Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dietary Supplements
2.2. Animal Experiment
- -
- CON and CONplus—control groups without diet supplementation, fed a standard diet and water ad libitum,
- -
- M and Mplus—animals fed a standard diet supplemented with 1% aqueous extract of bitter melon dried fruits (BME) ad libitum,
- -
- G and Gplus—animals were fed the standard diet and water ad libitum and were given 0.15 mL/d PSO via intragastric gavage,
- -
- GM and GMplus—animals were fed the standard diet and were supplemented with both 0.15 mL/d PSO via intragastric gavage and 1% BME ad libitum.
2.3. Fatty Acids Analysis by Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
2.4. Determination of CFA Content Using Ag+-HPLC-DAD
2.5. Calculation of Indices in Spleen Samples
2.6. Statistical Analysis
3. Results
3.1. FA Profile of Rats’ Spleen
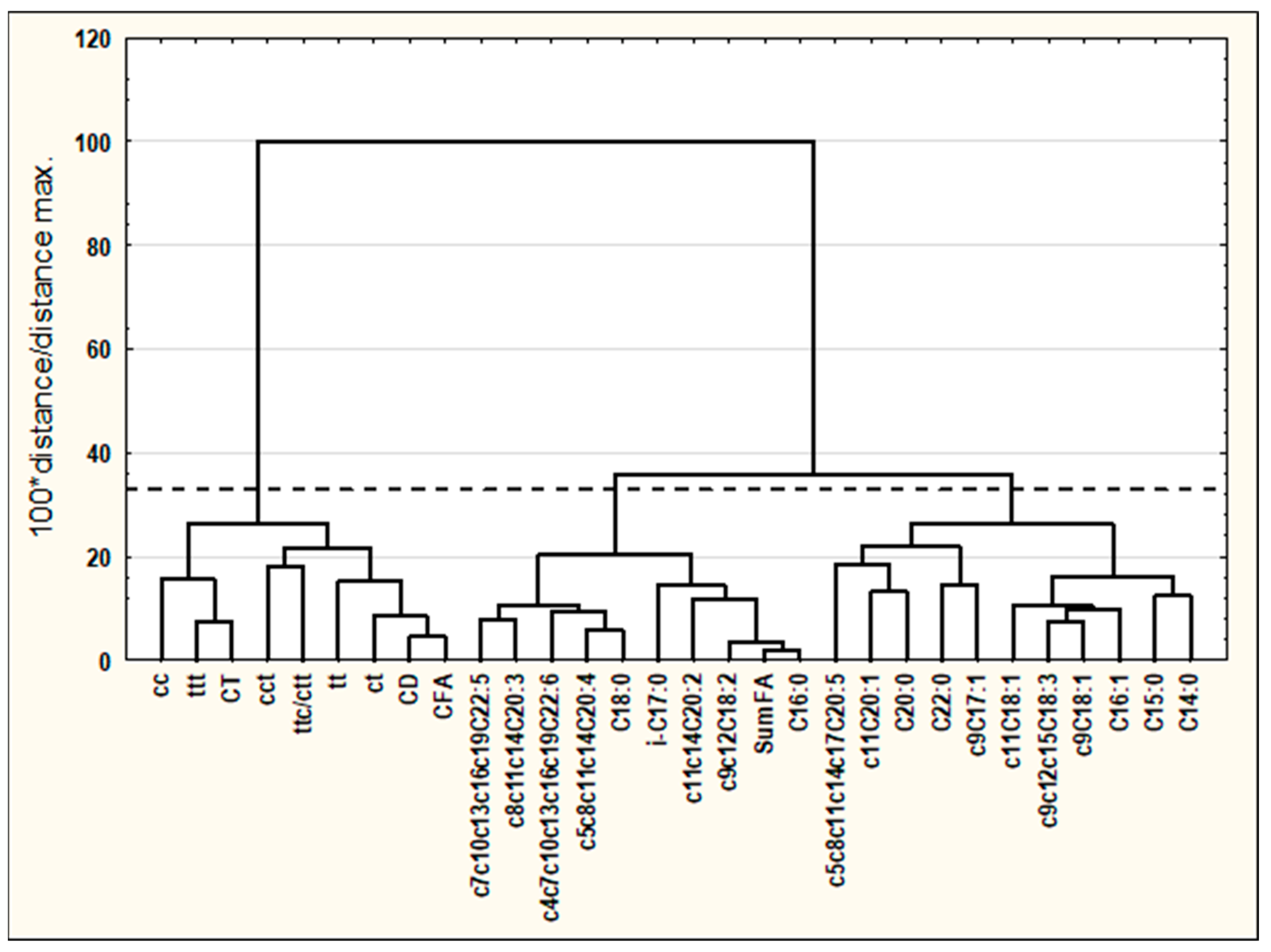
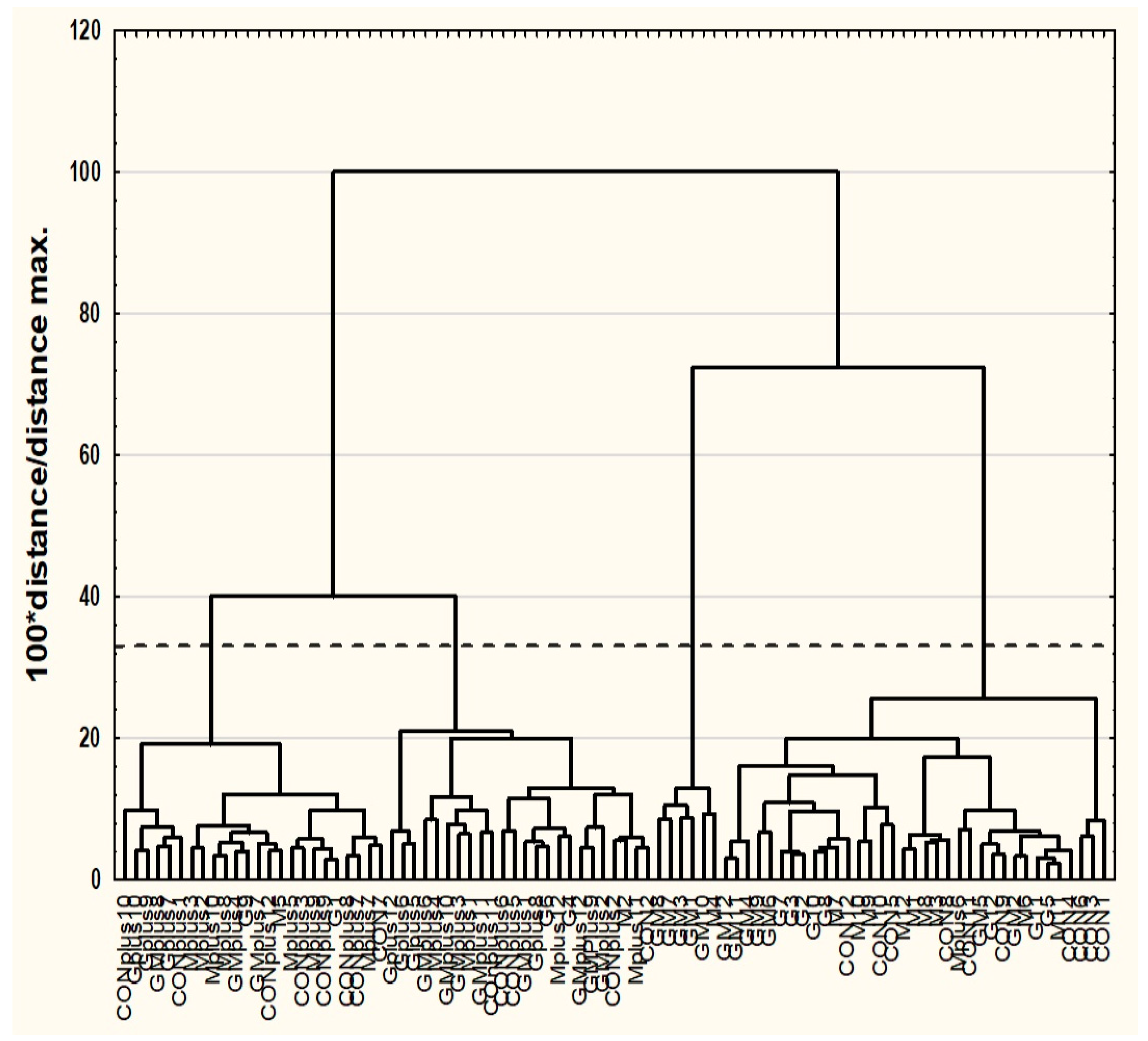
3.2. Cluster Analysis (CA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnum, K.J.; O’Connell, M.J. Cell Cycle Regulation by Checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [CrossRef]
- Mustafa, M.; Abbas, K.; Alam, M.; Ahmad, W.; Moinuddin; Usmani, N.; Siddiqui, S.A.; Habib, S. Molecular Pathways and Therapeutic Targets Linked to Triple-Negative Breast Cancer (TNBC). Mol. Cell. Biochem. 2023. ahead of print. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hoy, A.J.; Nagarajan, S.R.; Butler, L.M. Tumor Fatty Acid Metabolism in the Context of Therapy Resistance and Obesity. Nat. Rev. Cancer 2021, 21, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Ann, D.K. When Fats Commit Crimes: Fatty Acid Metabolism, Cancer Stemness and Therapeutic Resistance. Cancer Commun. 2018, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Lorito, N.; Smiriglia, A.; Morandi, A. Fat and Furious: Lipid Metabolism in Antitumoral Therapy Response and Resistance. Trends Cancer 2021, 7, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and Function of the Spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Hao, S.; Fang, H.; Fang, S.; Zhang, T.; Zhang, L.; Yang, L. Changes in Nuclear Factor Kappa B Components Expression in the Ovine Spleen during Early Pregnancy. J. Anim. Feed Sci. 2022, 31, 3–11. [Google Scholar] [CrossRef]
- Gunes, O.; Turgut, E.; Bag, Y.M.; Gundogan, E.; Gunes, A.; Sumer, F. The Impact of Splenectomy on Human Lipid Metabolism. Upsala J. Med. Sci. 2022, 127, e8500. [Google Scholar] [CrossRef]
- Ai, X.M.; Ho, L.C.; Han, L.L.; Lu, J.J.; Yue, X.; Yang, N.Y. The Role of Splenectomy in Lipid Metabolism and Atherosclerosis (AS). Lipids Health Dis. 2018, 17, 186. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A. Fine-Tuning the Lipogenic/Lipolytic Balance to Optimize the Metabolic Requirements of Cancer Cell Growth: Molecular Mechanisms and Therapeutic Perspectives. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging Roles of Lipid Metabolism in Cancer Metastasis. Mol. Cancer 2017, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Adipocyte and Lipid Metabolism in Cancer Drug Resistance. J. Clin. Investig. 2019, 129, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, L.; Eisenberg-Bord, M.; Eisenberg-Lerner, A.; Sagi-Eisenberg, R. Metabolic Alterations in the Tumor Microenvironment and Their Role in Oncogenesis. Cancer Lett. 2020, 484, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, J. The Expanded Role of Fatty Acid Metabolism in Cancer: New Aspects and Targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Teryks, M.; Tokarz, A. Sprzężone Trieny Kwasu Linolenowego (Conjugated Linolenic Acid—CLnA, Super CLA)—Źródła i Działanie Biologiczne. Postepy Hig. Med. Dosw. 2014, 68, 1238–1250. