Colorectal Neoplasia Detection Rates in Lynch Syndrome
Simple Summary
Abstract
1. Introduction
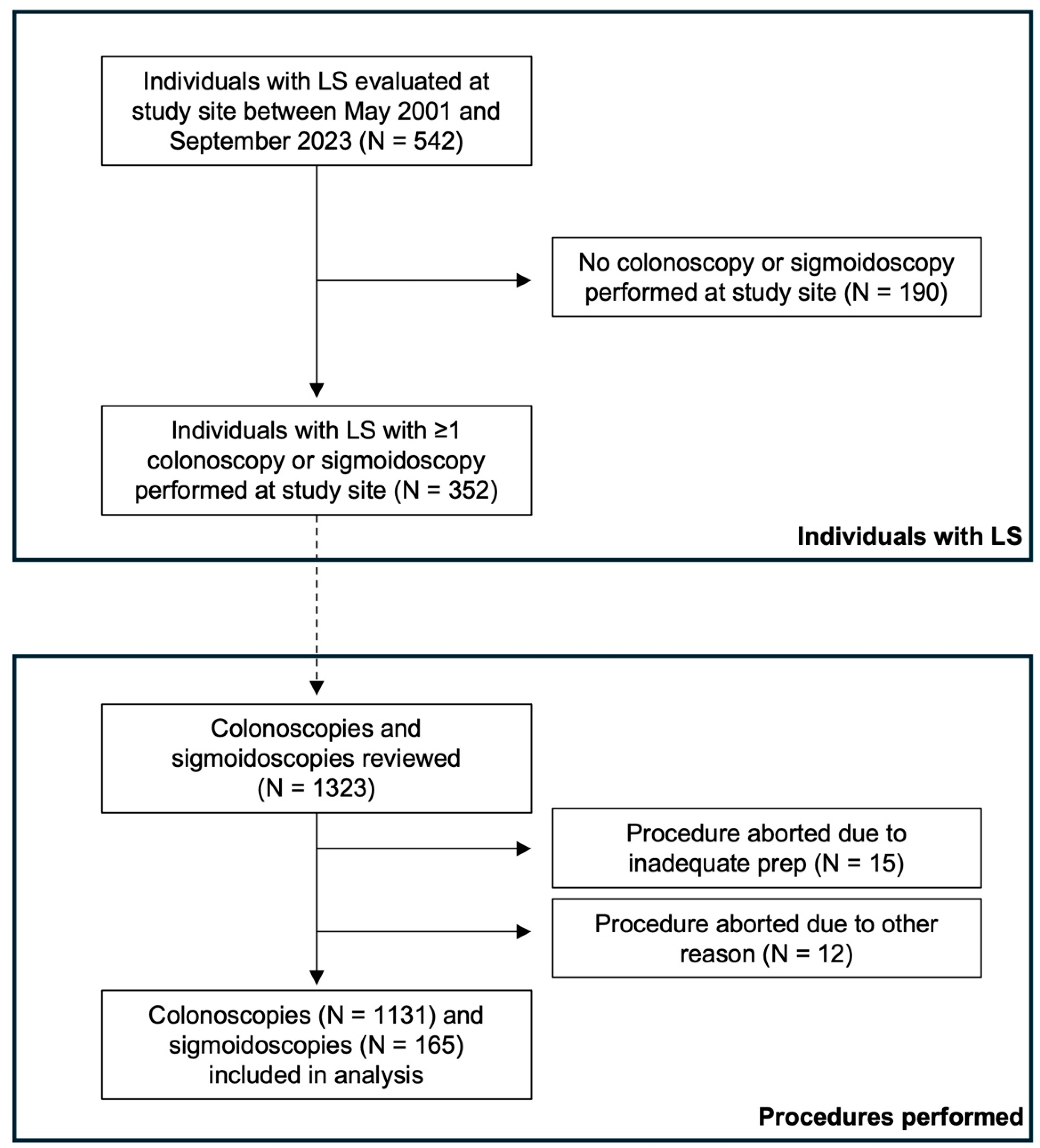
2. Methods
2.1. Study Population
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Cohort Demographics and Characteristics
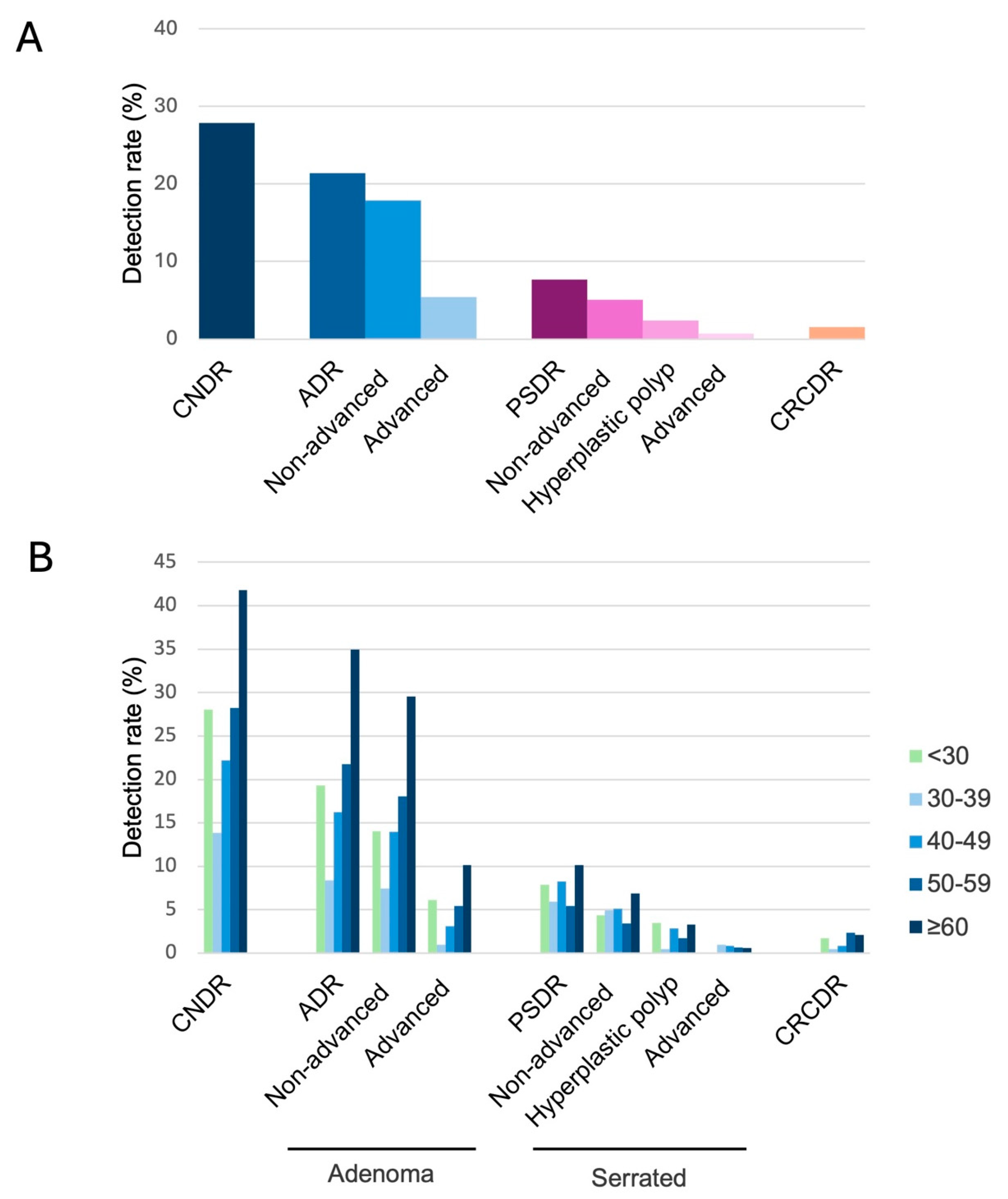
3.2. Neoplasia Detection Rates
3.3. Factors Associated with Colorectal Neoplasia
3.4. Surveillance-Detected Colorectal Cancers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Ghazaleh, N.; Kaushik, V.; Gorelik, A.; Jenkins, M.; Macrae, F. Worldwide prevalence of Lynch syndrome in patients with colorectal cancer: Systematic review and meta-analysis. Genet. Med. 2022, 24, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Stewart, S.M.; Stewart, J.; Lane, M.R. Hereditary non-polyposis colorectal cancer—Morphologies, genes and mutations. Mutat. Res. 1994, 310, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, D.L.; Axilbund, J.; Baxter, M.; Hylind, L.M.; Romans, K.; Griffin, C.A.; Cruz-Correa, M.; Giardiello, F.M. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin. Gastroenterol. Hepatol. 2011, 9, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Staffa, L.; Echterdiek, F.; Nelius, N.; Benner, A.; Werft, W.; Lahrmann, B.; Grabe, N.; Schneider, M.; Tariverdian, M.; von Knebel Doeberitz, M.; et al. Mismatch repair-deficient crypt foci in Lynch syndrome--molecular alterations and association with clinical parameters. PLoS ONE 2015, 10, e0121980. [Google Scholar] [CrossRef]
- Ahadova, A.; Gallon, R.; Gebert, J.; Ballhausen, A.; Endris, V.; Kirchner, M.; Stenzinger, A.; Burn, J.; von Knebel Doeberitz, M.; Bläker, H.; et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int. J. Cancer 2018, 143, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.A.J.; Morris, J.; Green, K.; Lalloo, F.; Woodward, E.R.; Hill, J.; Crosbie, E.J.; Evans, D.G. Association of Mismatch Repair Mutation With Age at Cancer Onset in Lynch Syndrome: Implications for Stratified Surveillance Strategies. JAMA Oncol. 2017, 3, 1702–1706. [Google Scholar] [CrossRef]
- Gupta, S.; Weiss, J.; Burke, C.; Chung, D.; Clayback, K.; Dallas, S.; Felder, S.; Giardiello, F.; Grady, W.; Hagemann, A.; et al. NCCN Guidelines Version 2.2023 Genetic/Familial High-Risk Assessment: Colorectal Continue; National Comprehensive Cancer Network: Fort Washington, PA, USA, 2023. [Google Scholar]
