Kelekis, N. MRECIST Criteria and Contrast-Enhanced US for the Assessment of the Response of Hepatocellular Carcinoma to Transarterial Chemoembolization. Diagn. Interv. Radiol. 2013, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Wiggermann, P.; Wohlgemuth, W.A.; Heibl, M.; Vasilj, A.; Loss, M.; Schreyer, A.G.; Stroszczynski, C.; Jung, E.M. Dynamic Evaluation and Quantification of Microvascularization during Degradable Starch Microspheres Transarterial Chemoembolisation (DSM-TACE) of HCC Lesions Using Contrast Enhanced Ultrasound (CEUS): A Feasibility Study. Clin. Hemorheol. Microcirc. 2013, 53, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Dong, Y.; Fan, P.; Mao, F.; Chen, K.; Chen, R.; Huang, B.; Cheng, Y.; Wang, W.-P. Early Evaluation of Treatment Response to Transarterial Chemoembolization in Patients with Advanced Hepatocellular Carcinoma: The Role of Dynamic Three-Dimensional Contrast-Enhanced Ultrasound. Clin. Hemorheol. Microcirc. 2021, 78, 365–377. [Google Scholar] [CrossRef] [PubMed]
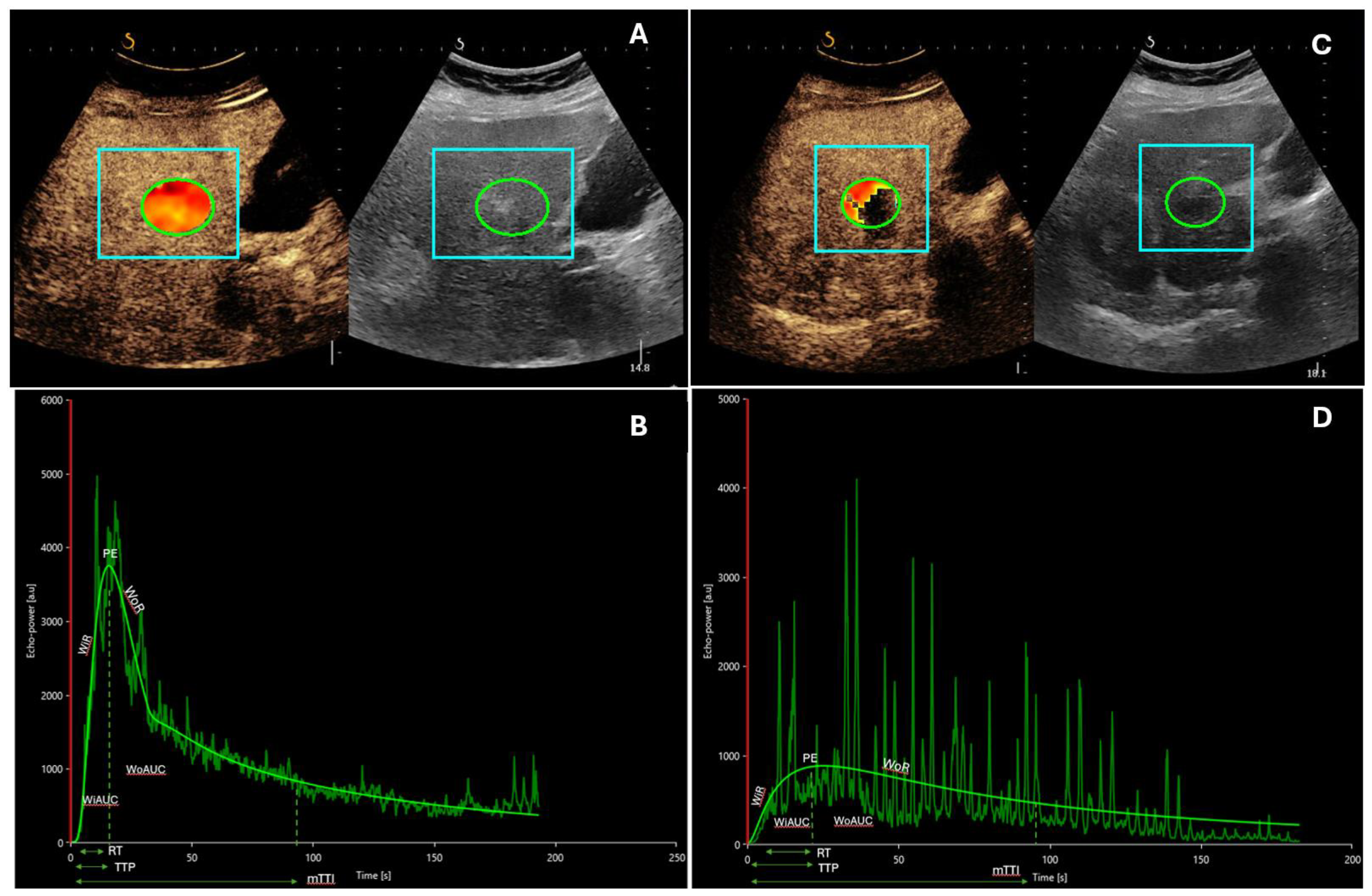
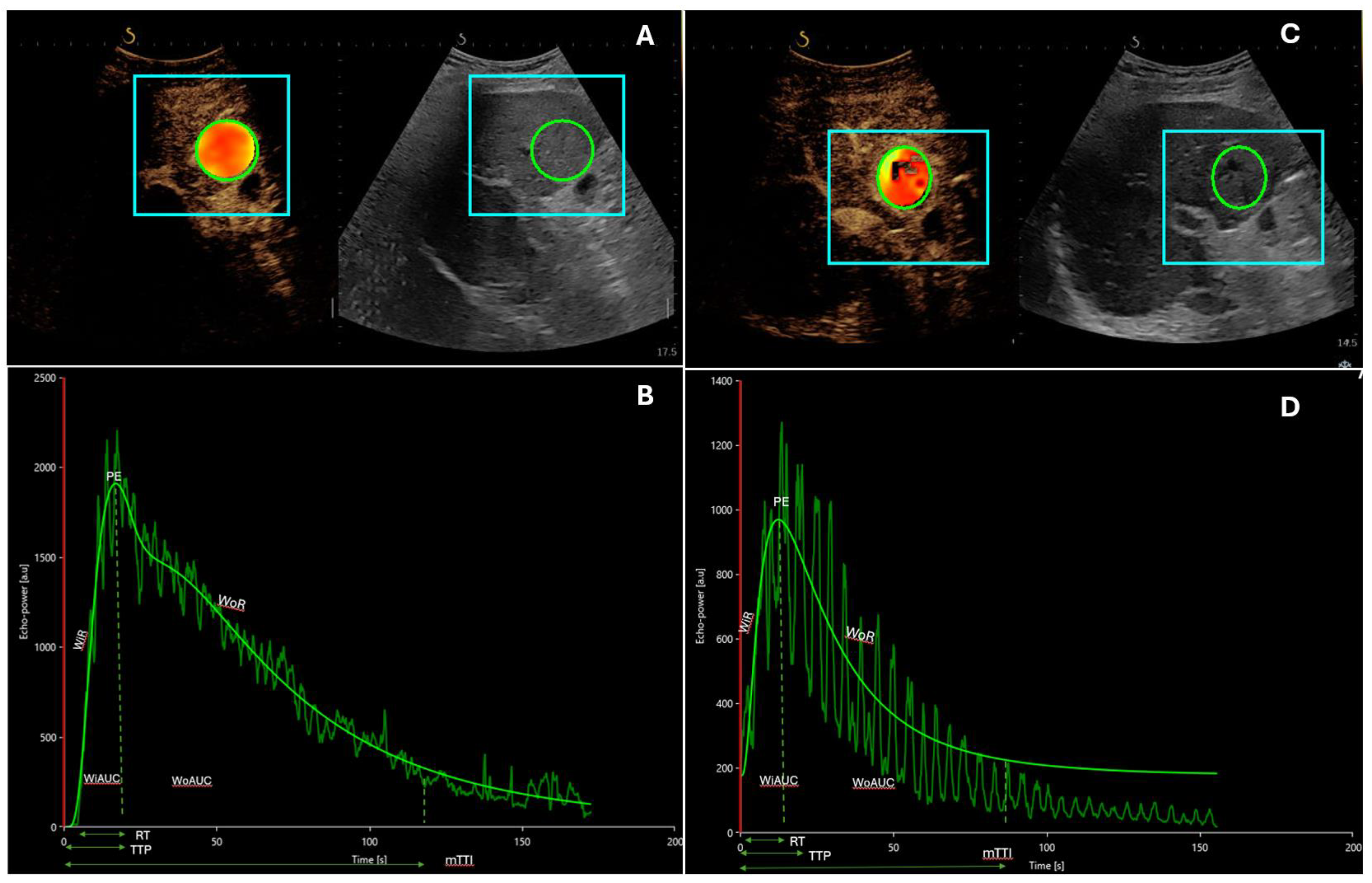
| Author | Patients (n) | US Contrast Agent | Therapy | |
|---|---|---|---|---|
| Frampas [27] | 16 3 | SonoVue®, Bracco, Italy | Sorafenib Sunitinib | Opposite modifications in non-progressors and progressors: mean AUC −38.3 vs. 436.3% (p = 0.06); mean AUCWI −37.5 vs. 1107.3% (p = 0.13); mean AUCWO −37.9 vs. 377.8% (p = 0.05). D-CEUS at one month: non-progressors: decrease in AUC > 40%; progressors: decrease in AUC < 40%; p = 0.015. |