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Keding, G.B. Bitter Gourd (Momordica charantia): A Dietary Approach to Hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- Peng, Y. Comparative Analysis of the Biological Components of Pomegranate Seed from Different Cultivars. Int. J. Food Prop. 2019, 22, 784–794. [Google Scholar] [CrossRef]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Antihyperglycemic Effects of Three Extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Yoshime, L.T.; Louise, I.; De Melo, P.; Augusto, J.; Sattler, G. Nutrire Bitter Gourd (Momordica charantia L.) Seed Oil as a Naturally Rich Source of Bioactive Compounds for Nutraceutical Purposes. Nutrire 2016, 41, 12. [Google Scholar] [CrossRef]
- Carvalho, A.; Moreira, M.M.; Deleure-Matos, C.; Gomes, A.; Freitas, A.; Grosso, C. Valorization of Lipid By-Product. In Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128173725. [Google Scholar]
- Ren, Q.; Yang, B.; Zhu, G.; Wang, S.; Fu, C.; Zhang, H.; Paul Ross, R.; Stanton, C.; Chen, H.; Chen, W. Antiproliferation Activity and Mechanism of C9, T11, C15-CLNA and T9, T11, C15-CLNA from Lactobacillus Plantarum ZS2058 on Colon Cancer Cells. Molecules 2020, 25, 1225. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Białek, M.; Lepionka, T.; Tober, E.; Czauderna, M. The Quality Determination of Selected Commercial Online Purchased Edible Pomegranate Seed Oils With New Argentometric Liquid Chromatography Method. J. Diet. Suppl. 2020, 18, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Lepionka, T.; Białek, A.; Białek, M.; Czauderna, M.; Stawarska, A.; Wrzesień, R.; Bielecki, W.; Paśko, P.; Galanty, A.; Bobrowska-Korczak, B. Mammary Cancer Risk and Serum Lipid Profile of Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract. Prostaglandins Other Lipid Mediat. 2019, 142, 33–45. [Google Scholar] [CrossRef]
- Białek, M.; Czauderna, M.; Białek, A. Partial Replacement of Rapeseed Oil with Fish Oil, and Dietary Antioxidants Supplementation Affects Concentrations of Biohydrogenation Products and Conjugated Fatty Acids in Rumen and Selected Lamb Tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Korniluk, K.; Wasowska, I. Improved Saponification Then Mild Base and Acid-Catalyzed Methylation Is a Useful Method for Quantifying Fatty Acids, with Special Emphasis on Conjugated Dienes. Acta Chromatogr. 2007, 18, 59–71. [Google Scholar]
- Czauderna, M.; Kowalczyk, J.; Marounek, M.; Mlchalski, J.P.; Rozbicka-Wieczorek, A.J.; Krajewska, K.A. A New Internal Standard for HPLC Assay of Conjugated Linoleic Acid in Animal Tissues and Milk. Czech J. Anim. Sci. 2011, 56, 23–29. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Wojtak, W.; Czauderna, M. Organic and Inorganic Selenium Compounds Affected Lipidomic Profile of Spleen of Lambs Fed with Diets Enriched in Carnosic Acid and Fish Oil. Animals 2023, 14, 133. [Google Scholar] [CrossRef]
- Catalano, O.A.; Soricelli, A.; Salvatore, M. Spleen Anatomy, Function and Development. In Abdominal Imaging; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642133275, pp. 1479–1494. ISBN 9783642133275. [Google Scholar]
- Sajdikova, M.; Novakova, L. 5. Spleen—Functions of Cells and Human Body. Available online: https://fblt.cz/en/skripta/v-krev-a-organy-imunitniho-systemu/5-slezina/ (accessed on 31 August 2023).