- Vasen, H.F.; Abdirahman, M.; Brohet, R.; Langers, A.M.; Kleibeuker, J.H.; van Kouwen, M.; Koornstra, J.J.; Boot, H.; Cats, A.; Dekker, E.; et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology 2010, 138, 2300–2306. [Google Scholar] [CrossRef]
- Dove-Edwin, I.; Sasieni, P.; Adams, J.; Thomas, H.J. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 2005, 331, 1047. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef]
- Aronson, M.; Gryfe, R.; Choi, Y.H.; Semotiuk, K.; Holter, S.; Ward, T.; Gallinger, S.; Cohen, Z.; Briollais, L. Evaluating colonoscopy screening intervals in patients with Lynch syndrome from a large Canadian registry. J. Natl. Cancer Inst. 2023, 115, 778–787. [Google Scholar] [CrossRef]
- Dominitz, J.A.; Ko, C.W. Managing the Measurement of Colonoscopy Quality. Am. J. Gastroenterol. 2019, 114, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Keswani, R.N.; Crockett, S.D.; Calderwood, A.H. AGA Clinical Practice Update on Strategies to Improve Quality of Screening and Surveillance Colonoscopy: Expert Review. Gastroenterology 2021, 161, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Wieszczy, P.; Rupinski, M.; Wojciechowska, U.; Didkowska, J.; Kraszewska, E.; Kobiela, J.; Franczyk, R.; Rupinska, M.; Kocot, B.; et al. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology 2017, 153, 98–105. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef]
- Goverde, A.; Eikenboom, E.L.; Viskil, E.L.; Bruno, M.J.; Doukas, M.; Dinjens, W.N.M.; Dubbink, E.J.; van den Ouweland, A.M.W.; Hofstra, R.M.W.; Wagner, A.; et al. Yield of Lynch Syndrome Surveillance for Patients With Pathogenic Variants in DNA Mismatch Repair Genes. Clin. Gastroenterol. Hepatol. 2020, 18, 1112–1120.e1. [Google Scholar] [CrossRef]
- Vleugels, J.L.A.; Sahin, H.; Hazewinkel, Y.; Koens, L.; van den Berg, J.G.; van Leerdam, M.E.; Dekker, E. Endoscopic detection rate of sessile serrated lesions in Lynch syndrome patients is comparable with an age- and gender-matched control population: Case-control study with expert pathology review. Gastrointest. Endosc. 2018, 87, 1289–1296. [Google Scholar] [CrossRef]
- Engel, C.; Vasen, H.F.; Seppälä, T.; Aretz, S.; Bigirwamungu-Bargeman, M.; de Boer, S.Y.; Bucksch, K.; Büttner, R.; Holinski-Feder, E.; Holzapfel, S.; et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries with Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018, 155, 1400–1409.e2. [Google Scholar] [CrossRef]
