Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Design
2.2. Treatment and Outcome Definition
2.3. Covariates
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Statistics. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast (accessed on 23 February 2023).
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Cohen, S.M.; Geradts, J.; Sun, X.; Khoury, T.; Bshara, W.; Zirpoli, G.R.; Miller, C.R.; Hwang, H.; Thorne, L.B.; et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiol. Biomark. Prev. 2016, 25, 470–478. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Castagnaviz, V.; Rinnerthaler, G.; Greil, R. Treatment Landscape for Patients with HER2-Positive Metastatic Breast Cancer: A Review on Emerging Treatment Options. Cancer Manag. Res. 2020, 12, 10615–10629. [Google Scholar] [CrossRef]
- Ahn, S.; Woo, J.W.; Lee, K.; Park, S.Y. HER2 status in breast cancer: Changes in guidelines and complicating factors for interpretation. J. Pathol. Transl. Med. 2020, 54, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- CADTH. CADTH Reimbursement Recommendation: Trastuzumab Deruxtecan; CADTH: Ottawa, ON, Canada, 2021. [Google Scholar]
- European Medicines Agency. Enhertu (Trastuzumab Deruxtecan): An Overview of Enhertu and Why It Is Authorised in the EU; European Medicines Agency: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Narayan, P.; Osgood, C.L.; Singh, H.; Chiu, H.-J.; Ricks, T.K.; Chow, E.C.Y.; Qiu, J.; Song, P.; Yu, J.; Namuswe, F.; et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 4478–4485. [Google Scholar] [CrossRef] [PubMed]
- Eiger, D.; Agostinetto, E.; Saúde-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Study of Trastuzumab Deruxtecan (T-DXd) vs. Investigator’s Choice Chemotherapy in HER2-Low, Hormone Receptor Positive, Metastatic Breast Cancer (DB-06). Available online: https://clinicaltrials.gov/ct2/show/NCT04494425 (accessed on 23 February 2023).
- A Study of MRG002 in the Treatment of Patients with HER2-Low Locally Advanced or Metastatic Breast Cancer (BC). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04742153?term=HER2+low&cond=Breast+Cancer&draw=2 (accessed on 23 February 2023).
- Cuthbert, C.A.; Watson, L.; Xu, Y.; Boyne, D.J.; Hemmelgarn, B.R.; Cheung, W.Y. Patient-Reported Outcomes in Alberta: Rationale, Scope, and Design of a Database Initiative. Curr. Oncol. 2019, 26, e503–e509. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, S.; Cheung, W.Y.; Bouchard-Fortier, A.; Dort, J.C.; Quan, H.; Buie, E.M.; McKinnon, G.; Quan, M.L. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Barcenas, C.H.; Song, J.; Murthy, R.K.; Raghavendra, A.S.; Li, Y.; Hsu, L.; Carlson, R.W.; Tripathy, D.; Hortobagyi, G.N. Prognostic Model for De Novo and Recurrent Metastatic Breast Cancer. JCO Clin. Cancer Inform. 