Hematologic Toxicity Profiles and the Impact of Hemoglobin Nadir and Transfusion on Oncologic Outcome in Definitive Radiochemotherapy for Cervical Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and RT Treatment
2.2. Chemotherapy and Hematotoxicity
2.3. Oncologic Outcomes
2.4. Statistical Analysis
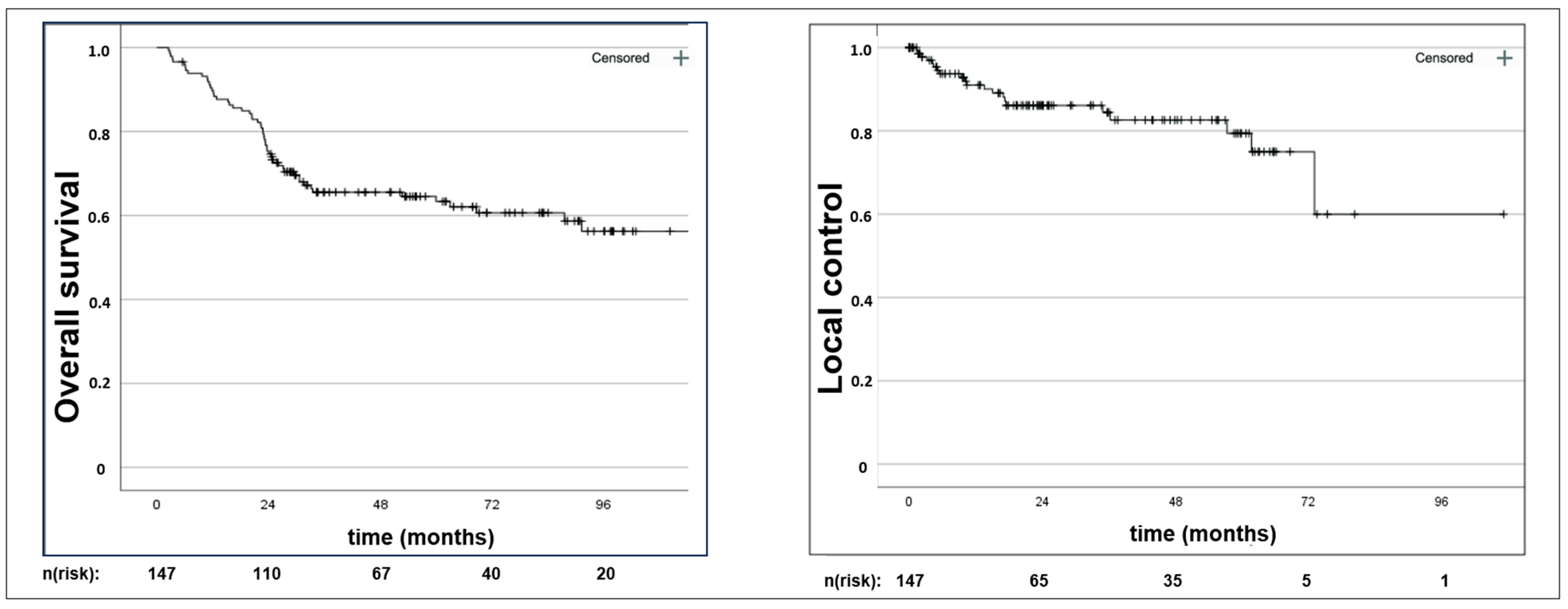
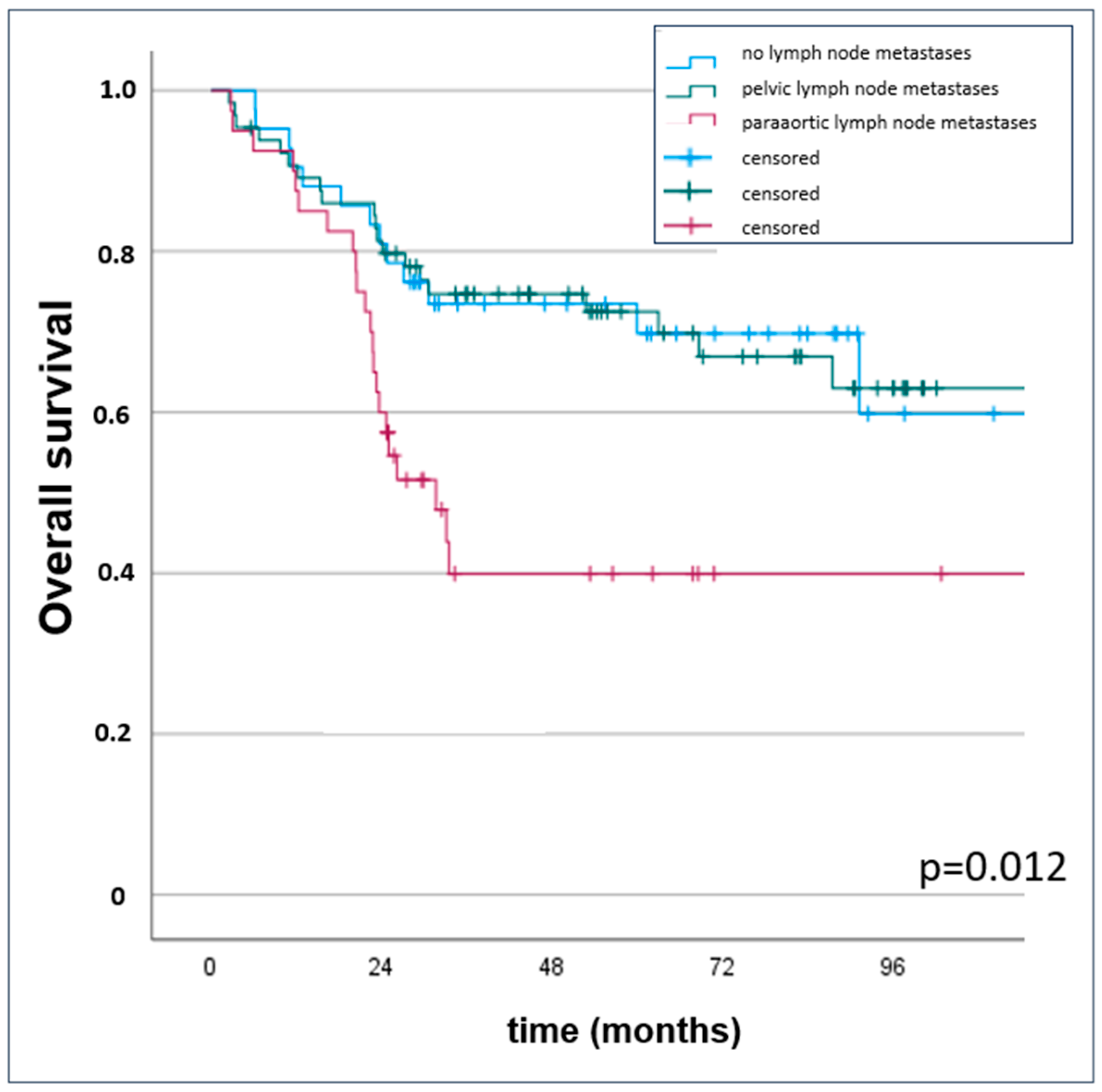
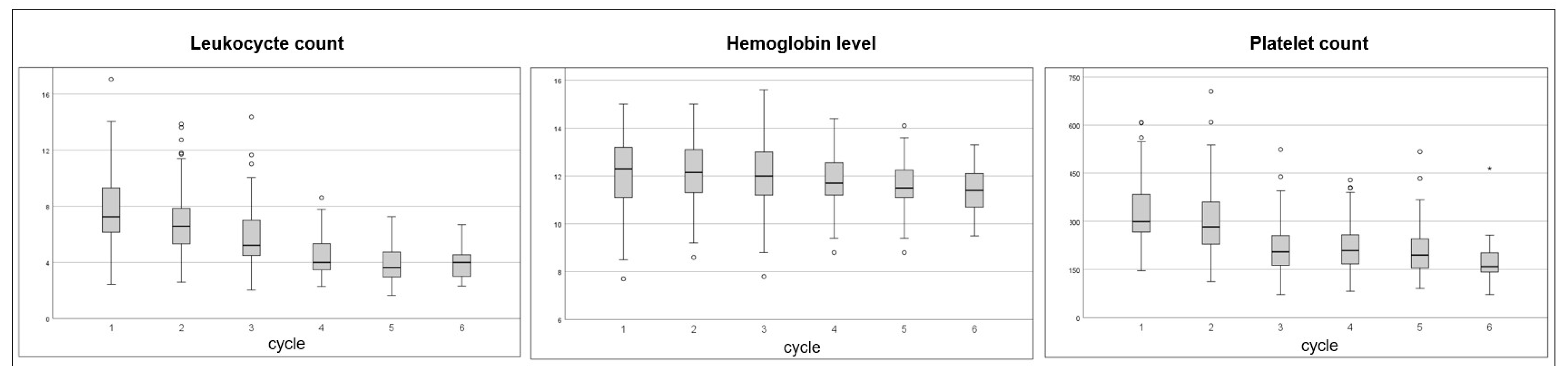
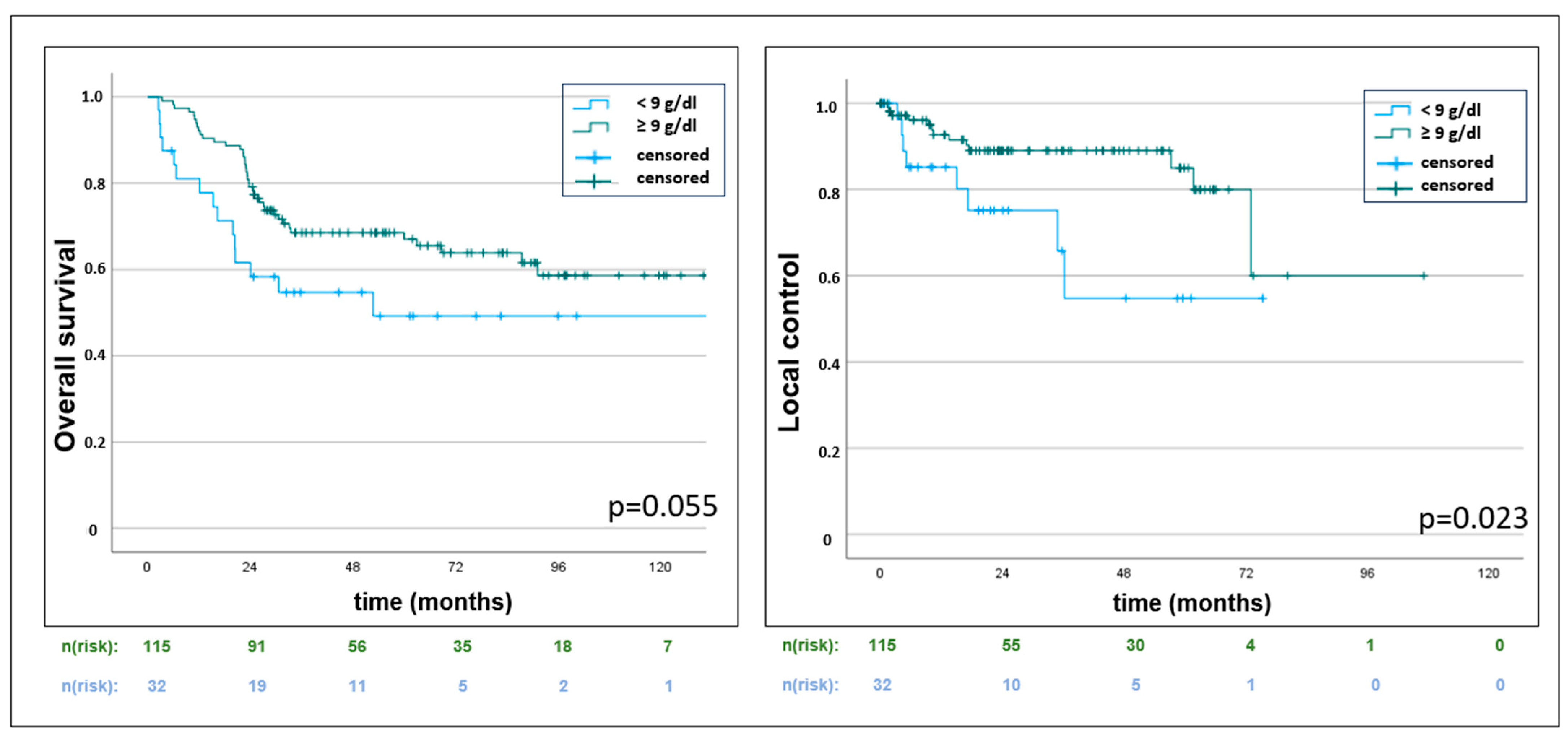
3. Results
3.1. Study Population
3.2. Oncologic Outcomes
3.3. Hematotoxicity and Hemoglobin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Cvek, J.; Randall, L.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 404, 1321–1332. [Google Scholar] [PubMed]
- McCormack, M.; Eminowicz, G.; Gallardo, D.; Diez, P.; Farrelly, L.; Kent, C.; Hudson, E.; Panades, M.; Mathew, T.; Anand, A.; et al. Induction chemotherapy followed by standard chemoradiotherapy versus standard chemoradiotherapy alone in patients with locally advanced cervical cancer (GCIG INTERLACE): An international, multicentre, randomised phase 3 trial. Lancet. 2024, 404, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef]
- Macdonald, O.K.; Chen, J.; Dodson, M.; Lee, C.M.; Gaffney, D.K. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am. J. Clin. Oncol. 2009, 32, 411–416. [Google Scholar] [CrossRef]
- Song, S.; Rudra, S.; Hasselle, M.D.; Dorn, P.L.; Mell, L.K.; Mundt, A.J.; Yamada, S.D.; Lee, N.K.; Hasan, Y. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer. 2013, 119, 325–331. [Google Scholar] [CrossRef]
- Perez, C.A.; Grigsby, P.W.; Castro-Vita, H.; Lockett, M.A. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1275–1288. [Google Scholar] [CrossRef]