- Liljegren, A.; Barker, G.; Elliott, F.; Bertario, L.; Bisgaard, M.L.; Eccles, D.; Evans, G.; Macrae, F.; Maher, E.; Lindblom, A.; et al. Prevalence of adenomas and hyperplastic polyps in mismatch repair mutation carriers among CAPP2 participants: Report by the colorectal adenoma/carcinoma prevention programme 2. J. Clin. Oncol. 2008, 26, 3434–3439. [Google Scholar] [CrossRef]
- Sánchez, A.; Roos, V.H.; Navarro, M.; Pineda, M.; Caballol, B.; Moreno, L.; Carballal, S.; Rodríguez-Alonso, L.; Ramon, Y.C.T.; Llort, G.; et al. Quality of Colonoscopy Is Associated With Adenoma Detection and Postcolonoscopy Colorectal Cancer Prevention in Lynch Syndrome. Clin. Gastroenterol. Hepatol. 2022, 20, 611–621.e9. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B.; Hazewinkel, Y.; Pellisé, M.; Rivero-Sánchez, L.; Balaguer, F.; Bisschops, R.; Tejpar, S.; Repici, A.; Ramsoekh, D.; Jacobs, M.; et al. Linked Colour imaging for the detection of polyps in patients with Lynch syndrome: A multicentre, parallel randomised controlled trial. Gut 2022, 71, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B.; Mostafavi, N.; Vleugels, J.L.A.; Hüneburg, R.; Lamberti, C.; Rivero-Sánchez, L.; Pellisé, M.; Stoffel, E.M.; Syngal, S.; Haanstra, J.F.; et al. Dye-Based Chromoendoscopy in Patients With Lynch Syndrome: An Individual Patient Data Meta-Analysis of Randomized Trials. Am. J. Gastroenterol. 2021, 116, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Haanstra, J.F.; Dekker, E.; Cats, A.; Nagengast, F.M.; Hardwick, J.C.; Vanhoutvin, S.A.; de Vos Tot Nederveen Cappel, W.H.; Vasen, H.F.; Kleibeuker, J.H.; Koornstra, J.J. Effect of chromoendoscopy in the proximal colon on colorectal neoplasia detection in Lynch syndrome: A multicenter randomized controlled trial. Gastrointest. Endosc. 2019, 90, 624–632. [Google Scholar] [CrossRef]
- Rivero-Sánchez, L.; Arnau-Collell, C.; Herrero, J.; Remedios, D.; Cubiella, J.; García-Cougil, M.; Alvarez, V.; Albéniz, E.; Calvo, P.; Gordillo, J.; et al. White-Light Endoscopy Is Adequate for Lynch Syndrome Surveillance in a Randomized and Noninferiority Study. Gastroenterology 2020, 158, 895–904.e1. [Google Scholar] [CrossRef]
