Endoscopic and Histological Characteristics of Gastric Cancer Detected Long After Helicobacter pylori Eradication Therapy
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Evaluation of Endoscopic Findings and Histological Features
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uemura, N.; Mukai, T.; Okamoto, S.; Yamaguchi, S.; Mashiba, H.; Taniyama, K.; Sasaki, N.; Haruma, K.; Sumii, K.; Kajiyama, G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol. Biomark. Prev. 1997, 6, 639–642. [Google Scholar] [CrossRef]
- Nakagawa, S.; Asaka, M.; Kato, M.; Nakamura, T.; Kato, C.; Fujioka, T.; Tatsuta, M.; Keida, K.; Terao, S.; Takahashi, S.; et al. Helicobacter pylori eradication and metachronous gastric cancer after endoscopic mucosal resection of early gastric cancer. Aliment. Pharmacol. Ther. 2006, 24, 214–218. [Google Scholar] [CrossRef]
- Tsuda, M.; Asaka, M.; Kato, M.; Matsushima, R.; Fujimori, K.; Akino, K.; Kikuchi, S.; Lin, Y.; Sakamoto, N. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017, 22, e12415. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Hata, J.; Sugiu, K.; Kusunoki, H.; Ito, M.; Tanaka, S.; Inoue, K.; Kawamura, Y.; Chayama, K.; Haruma, K. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: Results from a 9-year prospective follow-up study in Japan. Aliment. Pharmacol. Ther. 2005, 21, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Ito, M.; Tatsugami, M.; Boda, T.; Takata, S.; Tanaka, S.; Chayama, K. Gastric cancer development after Helicobacter pylori eradication therapy: A new form of gastric neoplasia. Digestion 2012, 85, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J. Gastroenterol. 2018, 24, 2163–2172. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hashimoto, S.; Nishikura, K.; Mizuno, K.-I.; Takeuchi, M.; Sato, Y.; Ajioka, Y.; Aoyagi, Y. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J. Gastroenterol. 2013, 48, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Saka, A.; Yagi, K.; Nimura, S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer 2016, 19, 524–530. [Google Scholar] [CrossRef]