- Nayak, B.N.; Buttar, H.S. Functions of Spleen in Health and Disease. J. Pharm. Sci. Technol. Manag. 2015, 1, 1–7. [Google Scholar]
- Karakuchi, N.; Yanagawa, S.; Takei, D.; Kodama, S.; Takeshima, Y.; Sumimoto, K. A Case of Peritoneal Dissemination and Splenic Metastasis after Gastric Cancer Surgery That Could Be Controlled with Multidisciplinary Treatment. Case Rep. Oncol. 2020, 13, 1164–1170. [Google Scholar] [CrossRef]
- Di Grande, A.; Peirs, S.; Donovan, P.D.; van Trimpont, M.; Morscio, J.; Lintermans, B.; Reunes, L.; Vandamme, N.; Goossens, S.; Nguyen, H.A.; et al. The Spleen as a Sanctuary Site for Residual Leukemic Cells Following ABT-199 Monotherapy in ETP-ALL. Blood Adv. 2021, 5, 1963–1976. [Google Scholar] [CrossRef] [PubMed]
- Velu, U.; Shetty, P.S.; Sharan, K.; Salins, S.; Singh, A. Spleen as an Organ at Risk in Adjuvant Chemoradiotherapy for Gastric Cancer: A Retrospective Dosimetric Study. J. Radiother. Pract. 2023, 22, e57. [Google Scholar] [CrossRef]
- El Hadad, S.; Al Rowily, E.; Aldahlawi, A.; Alrahimi, J.; Hassoubah, S. The Role of P53 and K-Ras in Regulating Spleen Innate Mediators in Mice with Colon Cancer. Pharmacophore 2021, 12, 19–27. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Gridley, G.; Hoover, R.N.; Check, D.; Landgren, O. Long-Term Risks after Splenectomy among 8,149 Cancer-Free American Veterans: A Cohort Study with up to 27 Years Follow-Up. Haematologica 2014, 99, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.; Akbar Saboor-Yaraghi, A. Spleen in Innate and Adaptive Immunity Regulation. AIMS Allergy Immunol. 2021, 5, 1–17. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of Tumor-Associated Macrophages and Neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Stöth, M.; Freire Valls, A.; Chen, M.; Hidding, S.; Knipper, K.; Shen, Y.; Klose, J.; Ulrich, A.; de Almodovar, C.R.; Schneider, M.; et al. Splenectomy Reduces Lung Metastases and Tumoral and Metastatic Niche Inflammation. Int. J. Cancer 2019, 145, 2509–2520. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Czauderna, M. Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically-Induced Mammary Tumors—Preliminary Study. Nutrients 2019, 11, 2032. [Google Scholar] [CrossRef]
- Białek, A.; Białek, M.; Lepionka, T.; Pachniewicz, P.; Czauderna, M. Oxysterols and Lipidomic Profile of Myocardium of Rats Supplemented with Pomegranate Seed Oil and/or Bitter Melon Aqueous Extract—Cardio-Oncological Animal Model Research. Chem. Phys. Lipids 2021, 235, 105057. [Google Scholar] [CrossRef]
- Lepionka, T.; Białek, M.; Czauderna, M.; Białek, A. Pomegranate Seed Oil and Bitter Melon Extract Supplemented in Diet Influence the Lipid Profile and Intensity of Peroxidation in Livers of SPRD Rats Exposed to a Chemical Carcinogen. Prostaglandins Other Lipid Mediat. 2021, 152, 106495. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Yamagishi, M.; Okazaki, K.; Umemura, T.; Imazawa, T.; Nishikawa, A.; Matsumoto, W.; Hirose, M. Lack of Chemopreventive Effects of α-Eleostearic Acid on 7,12-Dimethylbenz[a]Anthracene (DMBA) and 1,2-Dimethylhydrazine (DMH)-Induced Mammary and Colon Carcinogenesis in Female Sprague-Dawley Rats. Food Chem. Toxicol. 2006, 44, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fielding, B.A.; Calder, P.C.; Irvine, N.A.; Miles, E.A.; Lillycrop, K.A.; von Gerichten, J.; Burdge, G.C. How Does Polyunsaturated Fatty Acid Biosynthesis Regulate T-Lymphocyte Function? Nutr. Bull. 2019, 44, 350–355. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Sözener, Z.Ç.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.M.; Newsholme, E.A.; Calder, P.C. Level of Polyunsaturated Fatty Acids and the n-6 to n-3 Polyunsaturated Fatty Acid Ratio in the Rat Diet Alter Serum Lipid Levels and Lymphocyte Functions. In Prostaglandins, Leukotrienes and Essential Fatty Acids; Elsevier: Amsterdam, The Netherlands, 1997; Volume 57. [Google Scholar]
- West, A.L.; von Gerichten, J.; Irvine, N.A.; Miles, E.A.; Lillycrop, K.A.; Calder, P.C.; Fielding, B.A.; Burdge, G.C. Fatty Acid Composition and Metabolic Partitioning of α-Linolenic Acid Are Contingent on Life Stage in Human CD3+ T Lymphocytes. Front. Immunol. 2022, 13, 1079642. [Google Scholar] [CrossRef] [PubMed]
- Čolić, M.; Bekić, M.; Tomić, S.; Ðokić, J.; Radojević, D.; Šavikin, K.; Miljuš, N.; Marković, M.; Škrbić, R. Immunomodulatory Properties of Pomegranate Peel Extract in a Model of Human Peripheral Blood Mononuclear Cell Culture. Pharmaceutics 2022, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Ray, R.B. Bitter Melon (Momordica charantia), a Nutraceutical Approach for Cancer Prevention and Therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Ablimit, A.; Yu, Y.; Jin, X.; Li, J.-S. Effect of Momordica charantia Polysaccharide on Immunomodulatory Activity in Mice. Exp. Ther. Med. 2023, 26, 307. [Google Scholar] [CrossRef]