- Akyildiz, A.; Gultekin, M.; Yigit, E.; Demir, E.; Ismayilov, R.; Ahmed, M.; Buyukkor, M.; Yildirim, H.C.; Yildirim, N.; Ucar, G.; et al. Efficacy of cumulative cisplatin dose on survival in patients with locally advanced cervical cancer treated with definitive chemoradiotherapy: Multicenter study by Turkish Oncology Group. Int. J. Gynecol. Cancer 2024, 34, 1359–1365. [Google Scholar] [CrossRef]
- Nanda, R.; Katke, A.; Thejaswini, B.; Giri, G.V.; Pawar, Y.; Manjula, M.V.; Babu, G. Ten years survival results of randomized study comparing weekly vs. triweekly cisplatin with concurrent radiation in locally advanced carcinoma cervix. Rep. Pract. Oncol. Radiother. 2023, 28, 322–331. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q.; Liu, Y.; Li, J.; Deng, W.; Wang, J.; Zhou, S.; Yu, Z.; Huang, X.; Huang, Y.; et al. A prospective single-arm study on the relationship between dose-volume parameters of pelvic functional bone marrow and acute hematological toxicities during intensity-modulated radiotherapy with or without concurrent chemotherapy for uterine cervical/endometrial cancer. Radiat. Oncol. 2023, 18, 193. [Google Scholar] [PubMed]
- Gao, H.; Wu, H.; Zhang, Y.; Li, X.; Qi, Z.; Wang, M.; Wang, S. Long-term survival in patients with para-aortic metastatic cervical cancer receiving simultaneous integrated boost chemoradiation to positive lymph nodes: A single-center experience. Int. J. Gynecol. Cancer 2024, 34, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, Ș.A.; Toma, R.V.; Trifănescu, O.G.; Galeș, L.N.; Folea, A.R.; Sima, A.; Bîlteanu, L.; Anghel, R. Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer-A Risk Analysis. Cancers 2024, 16, 3032. [Google Scholar] [CrossRef] [PubMed]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Sobel, E.; Rose, G.S.; Thomas, G.; Carlson, J.W. Association of hemoglobin level with survival in cervical carcinoma patients treated with concurrent cisplatin and radiotherapy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2004, 94, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, G.; Zhou, Q.; Kuang, W. Outcomes and prognostic factors in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Radiat. Oncol. 2022, 17, 142. [Google Scholar] [CrossRef]