| Knieling [43] | 9 10 | SonoVue®, Bracco, Italy | Sorafenib TACE | Increase: Pw → baseline 11.28 s ± 2.03 s (1.00); after 1 month 13.60 s ± 1.52 s (1.53 ± 0.08; p = 0.0405); after 3 months 16.17 s ± 2.35 s (1.46 ± 0.07; p = 0.0071). |
| Knieling [36] | 1 | NA | Sorafenib | Increase: mTT → baseline: 11.04 s; after 3 months: 17.48 s; after 5 months: 26.60 s); Pw → baseline: 8.83 s; after 3 months: 12.32 s; after 5 months: 15.25 s). |
| Lassau [25] | 42 | SonoVue®, Bracco, Italy | Bevacizumab | Pw reduction at day 3 revealed a trend of correspondence with PFS (p = 0.028) |
| Lassau [28] | 539 (107 HCC) | SonoVue®, Bracco, Italy | Bevacizumab (for HCC) | Changes in AUC (baseline → day 30): related to freedom from progression (p = 0.00002) |
| Lo [26] | 15 | Definity®; Lantheus, USA (perflutren lipid microspheres) | Axitinib | Median OS: 7.1 months (1.8–27.3 mo; 95% confidence interval [CI]: 0, 14.270)—(p = 0.050) Median PFS: 3.6 months (1.8–17.4 mo; 95% CI: 2.085, 5.115)—(p = 0.310) No significant association with DCE-US quantitative parameters |
| Sugimoto [44] | 37 16: intermediate HCC 21: advanced HCC | Sonazoid®; Daiichi-Sankyo, Japan | Sorafenib | Differences responders versus non-responder (from baseline to day-14): AUC: 0.66 [0.26, 0.82] vs. 1.33 [1.11, 3.65], p = 0.0095; AUCWI: 0.64 [0.27, 0.87] vs. 1.81 [1.11, 3.23], p = 0.0016; AUCWO: (0.68 [0.26, 0.86] vs. 1.26 [0.62, 3.58], p = 0.0177; PI: 0.68 [0.34, 0.88] vs. 1.62 [1.02, 2.26], p = 0.0211. Differences in responders versus non-responders (from baseline to day 28): AUCWI: 0.51 [0.32, 0.89] vs. 1.55 [1.17, 2.75], p = 0.0222; PI: 0.79 [0.39, 1.00] vs. 1.13 [0.66, 2.32], p = 0.1294. |