- Sleiman, J.; Farha, N.; Beard, J.; Bena, J.; Morrison, S.; Milicia, S.; Heald, B.; Kalady, M.F.; Church, J.; Liska, D.; et al. Incidence and prevalence of advanced colorectal neoplasia in Lynch syndrome. Gastrointest. Endosc. 2023, 98, 412–419.e8. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Turgeon, D.K.; Stockwell, D.H.; Zhao, L.; Normolle, D.P.; Tuck, M.K.; Bresalier, R.S.; Marcon, N.E.; Baron, J.A.; Ruffin, M.T.; et al. Missed adenomas during colonoscopic surveillance in individuals with Lynch Syndrome (hereditary nonpolyposis colorectal cancer). Cancer Prev. Res. 2008, 1, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.J.; Enestvedt, B.K.; Jiang, Z.; Holub, J.L.; Gupta, M.; Lieberman, D.A.; Eisen, G.M. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest. Endosc. 2011, 74, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: A report from the prospective Lynch syndrome database. Gut 2017, 66, 1657–1664. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Haupt, S.; Ahadova, A.; Kloor, M.; Sampson, J.R.; Sunde, L.; Seppälä, T.; Burn, J.; Bernstein, I.; Capella, G.; et al. Incidences of colorectal adenomas and cancers under colonoscopy surveillance suggest an accelerated “Big Bang” pathway to CRC in three of the four Lynch syndromes. Hered. Cancer Clin. Pract. 2024, 22, 6. [Google Scholar] [CrossRef]
| Variable | n = 352 |
|---|---|
| Biological sex (%) | |
| Female | 227 (64.5) |
| 125 (35.5) | |
| Age at first colonoscopy/sigmoidoscopy after LS diagnosis (%) | |
| <30 | 38 (10.8) |
| 30–39 | 73 (20.7) |
| 40–49 | 76 (21.6) |
| 50–59 | 68 (19.3) |
| ≥60 | 97 (27.6) |
| Race (%) | |
| White | 308 (87.5) |
| Black | 12 (3.4) |
| Other | 23 (6.5) |
| Not reported | 9 (2.6) |
| Marital Status (%) | |
| Single | 77 (21.9) |
| Married | 239 (67.9) |
| Divorced/Widowed/Other | 36 (10.2) |
| Insurance Type (%) | |
| Private | 268 (76.1) |
| Medicare | 69 (19.6) |
| Medicaid | 12 (3.4) |
| Other | 3 (0.9) |
| Median income of zip code of residence (%) | |
| 0–49,999 | 17 (4.8) |
| 50,000–74,999 | 43 (12.2) |
| 75,000–99,999 | 80 (22.7) |
| 100,000–124,999 | 121 (34.4) |
| 125,000–149,999 | 50 (14.2) |
| ≥150,000 | 41 (11.6) |
| Smoking status (%) | |
| Never | 240 (68.2) |
| Former | 96 (27.3) |
| Current | 16 (4.5) |
| BMI at first colonoscopy/sigmoidoscopy (median [IQR]) | 26.5 [23.3, 30.8] |
| ASA use ≥2 years (%) | 145 (41.2) |
| Gene (%) | |
| MLH1 | 76 (21.6) |