- Koulis, T.A.; Kornaga, E.N.; Banerjee, R.; Phan, T.; Ghatage, P.; Magliocco, A.M.; Lees-Miller, S.P.; Doll, C.M. Anemia, leukocytosis and thrombocytosis as prognostic factors in patients with cervical cancer treated with radical chemoradiotherapy: A retrospective cohort study. Clin. Transl. Radiat. Oncol. 2017, 12, 51–56. [Google Scholar] [CrossRef]
- Kunos, C.A.; Fabian, D.; Fredericks, T.; Baldwin, L.; Dietrich, C.; Miller, R.W.; Ueland, F.R. Hemoglobin level associates with survival in women from Appalachian Kentucky with uterine cervix cancer. Front. Oncol. 2023, 13, 1132135. [Google Scholar] [CrossRef]
- Gennigens, C.; De Cuypere, M.; Seidel, L.; Hermesse, J.; Barbeaux, A.; Forget, F.; Albert, A.; Jerusalem, G.; Kridelka, F. Correlation between hematological parameters and outcome in patients with locally advanced cervical cancer treated by concomitant chemoradiotherapy. Cancer Med. 2020, 9, 8432–8443. [Google Scholar] [CrossRef]
- AWMF S3-Guideline Diagnostic, Therapy and Follow Up for Women with Cervical Cancer—Version 2.2. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Zervixkarzinom/Version_2/LL_Zervixkarzinom_Langversion_2.2.pdf (accessed on 25 November 2024).
- James, R.M.; Cruickshank, M.E.; Siddiqui, N.; Guideline Development Group. Management of cervical cancer: Summary of SIGN guidelines. BMJ 2008, 336, 41–43. [Google Scholar] [CrossRef]
- Zayed, S.; Nguyen, T.K.; Lin, C.; Boldt, G.; Beriwal, S.; Creutzberg, C.L.; Kamrava, M.; Mendez, L.C.; Velker, V.; Doll, C.; et al. Red Blood Cell Transfusion Practices for Patients With Cervical Cancer Undergoing Radiotherapy. JAMA Netw. Open 2021, 4, e213531. [Google Scholar] [CrossRef]