- Fachinan, R.; Fagninou, A.; Nekoua, M.P.; Amoussa, A.M.; Adjagba, M.; Lagnika, L.; Lalèyè, A.; Moutairou, K.; Yessoufou, A. Evidence of Immunosuppressive and Th2 Immune Polarizing Effects of Antidiabetic Momordica charantia Fruit Juice. Biomed. Res. Int. 2017, 2017, 9478048. [Google Scholar] [CrossRef]
- Stefanou, V.; Papatheodorou, S.; Tsakni, A.; Lougovois, V. Anti-Inflammatory Properties of Pomegranate. Int. J. Adv. Res. Microbiol. Immunol. 2020, 2, 1–13. [Google Scholar]
- Hamouda, A.F.; Felemban, S. Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study. Molecules 2023, 28, 903. [Google Scholar] [CrossRef] [PubMed]
- de O Trovão, L.; Dos S Rodrigues, L.; Mendes, P.M.; Alves, P.C.S.; da S Oliveira, A.; Brito, J.M.; Vale, A.A.M.; Dimitrius, D.V.; Simão, G.; Dos Santos, A.P.S.A.; et al. The Immunomodulatory Activity of Punica granatum L. Peel Extract Increases the Lifespan of Mice with Lethal Sepsis. J. Immunol. Res. 2023, 2023, 2868707. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid Metabolism in the Rumen: New Insights on Lipolysis and Biohydrogenation with an Emphasis on the Role of Endogenous Plant Factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Lepionka, T.; Paśko, P.; Galanty, A.; Tokarz, A.; Czauderna, M. Punica granatum (Pomegranate) Seed Oil and Momordica charantia (Bitter Melon) Extract Affect the Lipid’s Profile and Oxidative Stability of Femoral Muscles of Rats. Eur. J. Lipid Sci. Technol. 2019, 121, 1800420. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Czauderna, M. Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors. Animals 2020, 10, 464. [Google Scholar] [CrossRef]
- Stawarska, A.; Białek, A.; Stanimirova, I.; Stawarski, T.; Tokarz, A. The Effect of Conjugated Linoleic Acids (CLA) Supplementation on the Activity of Enzymes Participating in the Formation of Arachidonic Acid in Liver Microsomes of Rats—Probable Mechanism of CLA Anticancer Activity. Nutr. Cancer 2015, 67, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Stawarska, A.; Bodecka, J.; Białek, M.; Tokarz, A. Pomegranate Seed Oil Influences the Fatty Acids Profile and Reduces the Activity of Desaturases in Livers of Sprague-Dawley Rats. Prostaglandins Other Lipid Mediat. 2017, 131, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Belury, M.A. Conjugated Linoleic Acid Reduces Arachidonic Acid Content and PGE2 Synthesis in Murine Keratinocytes. Cancer Lett. 1998, 127, 15–22. [Google Scholar] [CrossRef]
- Kucharski, M.; Kaczor, U. Desaturaza Stearylo-CoA—Regulatormetabolizmu Lipidów. Postepy Hig. Med. Dosw. 2014, 68, 334–342. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.; Park, S.S.; Chang, C.; Kim, E. Stearoyl CoA Desaturase (SCD) Facilitates Proliferation of Prostate Cancer Cells through Enhancement of Androgen Receptor Transactivation. Mol. Cells 2011, 31, 371–377. [Google Scholar] [CrossRef]
- Son, Y.M.; Cheon, I.S.; Goplen, N.P.; Dent, A.L.; Sun, J. Inhibition of Stearoyl-CoA Desaturases Suppresses Follicular Help T- and Germinal Center B- Cell Responses. Eur. J. Immunol. 2020, 50, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, X.; Li, C.; Li, Y.; Ye, Z.; Shapiro, V.S.; Copland, J.A.; Hitosugi, T.; Bernlohr, D.A.; Sun, J.; et al. Stearoyl-CoA Desaturase-Mediated Monounsaturated Fatty Acid Availability Supports Humoral Immunity. Cell Rep. 2021, 34, 108601. [Google Scholar] [CrossRef] [PubMed]
- Ronaldo Alberti, L.; Magalhães Veloso, D.F.; de Souza Vasconcellos, L.; Petroianu, A. Is There a Relationship between Lipids Metabolism and Splenic Surgeries? Acta Cir. Bras. 2012, 27, 751–756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wysocki, A.; Drożdż, W.; Dolecki, M. Spleen and Lipids Metabolism-Is There Any Correlation? Med. Sci. Monit. 1999, 5, 524–527. [Google Scholar]
- Grajchen, E.; Loix, M.; Baeten, P.; Côrte-Real, B.F.; Hamad, I.; Vanherle, S.; Haidar, M.; Dehairs, J.; Broos, J.Y.; Ntambi, J.M.; et al. Fatty Acid Desaturation by Stearoyl-CoA Desaturase-1 Controls Regulatory T Cell Differentiation and Autoimmunity. Cell. Mol. Immunol. 2023, 20, 666–679. [Google Scholar] [CrossRef]
- Czumaj, A.; Śledziński, T. Biological Role of Unsaturated Fatty Acid Desaturases in Health and Disease. Nutrients 2020, 12, 356. [Google Scholar] [CrossRef]
- Tarantino, G.; Scalera, A.; Finelli, C. Liver-Spleen Axis: Intersection between Immunity, Infections and Metabolism. World J. Gastroenterol. 2013, 19, 3534–3542. [Google Scholar] [CrossRef]

| Fatty Acids (µg/g) | CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p Value |
|---|---|---|---|---|---|---|---|---|---|
| C14:0 | 182.0 ± 69.1 | 228.8 ± 72.5 a,b | 130.0 ± 47.3 a | 259.2 ± 145.9 c | 172.9 ± 100.4 | 123.1 ± 37.2 b,c | 159.5 ± 71.4 | 211.7 ± 125.9 | 0.0020 |
| C15:0 | 96.5 ± 44.5 | 109.0 ± 25.2 a | 82.8 ± 28.4 | 114.5 ± 46.8 | 81.0 ± 27.4 | 64.0 ± 20.0 a | 73.6 ± 35.9 | 99.9 ± 21.2 | 0.0085 |
| C16:0 | 5839 ± 1302 a | 6129 ± 1617 b,c | 5374 ± 982 d | 8537 ± 2614 e,f,g,h | 3445 ± 713 a,b,d,e | 4021 ± 951 c,f | 4219 ± 1919 g | 4469 ± 856 h | <0.0001 |