| MSH2/EPCAM | 112 (31.8) |
| MSH6 | 74 (21.0) |
| PMS2 | 90 (25.6) |
| Surveillance interval in years (median [IQR]) | 1.2 [1.0, 1.7] |
| History of prior colon resection (%) | 93 (26.4) |
| Personal history of any prior cancer (%) | 188 (53.4) |
| Personal history of prior colon cancer (%) | 77 (21.9) |
| Personal history of other cancer (%) | 146 (41.5) |
| Family history of any cancer (%) | 342 (97.2) |
| Family history of colon cancer (%) | 262 (74.4) |
| Family history of other cancer (%) | 317 (90.1) |
| Number of colonoscopies/sigmoidoscopies | |
| 1 | 104 (29.5) |
| 2 | 64 (18.2) |
| 3 | 42 (11.9) |
| 4 | 42 (11.9) |
| 5 | 28 (8.0) |
| 6 | 21 (6.0) |
| ≥7 | 51 (14.6) |
| Variable | Colorectal Neoplasia Present (n = 204) | Colorectal Neoplasia Absent (n = 148) | p |
|---|---|---|---|
| Biological sex (%) | 0.11 | ||
| Female | 124 (60.8) | 103 (69.6) | |
| Male | 80 (39.2) | 45 (30.4) | |
| Median age (%) | <0.01 | ||
| <30 | 18 (8.8) | 20 (13.5) | |
| 30–39 | 22 (10.8) | 51 (34.5) | |
| 40–49 | 48 (23.5) | 28 (18.9) | |
| 50–59 | 44 (21.6) | 24 (16.2) | |
| ≥60 | 72 (35.3) | 25 (16.9) | |
| Race (%) | 0.48 | ||
| White | 181 (88.7) | 127 (85.8) | |
| Black | 8 (3.9) | 4 (2.7) | |
| Other | 10 (4.9) | 13 (8.8) | |
| Not reported | 5 (2.5) | 4 (2.7) | |
| Marital Status (%) | <0.01 | ||
| Single | 34 (16.7) | 43 (29.1) | |
| Married | 142 (69.6) | 97 (65.5) | |
| Divorced/Widowed/Other | 28 (13.7) | 8 (5.4) | |
| Insurance Type (%) | 0.02 | ||
| Private | 148 (72.5) | 120 (81.1) | |
| Medicare | 49 (24.0) | 20 (13.5) | |
| Medicaid | 7 (3.4) | 5 (3.4) | |
| Other | 0 (0.0) | 3 (2.0) | |
| Income (%) | 0.52 | ||
| 0–49,999 | 10 (4.9) | 7 (4.7) | |
| 50,000–74,999 | 27 (13.2) | 16 (10.8) | |
| 75,000–99,999 | 40 (19.6) | 40 (27.0) | |
| 100,000–124,999 | 73 (35.8) | 48 (32.4) | |
| 125,000–149,999 | 27 (13.2) | 23 (15.5) | |
| ≥150,000 | 27 (13.2) | 14 (9.5) | |
| Smoking status (%) | 0.13 | ||
| Never | 131 (64.2) | 109 (73.6) | |
| Former | 64 (31.4) | 32 (21.6) | |
| Current | 9 (4.4) | 7 (4.7) | |
| BMI at first colonoscopy/sigmoidoscopy (median [IQR]) | 26.5 [24.3, 30.9] | 26.3 [22.5, 30.5] | 0.28 |
| ASA use ≥2 years (%) | 86 (42.2) | 59 (39.9) | 0.75 |
| Gene (%) | 0.58 | ||
| MLH1 | 45 (22.1) | 31 (20.9) | |
| MSH2/EPCAM | 70 (34.3) | 42 (28.4) | |
| MSH6 | 41 (20.1) | 33 (22.3) | |