2021, 5, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Eggemann, H.; Burger, E.; Fettke, F.; Costa, S.D.; Ignatov, A. Moderate level of HER2 expression and its prognostic significance in breast cancer with intermediate grade. Breast Cancer Res. Treat. 2015, 151, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, H.; Ignatov, T.; Burger, E.; Kantelhardt, E.J.; Fettke, F.; Thomssen, C.; Costa, S.D.; Ignatov, A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr. Relat. Cancer 2015, 22, 725–733. [Google Scholar] [CrossRef]
- Gilcrease, M.Z.; Woodward, W.A.; Nicolas, M.M.; Corley, L.J.; Fuller, G.N.; Esteva, F.J.; Tucker, S.L.; Buchholz, T.A. Even Low-level HER2 Expression May be Associated With Worse Outcome in Node-positive Breast Cancer. Am. J. Surg. Pathol. 2009, 33, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef]
- Hein, A.; Hartkopf, A.D.; Emons, J.; Lux, M.P.; Volz, B.; Taran, F.-A.; Overkamp, F.; Hadji, P.; Tesch, H.; Häberle, L.; et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur. J. Cancer 2021, 155, 1–12. [Google Scholar] [CrossRef]
- Malmgren, J.A.; Mayer, M.; Atwood, M.K.; Kaplan, H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res. Treat. 2018, 167, 579–590. [Google Scholar] [CrossRef]
- Fourquet, A.; Campana, F.; Zafrani, B.; Mosseri, V.; Vielh, P.; Durand, J.-C.; Vilcoq, J.R. Prognostic factors of breast recurrence in the conservative management of early breast cancer: A 25-year follow-up. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.; van Kampen, R.J.; Voogd, A.C.; Dercksen, M.W.; van den Berkmortel, F.; Smilde, T.J.; van de Wouw, A.J.; Peters, F.P.; van Riel, J.M.; Peters, N.A.; et al. Prognosis of metastatic breast cancer: Are there differences between patients with de novo and recurrent metastatic breast cancer? Br. J. Cancer. 2015, 112, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Uno, H.; Cronin, A.M.; Carroll, N.M.; Hornbrook, M.C.; Ritzwoller, D.P. Comparing Survival After Recurrent vs De Novo Stage IV Advanced Breast, Lung, and Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky024. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, J.; Kamigaki, S.; Fujita, J.; Osato, H.; Komoike, Y. The Difference in Prognostic Outcomes Between De Novo Stage IV and Recurrent Metastatic Patients with Hormone Receptor-positive, HER2-negative Breast Cancer. In Vivo 2018, 32, 353–358. [Google Scholar] [CrossRef] [PubMed]