| i-C17:0 | 128.5 ± 56.2 a | 103.7 ± 37.0 | 99.5 ± 25.8 | 134.3 ± 53.1 b | 66.1 ± 31.3 a,b | 79.3 ± 33.3 | 76.7 ± 29.6 | 96.7 ± 28.9 | 0.0018 |
| C18:0 | 3830 ± 1118 | 4572 ± 1416 a,b,c,d | 3970 ± 507 e | 5919 ± 1377 f,g,h,i | 2683 ± 526 a,f | 2666 ± 393 b,e,g | 2614 ± 757 c,h | 2872 ± 519 d,i | <0.0001 |
| C20:0 | 30.6 ± 13.5 | 48.8 ± 19.6 a | 29.3 ± 10.9 | 49.5 ± 26.8 | 44.6 ± 15.1 b | 26.2 ± 11.8 | 20.2 ± 11.5 a,b | 31.8 ± 18.6 | 0.0013 |
| C22:0 | 35.9 ± 12.5 | 34.8 ± 15.9 | 27.2 ± 13.3 | 55.0 ± 26.7 a,b | 28.7 ± 25.4 | 23.4 ± 16.9 a | 21.5 ± 5.5 b | 29.7 ± 15.6 | 0.0041 |
| SFA (mg/g) | 10.14 ± 2.0 a,b | 11.21 ± 3.00 c,d | 9.71 ± 1.57 e | 15.07 ± 4.20 f,g,h,i | 6.52 ± 1.32 a,c,e,f | 7.00 ± 1.20 b,d,g | 7.18 ± 2.73 h | 7.81 ± 1.40 i | <0.0001 |
| ΣC16:1# | 503.7 ± 322.2 | 405.0 ± 215.4 | 321.6 ± 175.1 a | 694.2 ± 480.3 a,b,c | 248.1 ± 136.0 b | 302.1 ± 133.6 c | 437.0 ± 318.0 | 434.4 ± 276.0 | 0.0083 |
| c9C17:1 | 71.2 ± 34.7 a | 53.9 ± 21.9 b | 32.3 ± 8.5 a,b,c | 87.4 ± 37.2 c,d | 47.3 ± 8.9 | 47.0 ± 10.6 | 41.9 ± 2.5 | 40.2 ± 11.5 d | <0.0001 |
| c6C18:1 | 28.1 ± 15.7 | 25.1 ± 14.3 | n.d. | 27.7 ± 9.0 | 35.6 ± 25.1 | 23.7 ± 12.9 | 19.4 ± 9.7 | 21.8 ± 10.6 | 0.6736 |
| c7C18:1 | 23.9 ± 10.7 | 28.1 ± 11.6 | n.d. | 38.8 ± 22.3 a | 20.4 ± 15.5 | 14.4 ± 8.1 a | 13.9 ± 7.8 | 15.8 ± 8.9 | 0.0025 |
| c9C18:1 | 3702 ± 2152 a | 2923 ± 1265 b | 2171 ± 686 | 4254 ± 2214 b,d | 1480 ± 564 a,b,c | 1968 ± 565 d | 1750 ± 865 | 1988 ± 528 | <0.0001 |
| c11C18:1 | 634.4 ± 197.0 | 649.9 ± 227.7 | 488.1 ± 86.5 | 718.8 ± 232.9 a | 459.5 ± 95.4 a | 522.6 ± 144.4 | 486.5 ± 245.0 | 489.3 ± 83.2 | 0.0098 |
| c11C20:1 | 77.1 ± 20.9 | 95.0 ± 34.3 | 69.6 ± 15.1 | 91.0 ± 39.1 | 69.2 ± 23.5 | 63.8 ± 18.4 | 58.8 ± 37.7 | 71.3 ± 24.0 | <0.0001 |
| MUFA (mg/g) | 5.0 ± 2.65 a | 4.18 ± 1.67 | 3.08 ± 0.93 | 5.91 ± 2.96 b,c | 2.36 ± 0.76 a,b | 2.94 ± 0.79 c | 2.81 ± 1.36 | 3.06 ± 0.77 | 0.0002 |
| c9c12C18:2 | 7441 ± 2227 a,b | 7443 ± 2033 c,d | 6628 ± 1167 e | 11,752 ± 4059 f,g,h,i | 3536 ± 1195 a,c,e,f | 4633 ± 875 b,d,g | 5335 ± 2766 h | 5068 ± 1194 i | <0.0001 |
| c9c12c15C18:3 | 244.2 ± 202.2 a,b,c | 145.5 ± 64.6 | 103.0 ± 44.8 | 292.6 ± 193.2 d,e,f | 126.8 ± 41.0 | 71.4 ± 50.7 a,d | 62.3 ± 35.7 b,e | 68.4 ± 35.0 c,f | <0.0001 |
| c9t11C18:2 | n.d. | n.d. | 67.2 ± 29.4 | 104.5 ± 53.1 a,b,c | 38.2 ± 25.2 a | 45.1 ± 29.1 b | 30.2 ± 18.1 c | 48.1 ± 21.6 | 0.0004 |
| c11c14C20:2 | 80.2 ± 25.6 a | 78.8 ± 32.1 b | 85.6 ± 28.2 c | 113.4 ± 35.4 d,e,f | 38.1 ± 12.4 a,b,c,d | 52.4 ± 18.6 e | 65.2 ± 24.5 f | 61.3 ± 15.9 | <0.0001 |
| c8c11c14C20:3 | 117.5 ± 31.2 a,b,c,d | 171.8 ± 43.5 a,e,f,g,h | 158.7 ± 25.8 b,I,j,k,l | 191.3 ± 21.1 c,m,n,o,p | 86.8 ± 27.4 e,i,m | 75.5 ± 21.8 d,f,j,n | 88.7 ± 34.6 g,k,o | 88.4 ± 36.8 h,l,p | <0.0001 * |
| c5c8c11c14C20:4 | 3384 ± 1083 a | 4401 ± 1205 b,c | 4256 ± 555 d,e | 5665 ± 925 a,f,g,h,i | 3394 ± 490 f | 3069 ± 574 b,d,g | 2580 ± 655 c,e,h | 3327 ± 393 i | <0.0001 |
| c5c8c11c14c17C20:5 | 89.3 ± 56.0 | 193.2 ± 122.6 a,b,c | 96.7 ± 30.1 d | 107.3 ± 52.9 e | 103.9 ± 49.1 f | 59.4 ± 35.2 a | 27.7 ± 14.4 b,d,e,f | 46.8 ± 32.2 c | <0.0001 |
| c7c10c13c16c19C22:5 | 403.0 ± 122.6 | 609.8 ± 157.1 a,b,c,d | 516.0 ± 63.7 e,f,g | 742.9 ± 159.4 h,I,j,k | 227.3 ± 28.0 a,h | 210.8 ± 58.9 b,e,i | 164.2 ± 39.0 c,f,j | 198.7 ± 55.6 d,g,k | <0.0001 |
| c4c7c10c13c16c19C22:6 | 316.3 ± 79.2 | 362.9 ± 94.5 | 358.9 ± 74.7 | 504.1 ± 92.8 | 247.9 ± 67.8 | 265.5 ± 74.0 | 194.4 ± 71.2 | 244.8 ± 71.1 | <0.0001 * |
| PUFA (mg/g) | 12.01 ± 2.94 a | 13.37 ± 3.28 b,c | 12.29 ± 1.59 d,e | 19.47 ± 5.08 f,g,h,i | 7.84 ± 1.48 a,b,d,f | 8.50 ± 1.36 c,e,g | 8.55 ± 3.07 h | 9.14 ± 1.39 i | <0.0001 |
| Total FA (mg/g) | 27.52 ± 6.06 a,b | 28.90 ± 7.49 c,d | 25.07 ± 4.16 e | 40.43 ± 12.38 f,g,h,i | 16.45 ± 3.68 a,c,e,f | 18.89 ± 3.91 b,d,g | 19.39 ± 8.67 h | 19.63 ± 3.51 i | <0.0001 |
| Fatty Acids (%) | CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p Value |
|---|---|---|---|---|---|---|---|---|---|
| C14:0 | 0.65 ± 0.17 | 0.90 ± 0.37 a | 0.51 ± 0.12 a,b,c | 0.61 ± 0.18 | 0.89 ± 0.30 b | 0.66 ± 0.19 | 0.84 ± 0.27 | 0.85 ± 0.17 c | 0.0001 |
| C15:0 | 0.37 ± 0.09 a | 0.39 ± 0.09 b | 0.33 ± 0.10 c,d | 0.28 ± 0.06 e,f | 0.48 ± 0.09 g,c,e | 0.34 ± 0.09 h,g | 0.38 ± 0.13 | 0.51 ± 0.07 a,b,c,d,f,h | <0.0001 * |
| C16:0 | 21.25 ± 1.62 | 21.18 ± 0.98 | 21.38 ± 0.50 | 21.12 ± 0.56 | 20.50 ± 0.64 a | 21.20 ± 1.48 | 21.99 ± 0.50 | 22.86 ± 1.79 a | 0.0024 |
| i-C17:0 | 0.42 ± 0.10 | 0.37 ± 0.05 | 0.40 ± 0.03 | 0.33 ± 0.05 a,b | 0.39 ± 0.14 | 0.45 ± 0.12 a | 0.42 ± 0.13 | 0.46 ± 0.09 b | 0.0090 |
| C18:0 | 14.02 ± 3.16 | 15.82 ± 2.01 | 15.92 ± 0.62 | 14.97 ± 1.42 | 15.98 ± 1.97 | 15.11 ± 2.00 | 14.43 ± 2.93 | 14.29 ± 1.06 | 0.0736 |
| C20:0 | 0.13 ± 0.06 | 0.17 ± 0.06 | 0.11 ± 0.03 a | 0.12 ± 0.05 | 0.25 ± 0.09 a,b | 0.14 ± 0.07 | 0.12 ± 0.07 b | 0.15 ± 0.08 | 0.0065 |
| C22:0 | 0.12 ± 0.02 | 0.12 ± 0.05 | 0.11 ± 0.05 | 0.14 ± 0.06 | 0.11 ± 0.05 | 0.11 ± 0.05 | 0.13 ± 0.06 | 0.14 ± 0.07 | 0.7955 |