- Blohmer, J.U.; Paepke, S.; Sehouli, J.; Boehmer, D.; Kolben, M.; Würschmidt, F.; Petry, K.U.; Kimmig, R.; Elling, D.; Thomssen, C.; et al. Randomized phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin Alfa in patients with high-risk cervical cancer: Results of the NOGGO-AGO intergroup study. J. Clin. Oncol. 2011, 29, 3791–3797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strauss, H.G.; Haensgen, G.; Dunst, J.; Hayward, C.R.; Burger, H.U.; Scherhag, A.; Koelbl, H. Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Corrons, J.L.V.; Casafont, L.B.; Frasnedo, E.F. Concise review: How do red blood cells born, live, and die? Ann. Hematol. 2021, 100, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, D.; Bao, Z.; Yi, Z.; Mei, Z.; Sun, S.; Xiang, Q.; Yang, C.; Yang, H.; Qiu, H.; et al. The impact of bone marrow irradiation dose on acute haematologic toxicity in cervical cancer patients treated with concurrent chemoradiotherapy. Radiat. Oncol. 2023, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, D.; Kormann, C.; Pigorsch, S.; Kiechle, M.; Combs, S.E.; Habermehl, D. Bone marrow toxicity in patients with locally advanced cervical cancer undergoing multimodal treatment with VMAT/IMRT: Are there dosimetric predictors for toxicity? Eur. J. Med. Res. 2024, 29, 445. [Google Scholar] [CrossRef]
- Klopp, A.H.; Moughan, J.; Portelance, L.; Miller, B.E.; Salehpour, M.R.; Hildebrandt, E.; Nuanjing, J.; D’Souza, D.; Souhami, L.; Small, W.; et al. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 83–90. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, L.; Yu, H.; Guo, L. A machine learning model for grade 4 lymphopenia prediction during pelvic radiotherapy in patients with cervical cancer. Front. Oncol. 2022, 12, 905222. [Google Scholar] [CrossRef]