- File, D.M.; Pascual, T.; Deal, A.M.; Wheless, A.; Perou, C.M.; Claire Dees, E.; Carey, L.A. Clinical subtype, treatment response, and survival in De Novo and recurrent metastatic breast cancer. Breast Cancer Res. Treat. 2022, 196, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jia, H.; Zhang, H.; Chen, L.; Zhao, P.; Zhao, J.; Fu, G.; Xing, X.; Li, Y.; Wang, C. Is HER2 ultra-low breast cancer different from HER2 null or HER2 low breast cancer? A study of 1363 patients. Breast Cancer Res. Treat. 2023, 202, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lan, A.; Dai, Y.; Jiang, L.; Liu, S. Comparison of the pCR Rate and DFS Among Breast Cancer Patients with Different Hormone Receptor and HER2 Statuses. Breast Cancer 2023, 15, 327–335. [Google Scholar] [CrossRef]

| Variable | Overall | Not Treated | Treated | p-Value |
|---|---|---|---|---|
| n | 965 | 190 | 775 | |
| Age (mean (SD)) | 64.01 (14.89) | 72.19 (14.13) | 62.00 (14.38) | <0.001 |
| Age Category (%) | <0.001 | |||
| (22, 40] | 62 (6.4) | suppressed | suppressed | |
| (40, 50] | 111 (11.5) | suppressed | suppressed | |
| (50, 60] | 204 (21.1) | 26 (13.7) | 178 (23.0) | |
| (60, 70] | 221 (22.9) | 34 (17.9) | 187 (24.1) | |
| (70, 80] | 216 (22.4) | 45 (23.7) | 171 (22.1) | |
| (80, 99] | 151 (15.6) | 70 (36.8) | 81 (10.5) | |
| Year of Diagnosis (%) | 0.010 | |||
| 2010 | 73 (7.6) | 25 (13.2) | 48 (6.2) | |
| 2011 | 77 (8.0) | 18 (9.5) | 59 (7.6) | |
| 2012 | 65 (6.7) | 16 (8.4) | 49 (6.3) | |
| 2013 | 90 (9.3) | 17 (8.9) | 73 (9.4) | |
| 2014 | 89 (9.2) | 20 (10.5) | 69 (8.9) | |
| 2015 | 90 (9.3) | 14 (7.4) | 76 (9.8) | |
| 2016 | 125 (13.0) | 19 (10.0) | 106 (13.7) | |
| 2017 | 124 (12.8) | 23 (12.1) | 101 (13.0) | |
| 2018 | 117 (12.1) | 12 (6.3) | 105 (13.5) | |
| 2019 | 115 (11.9) | 26 (13.7) | 89 (11.5) | |
| ER Status | <0.001 | |||
| negative | 133 (13.8) | 54 (28.4) | 79 (10.2) | |
| positive | 830 (86.0) | 136 (71.6) | 694 (89.5) | |
| missing | 2 (0.2) | 0 (0.0) | 2 (0.3) | |
| PR Status | <0.001 | |||
| negative | 278 (28.8) | 85 (44.7) | 193 (24.9) | |
| positive | 683 (70.8) | 105 (55.3) | 578 (74.6) | |
| missing | 4 (0.4) | 0 (0.0) | 4 (0.5) | |
| ER/PR Status | <0.001 | |||
| ER− PR− | 129 (13.4) | 53 (27.9) | 76 (9.8) | |
| ER+ PR+ | 679 (70.4) | 104 (54.7) | 575 (74.2) | |
| Single+ | 153 (15.9) | 33 (17.4) | 120 (15.5) | |
| missing | 4 (0.4) | 0 (0.0) | 4 (0.5) | |
| No. of Metastases (%) | 0.020 | |||
| 1 | 507 (52.5) | 87 (45.8) | 420 (54.2) | |
| 2 | 232 (24.0) | 44 (23.2) | 188 (24.3) | |
| 3 | 127 (13.2) | 28 (14.7) | 99 (12.8) | |
| 4+ | 92 (9.5) | 28 (14.7) | 64 (8.3) | |
| missing | 7 (0.7) | 3 (1.6) | 4 (0.5) | |
| Location of Metastases (%) | NA | NA | NA | NA |
| bone (%) | 661 (68.5) | 127 (66.8) | 534 (68.9) | 0.645 |
| lung (%) | 329 (34.1) | 72 (37.9) | 257 (33.2) | 0.251 |
| hepatic (%) | 216 (22.4) | 50 (26.3) | 166 (21.4) | 0.176 |
| lymph nodes (%) | 254 (26.3) | 45 (23.7) | 209 (27.0) | 0.407 |
| brain (%) | 26 (2.7) | 11 (5.8) | 15 (1.9) | 0.007 |