| SFA | 36.97 ± 1.82 a | 38.94 ± 1.46 | 38.76 ± 0.52 | 37.56 ± 1.41 | 38.59 ± 2.32 | 38.02 ± 1.98 | 38.31 ± 3.32 | 39.27 ± 1.16 a | 0.0345 |
| ΣC16:1 # | 1.83 ± 1.12 | 1.25 ± 0.34 | 1.24 ± 0.46 | 1.56 ± 0.67 | 1.37 ± 0.54 | 1.57 ± 0.52 | 2.00 ± 1.05 | 2.00 ± 0.81 | 0.0686 |
| c9C17:1 | 0.25 ± 0.10 a | 0.16 ± 0.05 b | 0.10 ± 0.02 a,c,d,e | 0.18 ± 0.06 | 0.27 ± 0.05 b,c | 0.24 ± 0.09 d | 0.16 ± 0.02 | 0.21 ± 0.07 e | <0.0001 |
| c6C18:1 | 0.09 ± 0.04 | 0.09 ± 0.06 | n.d. | 0.07 ± 0.02 a | 0.18 ± 0.10 a | 0.11 ± 0.05 | 0.12 ± 0.09 | 0.10 ± 0.06 | 0.0240 |
| c7C18:1 | 0.09 ± 0.03 | 0.10 ± 0.05 | n.d. | 0.09 ± 0.04 | 0.10 ± 0.07 | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.8734 |
| c9C18:1 | 13.21 ± 6.85 | 9.07 ± 1.23 | 8.53 ± 1.39 | 9.96 ± 2.32 | 9.82 ± 4.60 | 10.47 ± 2.21 | 9.72 ± 2.11 | 10.74 ± 2.96 | 0.1987 |
| c11C18:1 | 2.31 ± 0.50 | 2.22 ± 0.35 | 1.95 ± 0.13 a,b,c | 1.77 ± 0.15 d,e,f,g | 2.76 ± 0.16 a,d | 2.75 ± 0.31 b,e | 2.46 ± 0.24 f | 2.60 ± 0.52 c,g | <0.0001 |
| c11C20:1 | 0.28 ± 0.07 a | 0.33 ± 0.09 | 0.28 ± 0.05 b | 0.23 ± 0.08 c,d | 0.41 ± 0.10 a,b,c | 0.35 ± 0.11 d | 0.29 ± 0.12 | 0.34 ± 0.10 | 0.0003 * |
| MUFA | 18.06 ± 8.32 | 13.22 ± 1.66 | 12.10 ± 1.78 a | 13.87 ± 3.02 | 14.92 ± 4.96 | 15.57 ± 2.72 a | 14.83 ± 3.20 | 16.06 ± 3.95 | 0.0389 |
| c9c12C18:2 | 26.72 ± 3.09 a | 25.70 ± 1.41 b | 26.85 ± 0.81 c | 28.81 ± 1.71 b,d,e | 22.83 ± 1.73 a,c,d,f | 24.68 ± 2.16 e | 27.01 ± 2.54 f | 26.31 ± 1.25 | <0.0001 |
| c9c12c15C18:3 | 0.87 ± 0.68 a,b | 0.49 ± 0.13 | 0.40 ± 0.12 | 0.66 ± 0.27 c | 0.76 ± 0.27 d,e,f | 0.38 ± 0.29 d | 0.27 ± 0.12 a,e | 0.30 ± 0.13 b,c,f | <0.0001 |
| c9t11C18:2 | n.d. | n.d. | 0.26 ± 0.09 | 0.29 ± 0.14 | 0.26 ± 0.18 | 0.24 ± 0.16 | 0.17 ± 0.11 | 0.23 ± 0.10 | 0.3948 |
| c11c14C20:2 | 0.30 ± 0.10 | 0.27 ± 0.06 | 0.34 ± 0.09 a | 0.27 ± 0.04 | 0.23 ± 0.06 a,b | 0.28 ± 0.09 | 0.35 ± 0.05 b | 0.32 ± 0.09 | 0.0129 |
| c8c11c14C20:3 | 0.44 ± 0.14 a | 0.61 ± 0.17 b,c | 0.64 ± 0.10 a,d,e | 0.49 ± 0.12 | 0.52 ± 0.14 | 0.40 ± 0.09 b,d | 0.50 ± 0.20 | 0.42 ± 0.17 c,e | 0.0003 * |
| c5c8c11c14C20:4 | 12.63 ± 4.21 a | 16.00 ± 1.43 | 17.10 ± 1.49 | 14.61 ± 2.37 b | 18.44 ± 4.02 a,b | 17.34 ± 2.39 | 14.72 ± 3.96 | 15.59 ± 2.66 | 0.0009 |
| c5c8c11c14c17C20:5 | 0.28 ± 0.15 a | 0.73 ± 0.53 b,c | 0.39 ± 0.11 d,e | 0.27 ± 0.10 f | 0.63 ± 0.29 a,f,g,h | 0.29 ± 0.14 | 0.13 ± 0.05 b,d,g | 0.18 ± 0.09 c,e,h | <0.0001 |
| c7c10c13c16c19C22:5 | 1.67 ± 0.73 | 2.14 ± 0.43 a,b,c,d | 2.08 ± 0.21 e,f,g,h | 1.91 ± 0.41 h,I,j | 1.35 ± 0.24 a, | 1.12 ± 0.25 b,e,h | 1.00 ± 0.45 c,f,i | 0.99 ± 0.31 d,g,j | <0.0001 |
| c4c7c10c13c16c19C22:6 | 1.25 ± 0.37 | 1.27 ± 0.23 | 1.45 ± 0.29 | 1.31 ± 0.29 | 1.48 ± 0.36 | 1.40 ± 0.26 | 1.06 ± 0.30 | 1.20 ± 0.37 | 0.0874 * |
| PUFA | 44.16 ± 6.40 | 47.22 ± 2.18 | 49.51 ± 1.57 a,b,c | 48.62 ± 1.88 | 46.48 ± 4.11 | 46.13 ± 2.24 a | 45.21 ± 3.25 b | 45.53 ± 3.29 c | 0.0034 |
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| ΣSFA | 10,143 ± 2094 a,b | 11,226 ± 2998 c,d | 9713 ± 1573 e | 15,069 ± 4202 f,g,h,i | 6521 ± 1320 a,c,e,f | 7003 ± 1197 b,d,g | 7184 ± 2731 h | 7811 ± 1403 i | <0.0001 |
| ΣMUFA | 5040 ± 2647 a | 4180 ± 1669 | 3082 ± 929 | 5912 ± 2960 b,c | 2360 ± 759 a,b | 2941 ± 793 c | 2807 ± 1365 | 3060 ± 770 | 0.0002 |
| ΣPUFA | 12,007 ± 2942 a | 13,367 ± 3280 b,c | 12,291 ± 1587 d,e | 19,475 ± 5081 f,g,h,i | 7835 ± 1482 a,b,d,f | 8497 ± 1356 c,e,g | 8545 ± 3069 h | 9144 ± 1391 i | <0.0001 |
| Σn-3 PUFA | 1053 ± 145 a,b,c | 1311 ± 313 d,e,f,g | 1075 ± 143 h,i,j | 1647 ± 400 k,l,m,n | 706 ± 146 d,k | 607 ± 157 a,e,h,l | 448 ± 94 b,f,i,m | 559 ± 113 c,g,j,n | <0.0001 |
| Σn-6 PUFA | 11,023 ± 2940 a | 12,095 ± 3156 b,c | 11,128 ± 1615 d | 17,722 ± 4909 e,f,g,h | 7055 ± 1560 a,b,d,e | 7830 ± 1293 c,f | 8069 ± 3101 g | 8545 ± 1425 h | <0.0001 |
| Σn-3 PUFA/Σn-6 PUFA | 0.101 ± 0.029 a | 0.110 ± 0.021 b,c,d | 0.097 ± 0.007 e,f | 0.094 ± 0.008 | 0.102 ± 0.018 g,h | 0.078 ± 0.018 b | 0.061 ± 0.020 c,e,g | 0.066 ± 0.016 a,d,f,h | <0.0001 |
| ΣLPUFA | 4390 ± 1296 a | 5818 ± 1433 b,c | 5472 ± 683 d,e | 7324 ± 1188 f,g,h,i,a | 4098 ± 580 f | 3733 ± 686 b,d,g | 3120 ± 763 c,e,h | 3967 ± 446 i | <0.0001 |
| Σn-6 LPUFA | 3582 ± 1124 a | 4652 ± 1258 b,c | 4500 ± 591 d,e | 5970 ± 969 f,g,h,i,a | 3519 ± 507 f | 3197 ± 593 b,d,g | 2734 ± 694 c,e,h | 3477 ± 419 i | <0.0001 |
| Σn-3 LPUFA | 809 ± 229 | 1166 ± 275 a,b,c,d | 972 ± 116 e,f,g | 1354 ± 251 h,i,j,k | 579 ± 114 a,h | 536 ± 134 b,e,i | 386 ± 81 c,f,j | 490 ± 103 d,g,k | <0.0001 |
| A-SFA | 6021 ± 1362 a | 6357 ± 1642 b,c | 5504 ± 1022 d | 8796 ± 2748 e,f,g,h | 3618 ± 758 a,b,d,e | 4144 ± 964 c,f | 4378 ± 1977 g | 4681 ± 949 h | <0.0001 |
| A-SFA/ΣFA | 0.219 ± 0.017 | 0.220 ± 0.009 | 0.219 ± 0.005 | 0.217 ± 0.006 | 0.221 ± 0.023 | 0.219 ± 0.015 | 0.224 ± 0.013 | 0.238 ± 0.020 | 0.0621 |
| T-SFA | 9851 ± 2030 a,b | 10,930 ± 2941 c,d | 9474 ± 1523 e | 14,715 ± 4076 f,g,h,i | 6301 ± 1262 a,c,e,f | 6810 ± 1160 b,d,g | 6992 ± 2675 h | 7553 ± 1349 i | <0.0001 |