| PMS2 | 48 (23.5) | 42 (28.4) | |
| Surveillance interval in years (median [IQR]) | 1.1 [1.0, 1.5] | 1.2 [1.0, 2.0] | 0.12 |
| History of prior colon resection (%) | 65 (31.9) | 28 (18.9) | 0.01 |
| Personal history of any prior cancer (%) | 127 (62.3) | 61 (41.2) | <0.01 |
| Personal history of prior colon cancer (%) | 52 (25.5) | 25 (16.9) | 0.07 |
| Personal history of other cancer (%) | 75 (36.8) | 36 (24.3) | 0.02 |
| Family history of any cancer (%) | 196 (96.1) | 146 (98.6) | 0.27 |
| Family history of colon cancer (%) | 150 (73.5) | 112 (75.7) | 0.74 |
| Family history of other cancer (%) | 46 (22.5) | 34 (23.0) | 1.00 |
| Number of colonoscopies/sigmoidoscopies | <0.01 | ||
| 1 | 34 (16.7) | 70 (47.3) | |
| 2 | 34 (16.7) | 30 (20.3) | |
| 3 | 28 (13.7) | 14 (9.5) | |
| 4 | 27 (13.2) | 15 (10.1) | |
| 5 | 25 (12.3) | 3 (2.0) | |
| 6 | 17 (8.3) | 4 (2.7) | |
| ≥7 | 37 (19.1) | 12 (8.2) |
| Variable | Adenoma Present (n = 163) | Adenoma Absent (n = 189) | p |
|---|---|---|---|
| Biological sex (%) | 0.05 | ||
| Female | 96 (58.9) | 131 (69.3) | |
| Male | 67 (41.1) | 58 (30.7) | |
| Median age (%) | <0.01 | ||
| <30 | 12 (7.4) | 26 (13.8) | |
| 30–39 | 15 (9.2) | 58 (30.7) | |
| 40–49 | 37 (22.7) | 39 (20.6) | |
| 50–59 | 31 (19.0) | 37 (19.6) | |
| ≥60 | 68 (41.7) | 29 (15.3) | |
| Race (%) | 0.09 | ||
| White | 146 (89.6) | 162 (85.7) | |
| Black | 8 (4.9) | 4 (2.1) | |
| Other | 6 (3.7) | 17 (9.0) | |
| Not reported | 3 (1.8) | 6 (3.2) | |
| Marital Status (%) | 0.01 | ||
| Single | 25 (15.3) | 52 (27.5) | |
| Married | 116 (71.2) | 123 (65.1) | |
| Divorced/Widowed/Other | 22 (13.5) | 14 (7.4) | |
| Insurance Type (%) | 0.01 | ||
| Private | 110 (67.5) | 158 (83.6) | |
| Medicare | 47 (28.8) | 22 (11.6) | |
| Medicaid | 6 (3.7) | 6 (3.2) | |
| Other | 0 (0.0) | 3 (1.6) | |
| Income (%) | 0.32 | ||
| 0–49,999 | 8 (4.9) | 9 (4.8) | |
| 50,000–74,999 | 22 (13.5) | 21 (11.1) | |
| 75,000–99,999 | 29 (17.8) | 50 (26.5) | |
| 100,000–124,999 | 56 (34.4) | 65 (34.4) | |
| 125,000–149,999 | 23 (14.1) | 27 (14.3) | |
| ≥150,000 | 24 (14.7) | 17 (9.0) | |
| Smoking status (%) | 0.29 | ||
| Never | 105 (64.4) | 135 (71.4) | |
| Former | 51 (31.3) | 45 (23.8) | |
| Current | 7 (4.3) | 9 (4.8) | |
| BMI at first colonoscopy/sigmoidoscopy (median [IQR]) | 26.7 [24.5, 31.3] | 25.8 [22.7, 29.9] | 0.04 |
| ASA use ≥2 years (%) | 74 (45.4) | 71 (37.6) | 0.17 |