| adrenals (%) | 30 (3.1) | 13 (6.8) | 17 (2.2) | 0.002 |
| skin (%) | 33 (3.4) | suppressed | suppressed | 0.999 |
| peritoneum (%) | 37 (3.8) | 12 (6.3) | 25 (3.2) | 0.076 |
| other (%) | 101 (10.5) | 32 (16.8) | 69 (8.9) | 0.002 |
| Systemic Tx Institution (%) | <0.001 | |||
| academic | 658 (68.2) | 0 (0.0) | 658 (84.9) | |
| community | 117 (12.1) | 0 (0.0) | 117 (15.1) | |
| did not initiate systemic Tx | 190 (19.7) | 190 (100.0) | 0 (0.0) |
| Variable | Overall | Recurrent | De Novo | p-Value |
|---|---|---|---|---|
| n | 3413 | 2448 | 965 | |
| No. lines (median [IQR]) | 1.00 [0.00, 2.00] | 1.00 [0.00, 2.00] | 2.00 [1.00, 3.00] | |
| Initiated 1L (%) | 2460 (72.1) | 1685 (68.8) | 775 (80.3) | <0.001 |
| 1L regimens (%) | <0.001 | |||
| AI mono | 900 (36.6) | 583 (34.6) | 317 (40.9) | |
| Tamoxifen mono | 457 (18.6) | 375 (22.3) | 82 (10.6) | |
| Capecitabine mono | 265 (10.8) | 213 (12.6) | 52 (6.7) | |
| AI + Palbociclib | 207 (8.4) | 112 (6.6) | 95 (12.3) | |
| Taxane mono | 137 (5.6) | 62 (3.7) | 75 (9.7) | |
| Taxane + chemo | 119 (4.8) | 95 (5.6) | 24 (3.1) | |
| Chemo combo | 92 (3.7) | 71 (4.2) | 21 (2.7) | |
| FEC | 78 (3.2) | 20 (1.2) | 58 (7.5) | |
| Other chemo mono | 60 (2.4) | 52 (3.1) | <10 | |
| Other AI combo | 42 (1.7) | 19 (1.1) | 23 (3.0) | |
| AI + Everolimus | 40 (1.6) | 36 (2.1) | <10 | |
| Other | 32 (1.3) | 21 (1.2) | 11 (1.4) | |
| Fulvestrant | 18 (0.7) | 15 (0.9) | <10 | |
| Targeted mono | 13 (0.5) | 11 (0.7) | <10 | |
| Initiated 2L (%) | 1207 (49.1) | 690 (40.9) | 517 (66.7) | <0.001 |
| 2L Regimens (%) | <0.001 | |||
| AI mono | 287 (23.8) | 171 (24.8) | 116 (22.4) | |
| Capecitabine mono | 190 (15.7) | 113 (16.4) | 77 (14.9) | |
| Tamoxifen mono | 154 (12.8) | 87 (12.6) | 67 (13.0) | |
| Taxane mono | 135 (11.2) | 63 (9.1) | 72 (13.9) | |
| Other chemo mono | 99 (8.2) | 81 (11.7) | 18 (3.5) | |
| AI + Palbociclib | 82 (6.8) | 26 (3.8) | 56 (10.8) | |
| Chemo combo | 62 (5.1) | 40 (5.8) | 22 (4.3) | |
| AI + Everolimus | 56 (4.6) | 27 (3.9) | 29 (5.6) | |
| Other AI combo | 56 (4.6) | 23 (3.3) | 33 (6.4) | |
| Fulvestrant | 37 (3.1) | 26 (3.8) | 11 (2.1) | |
| Taxane + chemo | 25 (2.1) | 19 (2.8) | <10 | |
| Targeted mono | 17 (1.4) | <10 | <10 | |
| Other | <10 | <10 | <10 | |
| Initiated 3L (%) 3L | 682 (56.5) | 343 (49.7) | 339 (65.6) | <0.001 |
| Regimens (%) | <0.001 | |||
| Capecitabine mono | 136 (19.9) | 59 (17.2) | 77 (22.7) | |
| AI mono | 109 (16.0) | 54 (15.7) | 55 (16.2) | |
| Taxane mono | 76 (11.1) | 39 (11.4) | 37 (10.9) | |
| Tamoxifen mono | 60 (8.8) | 25 (7.3) | 35 (10.3) | |
| Other chemo mono | 58 (8.5) | 33 (9.6) | 25 (7.4) | |
| Eribulin mono | 48 (7.0) | 42 (12.2) | <10 | |
| Chemo combo | 47 (6.9) | 25 (7.3) | 22 (6.5) | |