- Kitamura, Y.; Ito, M.; Matsuo, T.; Boda, T.; Oka, S.; Yoshihara, M.; Tanaka, S.; Chayama, K. Characteristic Epithelium with Low-Grade Atypia Appears on the Surface of Gastric Cancer after Successful Helicobacter pylori Eradication Therapy. Helicobacter 2014, 19, 289–295. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, S.; Takata, S.; Oka, S.; Imagawa, S.; Ueda, H.; Egi, Y.; Kitadai, Y.; Yasui, W.; Yoshihara, M.; et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short-term follow-up. Aliment. Pharmacol. Ther. 2015, 21, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.; Lam, S.K.; Wong, W.M.; Chen, J.S.; Zheng, T.T.; Feng, R.E.; Lai, K.C.; Hu, W.H.; Yuen, S.T.; Leung, S.Y.; et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA 2004, 291, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Haruma, K.; Take, S.; Nagahara, A.; Ito, K.; Oizumi, H.; Matsumoto, K.; Murakami, K.; Iijima, K.; Kato, M.; Inoue, K.; et al. A study of gastric cancer cases discovered more than 10 years after eradication of Helicobacter pylori. Jpn. Cho 2012, 47, 1623–1629. [Google Scholar]
- Kimura, K.; Takemoto, T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Haruma, K.; Kato, M.; Inoue, K.; Murakami, K.; Kamada, T. Kyoto Classification of Gastritis, 2nd ed.; Nihon Medical Center: Tokyo, Japan, 2018. [Google Scholar]
- Uedo, N.; Ishihara, R.; Iishi, H.; Yamamoto, S.; Yamada, T.; Imanaka, K.; Takeuchi, Y.; Higashino, K.; Ishiguro, S.; Tatsuta, M. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef]
- Yao, K.; Anagnostopoulos, G.; Ragunath, K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis: The updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Yagi, K.; Ajioka, Y. Endoscopic Diagnosis of Gastric Cancer Detected after Helicobacter pylori Eradication; Igaku-Shoin: Tokyo, Japan, 2016. (In Japanese) [Google Scholar]
- Yamamoto, K.; Kato, M.; Takahashi, M.; Haneda, M.; Shinada, K.; Nishida, U.; Yoshida, T.; Sonoda, N.; Ono, S.; Nakagawa, M.; et al. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter 2011, 16, 210–216. [Google Scholar] [CrossRef]