| Gene (%) | 0.33 | ||
| MLH1 | 36 (22.1) | 40 (21.2) | |
| MSH2/EPCAM | 59 (36.2) | 53 (28.0) | |
| MSH6 | 31 (19.0) | 43 (22.8) | |
| PMS2 | 37 (22.7) | 53 (28.0) | |
| Surveillance interval in years (median [IQR]) | 1.1 [1.0, 1.5] | 1.3 [1.0, 2.0] | 0.06 |
| History of prior colon resection (%) | 56 (34.4) | 37 (19.6) | <0.01 |
| Personal history of any prior cancer (%) | 107 (65.6) | 81 (42.9) | <0.01 |
| Personal history of prior colon cancer (%) | 45 (27.6) | 32 (16.9) | <0.01 |
| Personal history of other cancer (%) | 89 (54.6) | 57 (30.2) | 0.02 |
| Family history of any cancer (%) | 156 (95.7) | 186 (98.4) | 0.23 |
| Family history of colon cancer (%) | 123 (75.5) | 139 (73.5) | 0.77 |
| Family history of other cancer (%) | 150 (92.0) | 167 (88.4) | 0.33 |
| Number of colonoscopies/sigmoidoscopies | <0.01 | ||
| 1 | 23 (14.1) | 81 (42.9) | |
| 2 | 26 (16.0) | 38 (20.1) | |
| 3 | 20 (12.3) | 22 (11.6) | |
| 4 | 24 (14.7) | 18 (9.5) | |
| 5 | 20 (12.3) | 8 (4.2) | |
| 6 | 15 (9.2) | 6 (3.2) | |
| ≥7 | 22 (13.5) | 14 (7.4) |
| Variable | n = 18 |
|---|---|
| Gene (%) | |
| MLH1 | 7 (38.9) |
| MSH2/EPCAM | 10 (55.6) |
| MSH6 | 0 (0.0) |
| PMS2 | 1 (5.6) |
| Age at time of colonoscopy/sigmoidoscopy (%) | |
| <30 | 1 (5.6) |
| 30–39 | 1 (5.6) |
| 40–49 | 3 (16.7) |
| 50–59 | 7 (38.9) |
| ≥60 | 6 (33.3) |
| Prior colorectal neoplasia (%) | |
| No | 3 (16.7) |
| Yes | 10 (55.6) |
| N/A * | 5 (27.8) |
| Interval from prior lower endoscopic procedure in years (median [IQR]) | 1.2 [1.0, 1.4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirda, D.; Dungan, M.; Ren, Y.; Li, H.; Katona, B.W. Colorectal Neoplasia Detection Rates in Lynch Syndrome. Cancers 2024, 16, 4021. https://doi.org/10.3390/cancers16234021
Mirda D, Dungan M, Ren Y, Li H, Katona BW. Colorectal Neoplasia Detection Rates in Lynch Syndrome. Cancers. 2024; 16(23):4021. https://doi.org/10.3390/cancers16234021
Chicago/Turabian StyleMirda, Danielle, Michaela Dungan, Yue Ren, Hongzhe Li, and Bryson W. Katona. 2024. "Colorectal Neoplasia Detection Rates in Lynch Syndrome" Cancers 16, no. 23: 4021. https://doi.org/10.3390/cancers16234021
APA StyleMirda, D., Dungan, M., Ren, Y., Li, H., & Katona, B. W. (2024). Colorectal Neoplasia Detection Rates in Lynch Syndrome. Cancers, 16(23), 4021. https://doi.org/10.3390/cancers16234021