| Zocco [34] | 28 | SonoVue®, Bracco, Italy | Sorafenib | Mean overall survival (OS): responders > non-responders (382 versus 158 days; p = 0.003) Decrease at 15 and 30 days: peak-intensity (PI; p < 0.001), time to PI (Pw; p = 0.003), area under the curve (AUC; p = 0.002) Correlation between performance free survival (PFS), Pw, Tp, AUC |
| Study | Method | Findings |
|---|---|---|
| Sparchez [47] | DCE-US | Role in early assessment of tumor response to TACE. Early evaluation of disease burden after TACE. Possible superiority over CT. |
| Uller [48] | DCE-US (before and after DEB-TACE) | Peri-interventional instrument for the identification of extrahepatic tumor-feeding arteries. Early evaluation of treatment response. |
| Moschouris [49] | CEUS | Evaluation of treatment response after TACE. Elevated concordance with traditional imaging. Correlation with clinical outcomes. |
| Wiggermann [50] | DCE-US | Applied in a feasibility study for microcirculation changes after DSM-TACE. Reduction in post-TACE perfusion, regional blood flow, and blood volume. Transient occlusion leading to temporary storage of cytostatic agent in the targeted lesion. |
| Cao [51] | 3D CEUS | Analyzed microperfusional changes before and after TACE. Significant reduction in AUC, AUC during washout, and PE in responders. Potential future importance in early quantitative assessment of microvascularization changes after TACE. |