- Maehata, Y.; Nakamura, S.; Esaki, M.; Ikeda, F.; Moriyama, T.; Hida, R.; Washio, E.; Umeno, J.; Hirahashi, M.; Kitazono, T.; et al. Characteristics of Primary and Metachronous Gastric Cancers Discovered after Helicobacter pylori Eradication: A Multicenter Propensity Score-Matched Study. Gut Liver 2017, 11, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Ohno, A.; Miyoshi, J.; Kato, A.; Miyamoto, N.; Yatagai, T.; Hada, Y.; Kusuhara, M.; Jimbo, Y.; Ida, Y.; Tokunaga, K.; et al. Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication-analysis based on the Kyoto classification. BMC Gastroenterol. 2020, 20, 232. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Kusumoto, C.; Imada, T.; Hamada, F.; Yoshida, T.; Yokota, K.; Mitsuhashi, T.; Okada, H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J. Gastroenterol. 2020, 55, 281–288. [Google Scholar] [CrossRef]
- Kaji, K.; Hashiba, A.; Uotani, C.; Yamaguchi, Y.; Ueno, T.; Ohno, K.; Takabatake, I.; Wakabayashi, T.; Doyama, H.; Ninomiya, I.; et al. Grading of atrophic gastritis is useful for risk stratification in endoscopic screening for gastric Cancer. Am. J. Gastroenterol. 2019, 114, 71–79. [Google Scholar] [CrossRef]
- Toyokawa, T.; Suwaki, K.; Miyake, Y.; Nakatsu, M.; Ando, M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: A long-term prospective cohort study. J. Gastroenterol. Hepatol. 2010, 25, 544–547. [Google Scholar] [CrossRef]
- Cassaro, M.; Rugge, M.; Gutierrez, O.; Leandro, G.; Graham, D.Y.; Genta, R.M. Topographic patterns of intestinal metaplasia and gastric cancer. Am. J. Gastroenterol. 2000, 95, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Murakami, K.; Okimoto, T.; Abe, H.; Sato, R.; Ogawa, R.; Mizukami, K.; Shiota, S.; Nakagawa, Y.; Soma, W.; et al. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand. J. Gastroenterol. 2013, 48, 1249–1256. [Google Scholar] [CrossRef]
- Rokkas, T.; Pistiolas, D.; Sechopoulos, P.; Robotis, I.; Margantinis, G. The long-term impact of Helicobacter pylori eradication on gastric histology: A systematic review and meta-analysis. Helicobacter 2007, 12, 32–38. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Shi, R.; Huang, X.; Li, S.W.H.; Huang, Z.; Zhang, G. Helicobacter pylori Eradication Improves Gastric Atrophy and Intestinal Metaplasia in Long-Term Observation. Digestion 2012, 85, 126–130. [Google Scholar]