- Kumar, A.; Gurram, L.; Naga Ch, P.; Nayak, P.; Mulye, G.; Chopra, S.; Engineer, R.; Shrivastava, S.K.; Gupta, S.; Ghosh, J.; et al. Correlation of Hematological Parameters With Clinical Outcomes in Cervical Cancer Patients Treated With Radical Radio(chemo)therapy: A Retrospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 182–191. [Google Scholar] [CrossRef]
- Elmali, A.; Guler, O.C.; Demirhan, B.; Yavuz, M.; Onal, C. Long-term analysis of hematological parameters as predictors of recurrence patterns and treatment outcomes in cervical cancer patients undergoing definitive chemoradiotherapy. Strahlenther. Onkol. 2024, 11, 949–957. [Google Scholar] [CrossRef]
| Characteristics | Value, Percentage, or Range |
|---|---|
| Median age; years | 54 (31–81) |
| Median body-mass-index | 26.5 (16.3–56.8) |
| Histology | |
| squamous cell carcinoma | 123 (83.7%) |
| adenocarcinoma | 18 (12.2%) |
| neuroendocrine | 2 (1.4%) |
| mixed | 4 (2.7%) |
| FIGO stage | |
| I | 4 (2.7%) |
| II | 22 (15.0%) |
| III | 105 (71.4%) |
| IVa | 16 (10.9%) |
| T-stage | |
| 1/2 | 103 (70.1%) |
| 3/4 | 44 (29.9%) |
| Grading | |
| G1 | 5 (3.4%) |
| G2 | 65 (44.2%) |
| G3 | 57 (38.8%) |
| unknown | 20 (13.6%) |
| Parametrial infiltration | |
| yes | 106 (72.1%) |
| No | 41 (27.9%) |
| Lymph node metastases | |
| yes | 105 (71.4%) |
| no | 42 (28.6%) |
| Localization lymph node metastases | |
| pelvic | 65 (61.9%) |
| pelvic + paraaortic | 40 (38.1%) |
| Size lymph node metastases | |
| ≤10 mm | 21 (20.0%) |
| 11–20 mm | 59 (56.2%) |
| >20 mm | 24 (22.9%) |
| unknown | 1 (0.9%) |
| Lymph node dissection performed | |
| yes | 76 (51.7%) |
| No | 71 (48.3%) |
| Characteristics | Value, Percentage, or Range |
|---|---|
| Median total dose; Gy | 45.0 (34.2–54.0) |
| Median SIB dose; Gy | 55 (54–58.8) |
| Median treatment duration; days | 53 (29–152) |
| Extended paraaortic RT field | |
| yes | 39 (26.5%) |
| no | 108 (73.5%) |
| RT technique | |
| IMRT | 138 (93.9%) |
| 3D | 9 (6.1%) |
| Brachytherapy | |
| yes | 146 (99.3%) |
| no | 1 (0.7%) |
| Chemotherapy | |