| Nam [42] | 2D CEUS 3D CEUS | Compared dynamic 2D and 3D CEUS at different time points after TACE. The 3D-CEUS demonstrated more pronounced changes in responders during follow-up. Good agreement between 2D-CEUS and 3D-CEUS and MRI at 1 month. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrito, L.; Ainora, M.E.; Cuccia, G.; Galasso, L.; Mignini, I.; Esposto, G.; Garcovich, M.; Riccardi, L.; Gasbarrini, A.; Zocco, M.A. Dynamic Contrast-Enhanced Ultrasound in the Prediction of Advanced Hepatocellular Carcinoma Response to Systemic and Locoregional Therapies. Cancers 2024, 16, 551. https://doi.org/10.3390/cancers16030551
Cerrito L, Ainora ME, Cuccia G, Galasso L, Mignini I, Esposto G, Garcovich M, Riccardi L, Gasbarrini A, Zocco MA. Dynamic Contrast-Enhanced Ultrasound in the Prediction of Advanced Hepatocellular Carcinoma Response to Systemic and Locoregional Therapies. Cancers. 2024; 16(3):551. https://doi.org/10.3390/cancers16030551
Chicago/Turabian StyleCerrito, Lucia, Maria Elena Ainora, Giuseppe Cuccia, Linda Galasso, Irene Mignini, Giorgio Esposto, Matteo Garcovich, Laura Riccardi, Antonio Gasbarrini, and Maria Assunta Zocco. 2024. "Dynamic Contrast-Enhanced Ultrasound in the Prediction of Advanced Hepatocellular Carcinoma Response to Systemic and Locoregional Therapies" Cancers 16, no. 3: 551. https://doi.org/10.3390/cancers16030551
APA StyleCerrito, L., Ainora, M. E., Cuccia, G., Galasso, L., Mignini, I., Esposto, G., Garcovich, M., Riccardi, L., Gasbarrini, A., & Zocco, M. A. (2024). Dynamic Contrast-Enhanced Ultrasound in the Prediction of Advanced Hepatocellular Carcinoma Response to Systemic and Locoregional Therapies. Cancers, 16(3), 551. https://doi.org/10.3390/cancers16030551