| T-SFA/ΣFA | 0.359 ± 0.018 a | 0.378 ± 0.015 | 0.378 ± 0.005 | 0.367 ± 0.014 | 0.386 ± 0.031 a | 0.363 ± 0.020 | 0.369 ± 0.027 | 0.385 ± 0.021 | 0.0162 |
| indexASFA | 0.384 ± 0.024 | 0.401 ± 0.020 | 0.384 ± 0.015 | 0.378 ± 0.015 a | 0.408 ± 0.045 | 0.396 ± 0.046 | 0.422 ± 0.039 | 0.434 ± 0.051 a | 0.0041 |
| indexTSFA | 9813 ± 3109 | 11,011 ± 3499 a | 10,208 ± 1561 b | 15,582 ± 4013 c,d,e,f | 6516 ± 1531 a,b,c | 7422 ± 1336 d | 8351 ± 3590 e | 8637 ± 1709 f | <0.0001 |
| ΣSFA/ΣUFA | 0.601 ± 0.058 | 0.640 ± 0.037 | 0.631 ± 0.017 | 0.599 ± 0.032 | 0.638 ± 0.048 | 0.614 ± 0.050 | 0.636 ± 0.050 | 0.638 ± 0.028 | 0.0589 |
| ΣSFA/ΣPUFA | 0.855 ± 0.088 a | 0.839 ± 0.053 | 0.788 ± 0.040 | 0.771 ± 0.033 a,b | 0.834 ± 0.089 | 0.826 ± 0.069 | 0.835 ± 0.065 | 0.853 ± 0.068 b | 0.0035 |
| ΣSFA/ΣMUFA | 2.422 ± 0.927 | 2.882 ± 0.644 | 3.258 ± 0.464 a | 2.817 ± 0.618 | 2.906 ± 0.589 | 2.463 ± 0.467 a | 2.792 ± 0.658 | 2.619 ± 0.409 | 0.0362 |
| ΣSFA/ΣFA | 0.370 ± 0.019 a,b | 0.388 ± 0.015 | 0.388 ± 0.006 | 0.376 ± 0.014 | 0.399 ± 0.031 b | 0.373 ± 0.020 | 0.379 ± 0.029 | 0.398 ± 0.021 a | 0.0076 |
| ΣMUFA/ΣFA | 0.181 ± 0.083 | 0.143 ± 0.043 | 0.121 ± 0.018 | 0.139 ± 0.030 | 0.147 ± 0.056 | 0.156 ± 0.027 | 0.143 ± 0.035 | 0.156 ± 0.032 | 0.0525 |
| C18:0Δ9index | 0.464 ± 0.169 | 0.382 ± 0.094 | 0.347 ± 0.043 | 0.397 ± 0.075 | 0.348 ± 0.079 | 0.420 ± 0.069 | 0.383 ± 0.085 | 0.406 ± 0.068 | 0.0552 |
| ΣΔ9,6,5,4FAindex | 0.635 ± 0.023 | 0.620 ± 0.014 | 0.621 ± 0.006 | 0.633 ± 0.013 | 0.623 ± 0.017 | 0.629 ± 0.019 | 0.622 ± 0.019 | 0.623 ± 0.009 | 0.1638 |
| n6ElongC20/C18 index | 0.011 ± 0.004 | 0.010 ± 0.002 | 0.013 ± 0.004 | 0.010 ± 0.003 | 0.011 ± 0.003 | 0.011 ± 0.004 | 0.013 ± 0.003 | 0.012 ± 0.003 | 0.2302 |
| n3ElongC22/C20index | 0.828 ± 0.057 | 0.769 ± 0.128 | 0.844 ± 0.038 | 0.877 ± 0.045 a | 0.700 ± 0.096 a | 0.786 ± 0.094 | 0.851 ± 0.081 | 0.819 ± 0.090 | 0.0015 |
| Δ4index | 0.443 ± 0.039 | 0.373 ± 0.027 a,b,c,d | 0.408 ± 0.054 e,f,g | 0.407 ± 0.050 h,i | 0.513 ± 0.083 a,e | 0.556 ± 0.054 b,f,h | 0.529 ± 0.128 c | 0.548 ± 0.094 d,g,i | <0.0001 |
| Δ5index | 0.965 ± 0.006 | 0.962 ± 0.007 a,b,c | 0.964 ± 0.005 d,e | 0.967 ± 0.004 | 0.975 ± 0.007 a,d | 0.976 ± 0.006 b,e | 0.967 ± 0.008 | 0.974 ± 0.010 c | <0.0001 |
| h/H-Ch | 2.566 ± 0.171 | 2.511 ± 0.128 | 2.540 ± 0.089 | 2.617 ± 0.095 a | 2.498 ± 0.184 | 2.491 ± 0.279 | 2.370 ± 0.221 | 2.342 ± 0.198 a | 0.0090 |
| µg/g Sample | CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p Value |
|---|---|---|---|---|---|---|---|---|---|
| CFAs | 1008 ± 264 a,b,c | 491.1 ± 119.7 a | 599.6 ± 155.7 b | 690.7 ± 177.6 | 685.4 ± 163.1 | 646.0 ± 289.0 c | 826.9 ± 130.0 | 766.8 ± 287.1 | <0.0001 |
| CDs | 805.3 ± 241.5 a | 483.2 ± 117.5 a,b | 585.5 ± 155.6 | 592.6 ± 156.5 | 602.6 ± 145.7 | 556.2 ± 217.4 | 729.5 ± 122.6 b | 605.7 ± 235.0 | 0.0040 |
| tt | 294.9 ± 149.2 | 206.3 ± 47.1 a | 207.1 ± 57.7 b | 205.1 ± 41.1 | 280.5 ± 121.7 | 224.2 ± 76.2 | 306.8 ± 54.5 a,b | 271.9 ± 94.2 | 0.0075 |
| ct | 478.4 ± 81.3 a,b,c,d | 258.7 ± 73.8 a | 354.4 ± 106.4 | 355.6 ± 140.6 | 292.7 ± 42.4 b | 290.3 ± 146.2 c | 400.3 ± 89.0 | 297.0 ± 166.0 d | 0.0004 |
| cc | 32.01 ± 26.07 | 18.28 ± 8.72 a | 24.65 ± 11.16 | 31.80 ± 13.23 | 29.45 ± 15.37 | 41.63 ± 20.21 a | 33.64 ± 12.57 | 36.86 ± 18.56 | 0.0213 |
| CTs | 187.2 ± 62.8 a,b,c | 7.89 ± 3.50 a,d,e,f,g | 31.52 ± 26.82 b,h | 98.18 ± 50.47 d | 61.5 ± 41.8 c | 89.86 ± 101.26 e | 97.32 ± 26.60 f | 161.1 ± 56.6 g,h | <0.0001 |
| ttt | 168.0 ± 58.1 a,b,c,d | 3.55 ± 1.98 a,e,f,g | 27.52 ± 21.82 b,h | 81.77 ± 51.75 e | 39.5 ± 16.8 c,i | 55.36 ± 54.43 d | 67.94 ± 21.35 f | 145.4 ± 52.5 g,h,i | <0.0001 |
| ttc/ctt | 11.41 ± 9.80 a | 1.54 ± 0.65 a,b,c,d | 4.24 ± 2.54 | 10.73 ± 11.37 | 9.53 ± 11.59 b | 6.33 ± 6.00 | 10.07 ± 5.24 c | 6.76 ± 5.47 d | 0.0004 |
| cct | 7.20 ± 5.38 a | 2.88 ± 1.93 b | 1.44 ± 0.53 a,c,d | 4.64 ± 2.60 d | 5.02 ± 5.49 e | 3.34 ± 1.41 f | 21.69 ± 16.23 b,d,e,f | 3.65 ± 1.56 | <0.0001 |
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| (% CFAs) | |||||||||
| CDs | 79.5 ± 4.4 a,b | 98.4 ± 0.5 a,c,d,e,f,g | 97.6 ± 6.9 b | 85.9 ± 6.6 c | 88.5 ± 8.8 d | 87.5 ± 9.8 e | 88.1 ± 3.0 f | 78.8 ± 2.7 g | <0.0001 |
| tt | 28.0 ± 5.4 a,b | 42.2 ± 4.4 a,c | 35.1 ± 7.0 | 30.7 ± 6.3 b,c | 40.1 ± 9.2 | 37.1 ± 9.4 | 37.2 ± 3.9 | 37.1 ± 8.7 | <0.0001 * |
| ct | 48.4 ± 5.3 | 52.5 ± 5.0 a | 58.6 ± 6.0 b,c,d | 50.7 ± 10.3 | 43.7 ± 6.3 b | 43.9 ± 5.8 | 48.3 ± 7.2 c | 36.9 ± 10.2 a,d | <0.0001 |
| cc | 3.00 ± 1.42 a | 3.71 ± 1.36 b | 4.03 ± 1.41 | 4.55 ± 1.40 | 4.63 ± 2.74 | 6.55 ± 1.87 a,b | 4.02 ± 0.99 | 4.84 ± 1.7 | 0.0010 |
| CTs | 19.1 ± 6.1 a,b | 1.60 ± 0.5 a,c,d,e,f | 5.19 ± 4.12 b | 14.1 ± 6.62 c | 9.26 ± 5.79 | 12.5 ± 9.8 d | 11.9 ± 3.0 e | 21.2 ± 2.7 f | <0.0001 |
| ttt | 17.2 ± 5.5 a,b | 0.72 ± 0.31 a,c,d,e,f | 4.79 ± 4.08 b | 11.8 ± 7.39 c | 6.07 ± 2.67 | 8.99 ± 8.61 d | 8.43 ± 2.96 e | 19.3 ± 3.2 f | <0.0001 |
| ttc/ctt | 1.17 ± 1.00 | 0.33 ± 0.17 a | 0.70 ± 0.39 | 1.51 ± 1.58 | 1.47 ± 1.98 | 0.94 ± 0.60 | 1.23 ± 0.61 a | 0.81 ± 0.41 | 0.0162 |