- Wang, J.; Xu, L.; Shi, R.; Huang, X.; Li, S.W.H.; Huang, Z.; Zhang, G. Gastric Atrophy and Intestinal Metaplasia before and after Helicobacter pylori Eradication: A Meta-Analysis. Digestion 2011, 83, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Zuo, X.L.; Yu, T.; Gu, X.M.; Li, Z.; Zhou, C.J.; Li, Y.Q. Mucosal barrier defects in gastric intestinal metaplasia: In vivo evaluation by confocal endomicroscopy. Gastrointest. Endosc. 2012, 75, 980–987. [Google Scholar] [CrossRef]
- Uno, K.; Iijima, K.; Shimosegawa, T. Gastric cancer development after the successful eradication of Helicobacter pylori. World J. Gastrointest. Oncol. 2016, 8, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T. Epigenetic field for cancerization. J. Biochem. Mol. Biol. 2007, 40, 142–150. [Google Scholar] [CrossRef] [PubMed]
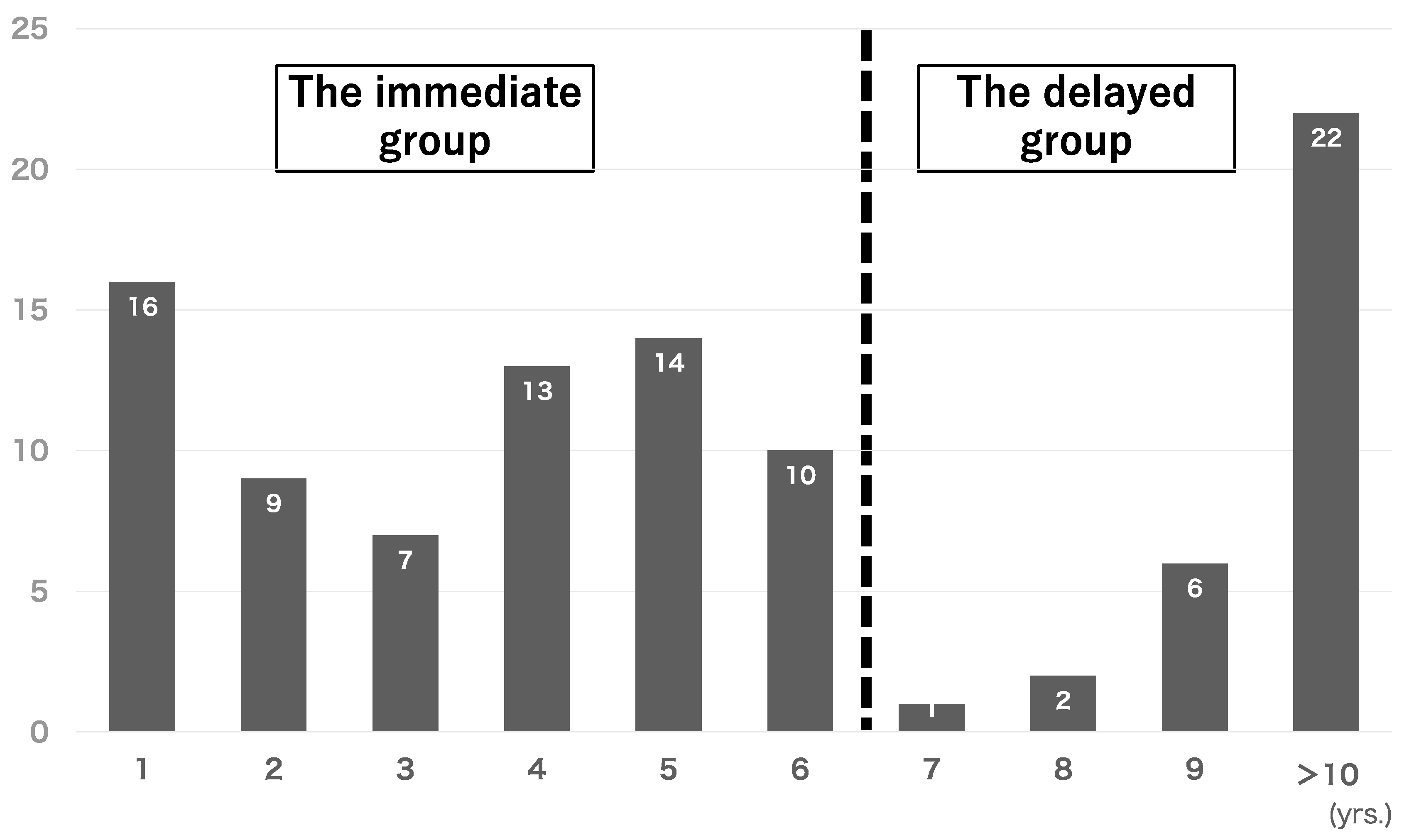

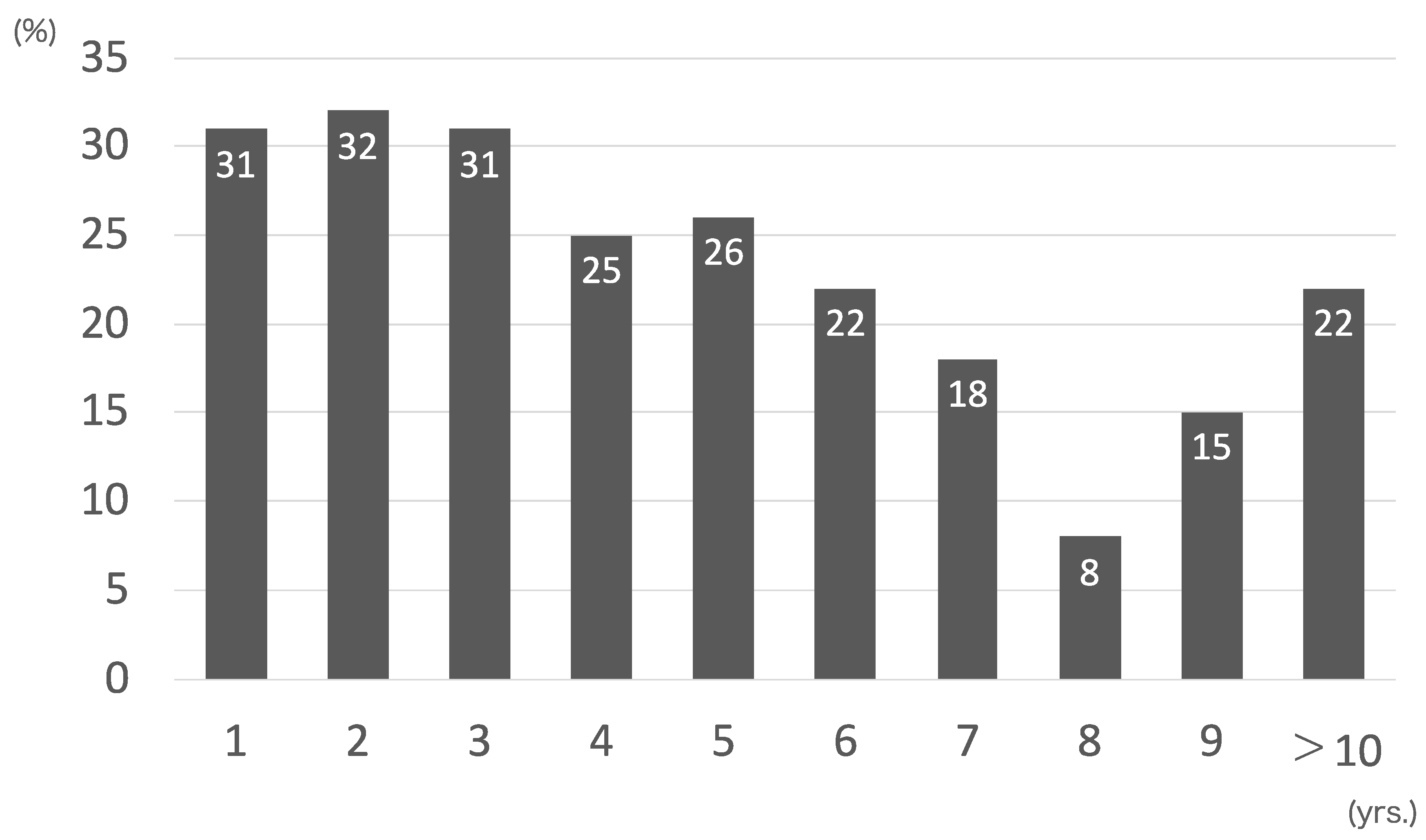
| The Immediate Group | The Delayed Group | p | |
|---|---|---|---|
| (n = 69) | (n = 31) | ||
| Patient characteristics | |||
| Age, median [range] (y) | 68 [39–80] | 72 [52–80] | 0.014 * |
| Sex | |||
| Male/Female | 53/16 | 23/8 | 0.745 † |
| Previous history (positive/negative) | |||
| Smoking | 33/36 | 20/11 | 0.071 † |
| Drinking | 44/25 | 25/6 | 0.136 † |
| Peptic ulcers | 4/65 | 3/18 | 0.351 † |
| Acid secretion inhibitor | 30/39 | 17/14 | 0.249 † |
| Endocopic resection | 6/63 | 14/17 | 0.0004 † |
| Multiple gastric cancers | 4/65 | 3/28 | 0.018 † |
| Endoscopic gastric atrophy | |||
| C-I, II/C-III, O-I/O-II, III | 1/17/51 | 1/9/21 | 0.541 † |
| Lesion characteristics | |||
| Lesion location | |||
| U/M/L | 8/20/41 | 1/15/15 | 0.004 † |
| Lesion circumference | |||
| A/P/G/L | 9/14/16/30 | 3/3/5/20 | 0.337 † |
| Macroscopic type | |||
| Elevated/Flat/Depressed | 13/5/51 | 7/3/21 | 0.937 † |
| Specimen size | |||
| mean [SD] (mm2) | 250 [435] | 200 [772] | 0.080 * |
| Curability (eCura) | |||
| A/B/C1/C2 | 61/5/0/3 | 24/3/0/4 | 0.813 † |
| Background Mucosa | The Immediate Group | The Delayed Group | p | |
|---|---|---|---|---|
| (n = 69) | (n = 31) | |||
| Endoscopic findings | enlarged fold | 31 (44.9%) | 13 (41.9%) | 0.54 * |
| map-like redness | 31 (44.9%) | 13 (41.9%) | 0.54 * | |
| intermediate zone irregularity | 17 (24.6%) | 10 (32.3%) | 0.31 * | |
| regular arrangement of collecting venules | 0 (0.0%) | 0 (0.0%) | 1.00 * | |
| light blue crest | 61 (88.4%) | 24 (77.9%) | 0.19 * | |
| Histological findings | the remnant rate of the fundic glands [SD] | 26.1 [22.2]% | 19.8 [15.6]% | 0.62 * |