| Fulvestrant | 43 (6.3) | 17 (5.0) | 26 (7.7) | |
| Other AI combo | 34 (5.0) | 19 (5.5) | 15 (4.4) | |
| AI + Evrolimus | 26 (3.8) | <10 | 18 (5.3) | |
| AI + Palbociclib | 26 (3.8) | <10 | 18 (5.3) | |
| Other | 19 (2.8) | 14 (4.1) | <10 | |
| Initiated 4L (%) | 391 (57.3) | 175 (51.0) | 216 (63.7) | 0.001 |
| 4L regimens (%) | 0.036 | |||
| AI mono or combo | 79 (20.2) | 28 (16.0) | 51 (23.6) | |
| Taxane mono | 66 (16.9) | 34 (19.4) | 32 (14.8) | |
| Other chemo mono | 60 (15.3) | 30 (17.1) | 30 (13.9) | |
| Capecitabine mono | 51 (13.0) | 17 (9.7) | 34 (15.7) | |
| Eribulin mono | 43 (11.0) | 26 (14.9) | 17 (7.9) | |
| Chemo combo | 32 (8.2) | 18 (10.3) | 14 (6.5) | |
| Other | 31 (7.9) | 11 (6.3) | 20 (9.3) | |
| Fulvestrant | 29 (7.4) | 11 (6.3) | 18 (8.3) | |
| Initiated 5L (%) | 224 (57.3) | 90 (51.4) | 134 (62.0) | 0.045 |
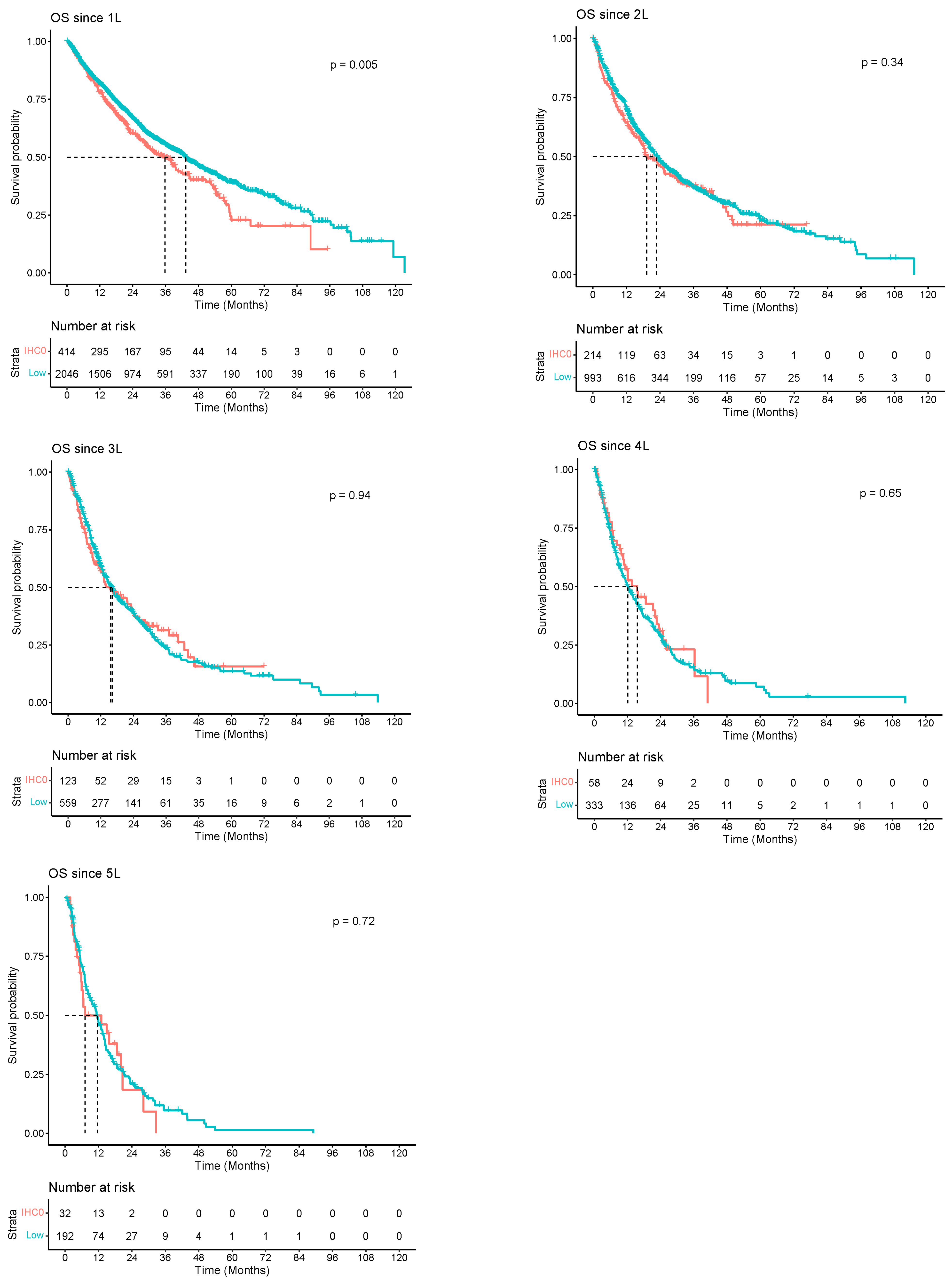
| Time Zero | Median Overall Survival, Months (95% CI) | 1-Year Overall Survival (95% CI) | 2-Year Overall Survival (95% CI) | 5-Year Overall Survival (95% CI) |
|---|---|---|---|---|
| diagnosis | 28.2 (26.0–30.7) | 0.7291 (0.7016–0.7577) | 0.5586 (0.5276–0.5914) | 0.2426 (0.2122–0.2773) |
| 1L | 42.2 (38.5–44.5) | 0.8109 (0.7952–0.8269) | 0.6568 (0.6369–0.6773) | 0.3715 (0.3440–0.4013) |
| 2L | 22.7 (20.3–24.8) | 0.6945 (0.6682–0.7219) | 0.4770 (0.4472–0.5088) | 0.2274 (0.1950–0.2652) |
| 3L | 15.9 (13.9–18.1) | 0.5875 (0.5500–0.6276) | 0.3891 (0.3502–0.4322) | 0.1399 (0.1048–0.1867) |
| 4L | 12.2 (10.6–15.1) | 0.5102 (0.4603–0.5655) | 0.2902 (0.2434–0.3461) | 0.0679 (0.0364–0.1267) |
| 5L | 11.5 (8.8–13.4) | 0.4735 (0.4086–0.5488) | 0.2094 (0.1559–0.2812) | 0.0127 (0.0019–0.0847) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farah, E.; Carbonell, C.; Boyne, D.J.; Brenner, D.R.; Henning, J.-W.; Moldaver, D.; Shokar, S.; Cheung, W.Y. Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer. Cancers 2024, 16, 518. https://doi.org/10.3390/cancers16030518
Farah E, Carbonell C, Boyne DJ, Brenner DR, Henning J-W, Moldaver D, Shokar S, Cheung WY. Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer. Cancers. 2024; 16(3):518. https://doi.org/10.3390/cancers16030518
Chicago/Turabian StyleFarah, Eliya, Chantelle Carbonell, Devon J. Boyne, Darren R. Brenner, Jan-Willem Henning, Daniel Moldaver, Simran Shokar, and Winson Y. Cheung. 2024. "Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer" Cancers 16, no. 3: 518. https://doi.org/10.3390/cancers16030518
APA StyleFarah, E., Carbonell, C., Boyne, D. J., Brenner, D. R., Henning, J.-W., Moldaver, D., Shokar, S., & Cheung, W. Y. (2024). Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer. Cancers, 16(3), 518. https://doi.org/10.3390/cancers16030518