| cct | 0.73 ± 0.58 a | 0.56 ± 0.28 | 0.24 ± 0.06 a,b,c,d | 0.65 ± 0.24 b | 0.74 ± 0.82 | 0.55 ± 0.17 c | 2.49 ± 1.61 d | 0.50 ± 0.21 | <0.0001 |
| (% CDs) | |||||||||
| tt | 35.2 ± 5.8 a,b | 42.9 ± 4.6 | 35.9 ± 5.7 | 36.1 ± 8.9 | 45.2 ± 7.8 a | 41.9 ± 7.8 | 42.3 ± 5.1 | 47.1 ± 11.2 b | 0.0011 |
| ct | 61.0 ± 6.6 a,b,c | 53.3 ± 5.0 | 60.1 ± 5.7 | 58.6 ± 9.3 | 49.7 ± 6.7 a | 50.5 ± 7.4 b | 54.7 ± 7.1 | 46.8 ± 12.6 c | 0.0002 |
| cc | 3.72 ± 1.59 a | 3.77 ± 1.39 b | 4.15 ± 1.52 c | 5.32 ± 1.72 | 5.19 ± 2.93 | 7.56 ± 2.15 a,b,c | 4.58 ± 1.16 | 6.16 ± 2.22 | 0.0004 |
| (% CTs) | |||||||||
| ttt | 88.7 ± 9.3 a | 43.1 ± 13.9 a,b,c | 95.2 ± 72.7 | 78.1 ± 21.2 b | 70.4 ± 16.0 | 69.3 ± 23.9 | 70.5 ± 13.7 | 90.9 ± 9.0 c | <0.0001 |
| ttc/ctt | 8.27 ± 8.06 a | 22.1 ± 9.6 a,b | 15.7 ± 5.2 c | 15.6 ± 18.8 | 15.6 ± 15.5 | 12.2 ± 8.9 | 10.0 ± 3.3 | 3.93 ± 2.43 b,c | 0.0002 |
| cct | 4.45 ± 4.35 a,b | 35.9 ± 16.4 a,c,d,e,f,g | 7.27 ± 6.04 c | 6.20 ± 4.35 d | 8.72 ± 8.88 e | 9.44 ± 8.35 f | 21.9 ± 14.9 b | 2.39 ± 0.90 g | <0.0001 |
| Cl1 | Cl2 | Cl3 | Cl4 | p Value | |
|---|---|---|---|---|---|
| C14:0 | 387.3 ± 117.9 a,b,c | 123.4 ± 45.8 a,d,e | 201.9 ± 105.2 b,d | 179.8 ± 64.9 c,e | <0.0001 |
| C15:0 | 153.2 ± 23.1 a,b,c | 67.79 ± 20.74 a,d,e | 91.87 ± 29.29 b,d | 95.88 ± 34.76 c,e | <0.0001 |
| C16:0 | 11,034 ± 1336 a,b | 3663 ± 931 a,c | 4581 ± 1049 b,d | 6038 ± 1100 c,d | <0.0001 |
| i-C17:0 | 187.5 ± 28.7 a,b | 71.83 ± 30.19 a,c | 88.25 ± 27.27 b | 111.5 ± 40.0 c | <0.0001 |
| C18:0 | 7579 ± 803 a,b | 2736 ± 676 a,c | 2951 ± 502 b,d | 4254 ± 825 c,d | <0.0001 |
| C20:0 | 74.32 ± 22.69 a,b,c | 28.49 ± 16.98 a | 33.50 ± 16.29 b | 36.03 ± 14.16 c | 0.0015 |
| C22:0 | 68.80 ± 26.32 a,b | 20.23 ± 12.36 a,c | 30.71 ± 18.80 b | 36.41 ± 15.25 c | <0.0001 |
| ΣC16:1 # | 1056 ± 424 a,b,c | 252 ± 116 a,d | 456 ± 268 b | 399 ± 234 c,d | 0.0001 |
| c9C17:1 | 108.1 ± 35.5 a,b,c | 42.65 ± 9.69 a | 46.03 ± 13.40 b | 56.60 ± 29.59 c | 0.0008 |
| c9C18:1 | 6296 ± 1372 a,b | 1609 ± 555 a,c | 2232 ± 939 b | 2875 ± 1456 c | <0.0001 |
| c11C18:1 | 970.0 ± 136.4 a,b | 423.4 ± 94.7 a,c,d | 540.9 ± 130.0 b,c | 601.2 ± 168.2 d | <0.0001 |
| c11C20:1 | 128.3 ± 41.6 a,b | 59.41 ± 24.12 a,c | 72.46 ± 23.31 b | 79.40 ± 20.67 c | 0.0002 |
| c9c12C18:2 | 15,332 ± 2582 a,b | 4269 ± 1439 a,c | 5321 ± 1595 b,d | 7560 ± 1735 c,d | <0.0001 |
| c9c12c15C18:3 | 463.3 ± 119.2 a,b,c | 94.89 ± 40.86 a | 86.81 ± 62.43 b,d | 163.5 ± 134.4 c,d | <0.0001 |
| c11c14C20:2 | 143.6 ± 26.0 a,b | 47.33 ± 17.80 a,c | 62.69 ± 19.40 b,d | 84.94 ± 25.94 c,d | <0.0001 |
| c8c11c14C20:3 | 201.8 ± 24.9 a,b | 81.53 ± 28.09 a,c | 100.1 ± 36.8 b,d | 158.8 ± 39.0 c,d | <0.0001 |
| c5c8c11c14C20:4 | 6687 ± 505 a,b | 3125 ± 634 a,c | 3229 ± 578 b,d | 4248 ± 933 c,d | <0.0001 |
| c5c8c11c14c17C20:5 | 134.9 ± 60.0 a | 73.02 ± 37.82 | 66.48 ± 57.70 a,b | 121.3 ± 92.2 b | 0.0027 |
| c7c10c13c16c19C22:5 | 829.3 ± 111.8 a,b | 253.6 ± 95.4 a,c | 222.2 ± 98.2 b,d | 549.4 ± 161.5 c,d | <0.0001 |
| c4c7c10c13c16c19C22:6 | 544.8 ± 95.8 a,b | 239.5 ± 78.1 a,c | 256.2 ± 66.1 b,d | 380.2 ± 86.3 c,d | <0.0001 |
| Sum FA | 52,637 ± 5506 a,b | 17,264 ± 4245 a,c | 20,827 ± 4594 b,d | 28,351 ± 4855 c,d | <0.0001 |
| CFA | 755.5 ± 222.4 | 563.7 ± 154.9 a | 855.4 ± 186.1 a,b | 516.6 ± 129.0 b | <0.0001 |
| CD | 670.3 ± 181.4 | 492.8 ± 130.0 a | 745.2 ± 142.6 a,b | 488.1 ± 112.8 b | <0.0001 |
| CT | 85.23 ± 47.43 a | 61.11 ± 40.68 b | 118.9 ± 85.2 c | 28.53 ± 44.16 a,b,c | <0.0001 |
| tt | 199.2 ± 22.4 a | 216.3 ± 50.8 b | 318.1 ± 89.4 a,b,c | 195.6 ± 43.3 c | <0.0001 |
| ct | 433.8 ± 155.9 | 249.5 ± 88.9 a | 390.4 ± 109.1 a,b | 271.7 ± 86.6 b | <0.0001 |
| cc | 37.28 ± 15.68 | 26.99 ± 13.34 a | 40.66 ± 18.33 a,b | 20.82 ± 9.34 b | <0.0001 |
| ttt | 66.32 ± 44.08 | 50.11 ± 37.84 a | 83.23 ± 64.06 b | 23.72 ± 41.99 a,b | <0.0001 |
| ttc/ctt | 13.12 ± 13.47 | 6.43 ± 8.53 a | 8.52 ± 5.85 b | 3.17 ± 4.46 a,b | 0.0001 |
| cct | 5.79 ± 3.24 | 3.74 ± 3.85 a | 9.75 ± 12.54 a,b | 2.48 ± 1.53 b | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepionka, T.; Białek, M.; Czauderna, M.; Wojtak, W.; Maculewicz, E.; Białek, A. Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen. Cancers 2024, 16, 479. https://doi.org/10.3390/cancers16030479
Lepionka T, Białek M, Czauderna M, Wojtak W, Maculewicz E, Białek A. Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen. Cancers. 2024; 16(3):479. https://doi.org/10.3390/cancers16030479
Chicago/Turabian StyleLepionka, Tomasz, Małgorzata Białek, Marian Czauderna, Wiktoria Wojtak, Ewelina Maculewicz, and Agnieszka Białek. 2024. "Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen" Cancers 16, no. 3: 479. https://doi.org/10.3390/cancers16030479
APA StyleLepionka, T., Białek, M., Czauderna, M., Wojtak, W., Maculewicz, E., & Białek, A. (2024). Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen. Cancers, 16(3), 479. https://doi.org/10.3390/cancers16030479