| Cisplatin | 140 (95.3%) |
| Carboplatin | 3 (2%) |
| Cis- and Carboplatin | 1 (0.7%) |
| Mitomycin C/5-FU | 3 (2%) |
| Cisplatin cycles | |
| ≤4 | 38 (27.1%) |
| ≥5 | 97 (69.3%) |
| unclear documentation | 5 (3.6%) |
| Median hemoglobin nadir; g/dL | 9.8 (7.5–13.5) |
| Red blood cell transfusion | |
| median | 1.5 (0–17) |
| 0 | 81 (55.1%) |
| 2–4 | 54 (36.7%) |
| ≥5 | 12 (8.2%) |
| Median time from lymph node dissection to RT start; days | 40 (11–100) |
| Characteristics | Overall Survival | Local Control | Distant Control | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| FIGO stage (1/2 vs. 3/4) | 2.04 (1.29–3.25) | 0.003 | 2.90 (1.31–6.40) | 0.008 | 2.18 (1.33–3.56) | 0.002 |
| T stage (1/2 vs. 3/4) | 1.45 (1.08–1.95) | 0.013 | 1.87 (1.18–2.99) | 0.008 | 1.25 (0.90–1.72) | 0.179 |
| Histology (squamous cell carcinoma vs. other) | 1.00 (0.71–1.41) | 0.986 | 0.74 (0.31–1.75) | 0.492 | 1.19 (0.89–1.59) | 0.239 |
| nodal status (N0 vs. N1) | 1.43 (0.77–2.66) | 0.246 | 1.36 (0.45–3.72) | 0.549 | 2.16 (1.05–4.45) | 0.037 |
| lymph node size (≤20 mm vs. >20 mm) | 1.04 (1.01–1.07) | 0.005 | 1.01 (0.92–1.05) | 0.790 | 1.04 (1.02–1.07) | 0.003 |
| localization lymph node (pelvic vs. paraaortic) | 1.62 (1.11–2.35) | 0.012 | 1.78 (0.95–3.34) | 0.071 | 1.88 (1.27–2.79) | 0.002 |
| lymph node dissection (yes vs. no) | 0.98 (0.58–1.67) | 0.942 | 1.11 (0.47–2.61) | 0.819 | 1.40 (0.79–2.46) | 0.247 |
| Parametrial infiltration (yes. vs. no) | 1.21 (0.65–2.26) | 0.543 | 3.61 (0.84–15.56) | 0.085 | 0.97 (0.52–1.80) | 0.922 |
| RT technique (IMRT vs. 3D) | 1.55 (0.62–3.88) | 0.353 | 0.05 (0–529.4) | 0.518 | 1.31 (0.32–5.40) | 0.709 |
| extended RT field (yes vs. no) | 2.50 (1.45–4.33) | 0.001 | 3.03 (1.24–7.43) | 0.016 | 2.51 (1.41–4.55) | 0.002 |
| Treatment duration (≤ 56 days vs. >56 days) | 1.59 (0.90–2.82) | 0.114 | 2.96 (1.21–7.26) | 0.018 | 1.18 (0.60–2.31) | 0.626 |