| crypt enlargement | 46 (63.7%) | 25 (80.6%) | 0.19 * | |
| intestinal metaplasia | 63 (91.3%) | 27 (87.1%) | 0.23 * | |
| neutrophil infiltration | 51 (73.9%) | 28 (90.3%) | 0.10 * | |
| intestinal metaplasia with neutrophil infiltration | 50 (79.4%) | 20 (74.1%) | 0.245 * | |
| Tumor Mucosa | The Immediate Group | The Delayed Group | p | |
|---|---|---|---|---|
| (n = 69) | (n = 31) | |||
| Endoscopic findings | surface structure (villous/pit/mixed) | 22:15:32 | 8:7:16 | 0.38 † |
| irregular vascular pattern | 56 (81.2%) | 19 (61.3%) | 0.07 * | |
| irregular surface pattern | 8 (11.6%) | 4 (12.9%) | 0.55 * | |
| a gastritis-like appearance | 45 (65.2%) | 21 (67.7%) | 0.42 * | |
| Histological findings | mosaicism | 37 (53.6%) | 20 (64.5%) | 0.21 * |
| elongation of the noncancer ducts | 0 (0.0%) | 0 (0.0%) | 1.00 * | |
| overlying non-neoplastic epithelium | 27 (39.1%) | 13 (41.9%) | 0.48 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, R.; Uchikoshi, S.; Horikawa, Y.; Mimori, N.; Kato, Y.; Tahata, Y.; Fushimi, S.; Saito, M.; Takahashi, S. Endoscopic and Histological Characteristics of Gastric Cancer Detected Long After Helicobacter pylori Eradication Therapy. Cancers 2024, 16, 4153. https://doi.org/10.3390/cancers16244153
Abe R, Uchikoshi S, Horikawa Y, Mimori N, Kato Y, Tahata Y, Fushimi S, Saito M, Takahashi S. Endoscopic and Histological Characteristics of Gastric Cancer Detected Long After Helicobacter pylori Eradication Therapy. Cancers. 2024; 16(24):4153. https://doi.org/10.3390/cancers16244153
Chicago/Turabian StyleAbe, Ryo, Shu Uchikoshi, Yohei Horikawa, Nobuya Mimori, Yuhei Kato, Yuta Tahata, Saki Fushimi, Masahiro Saito, and Satsuki Takahashi. 2024. "Endoscopic and Histological Characteristics of Gastric Cancer Detected Long After Helicobacter pylori Eradication Therapy" Cancers 16, no. 24: 4153. https://doi.org/10.3390/cancers16244153
APA StyleAbe, R., Uchikoshi, S., Horikawa, Y., Mimori, N., Kato, Y., Tahata, Y., Fushimi, S., Saito, M., & Takahashi, S. (2024). Endoscopic and Histological Characteristics of Gastric Cancer Detected Long After Helicobacter pylori Eradication Therapy. Cancers, 16(24), 4153. https://doi.org/10.3390/cancers16244153