| Hemoglobin nadir (<9 vs. ≥9 g/dL) | 0.56 (0.31–1.01) | 0.055 | 0.356 (0.15–0.87) | 0.023 | 0.64 (0.34–1.21) | 0.167 |
| red blood cell transfusion (yes vs. no) | 1.16 (1.04–1.29) | 0.008 | 1.28 (1.10–1.50) | 0.002 | 1.20 (1.08–1.33) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meixner, E.; Wermes, L.; Hoeltgen, L.; von Diest, L.A.; Sandrini, E.; Harrabi, S.; Seidensaal, K.; Hoegen-Saßmannshausen, P.; Vinsensia, M.; König, L.; et al. Hematologic Toxicity Profiles and the Impact of Hemoglobin Nadir and Transfusion on Oncologic Outcome in Definitive Radiochemotherapy for Cervical Cancer. Cancers 2024, 16, 3986. https://doi.org/10.3390/cancers16233986
Meixner E, Wermes L, Hoeltgen L, von Diest LA, Sandrini E, Harrabi S, Seidensaal K, Hoegen-Saßmannshausen P, Vinsensia M, König L, et al. Hematologic Toxicity Profiles and the Impact of Hemoglobin Nadir and Transfusion on Oncologic Outcome in Definitive Radiochemotherapy for Cervical Cancer. Cancers. 2024; 16(23):3986. https://doi.org/10.3390/cancers16233986
Chicago/Turabian StyleMeixner, Eva, Laura Wermes, Line Hoeltgen, Lisa Antonia von Diest, Elisabetta Sandrini, Semi Harrabi, Katharina Seidensaal, Philipp Hoegen-Saßmannshausen, Maria Vinsensia, Laila König, and et al. 2024. "Hematologic Toxicity Profiles and the Impact of Hemoglobin Nadir and Transfusion on Oncologic Outcome in Definitive Radiochemotherapy for Cervical Cancer" Cancers 16, no. 23: 3986. https://doi.org/10.3390/cancers16233986
APA StyleMeixner, E., Wermes, L., Hoeltgen, L., von Diest, L. A., Sandrini, E., Harrabi, S., Seidensaal, K., Hoegen-Saßmannshausen, P., Vinsensia, M., König, L., Arians, N., Debus, J., & Hörner-Rieber, J. (2024). Hematologic Toxicity Profiles and the Impact of Hemoglobin Nadir and Transfusion on Oncologic Outcome in Definitive Radiochemotherapy for Cervical Cancer. Cancers, 16(23), 3986. https://doi.org/10.3390/cancers16233986







