Frailty and Overall Survival of Older Patients Undergoing Radiotherapy for Head and Neck Cancer: A Prospective Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
- Patients aged 65 years or older;
- Histologically confirmed diagnosis of head and neck cancer;
- No prior active cancer treatments;
- Eligible for radiotherapy.
- Age under 65 years;
- Previous antineoplastic treatment or head and neck irradiation;
- Metastatic disease;
- Inability to provide valid informed consent.
2.2. Geriatric Assessment
2.3. Radiotherapy and Systemic Therapy
2.4. Statistical Analysis
3. Results
3.1. Patient Demographic and Clinical Characteristics
3.2. Outcomes
3.2.1. Overall Survival Analysis
3.2.2. RT-Related Toxicity
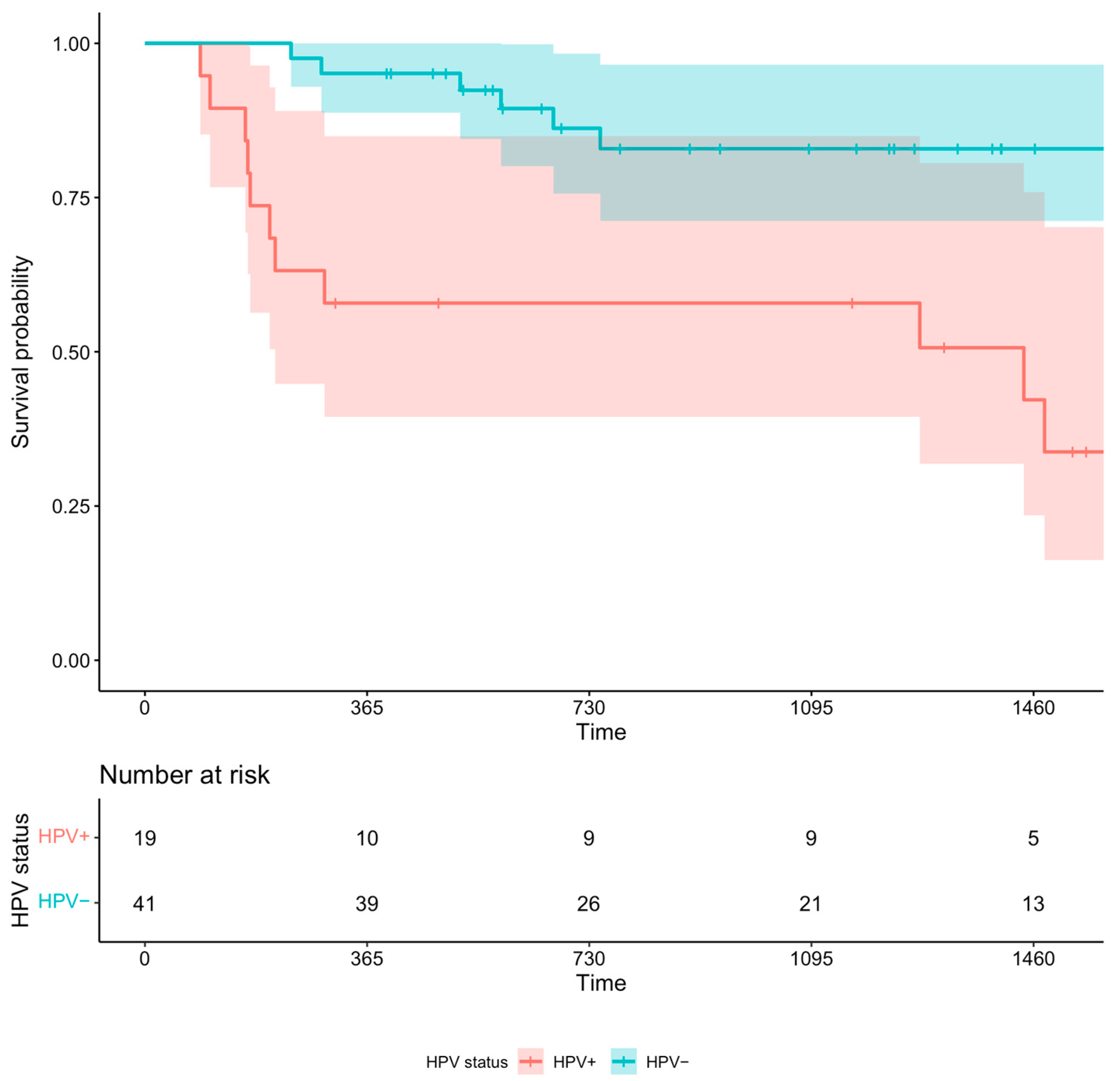
3.2.3. Sub-Analysis: HPV Status and OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grénman, R.; Chevalier, D.; Gregoire, V.; Myers, E.; Rogers, S. Treatment of Head and Neck Cancer in the Elderly: European Consensus (Panel 6) at the EUFOS Congress in Vienna 2007. Eur. Arch. Otorhinolaryngol. 2010, 267, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Syrigos, K.N.; Karachalios, D.; Karapanagiotou, E.M.; Nutting, C.M.; Manolopoulos, L.; Harrington, K.J. Head and Neck Cancer in the Elderly: An Overview on the Treatment Modalities. Cancer Treat. Rev. 2009, 35, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Dua, D.; Kelly, C.; Bossi, P. Managing Older Patients with Head and Neck Cancer: The Non-Surgical Curative Approach. J. Geriatr. Oncol. 2018, 9, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.L.; Sanabria, A.; Robbins, K.T.; Halmos, G.B.; Strojan, P.; Ng, W.T.; Takes, R.P.; Angelos, P.; Piazza, C.; De Bree, R.; et al. Management of Older Patients with Head and Neck Cancer: A Comprehensive Review. Adv. Ther. 2023, 40, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Vermorken, J.B. Treatment of Elderly Patients with Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2016, 6, 199. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Halmos, G.B.; Strojan, P.; De Bree, R.; Bossi, P.; Bradford, C.R.; Rinaldo, A.; Vander Poorten, V.; Sanabria, A.; Takes, R.P.; et al. The Role of Age in Treatment-related Adverse Events in Patients with Head and Neck Cancer: A Systematic Review. Head Neck 2019, 41, 2410–2429. [Google Scholar] [CrossRef]
- Pignon, J.-P.; Maître, A.L.; Maillard, E.; Bourhis, J. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Haddad, R.I. Management of Elderly Patients with Locoregionally Confined Head and Neck Cancer. Lancet Oncol. 2017, 18, e274–e283. [Google Scholar] [CrossRef]
- Belgioia, L.; De Felice, F.; Bacigalupo, A.; Alterio, D.; Argenone, A.; D’Angelo, E.; Desideri, I.; Franco, P.F.; Merlotti, A.; Musio, D.; et al. Results of a Survey on Elderly Head and Neck Cancer Patients on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Acta Otorhinolaryngol. Ital. 2020, 40, 405–409. [Google Scholar] [CrossRef]
- Neve, M.; Jameson, M.B.; Govender, S.; Hartopeanu, C. Impact of Geriatric Assessment on the Management of Older Adults with Head and Neck Cancer: A Pilot Study. J. Geriatr. Oncol. 2016, 7, 457–462. [Google Scholar] [CrossRef]
- Belgioia, L.; Bacigalupo, A.; Missale, F.; Vecchio, S.; Chiola, I.; Callegari, S.; Verzanini, E.; Peretti, G.; Corvò, R. Individualized Treatment of Head Neck Squamous Cell Carcinoma Patients Aged 70 or Older with Radiotherapy Alone or Associated to Cisplatin or Cetuximab: Impact of Weekly Radiation Dose on Loco-Regional Control. Med. Oncol. 2019, 36, 42. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.R.; Powers, A.E.; Vujovic, D.; Roof, S.; Bakst, R.L. Clinical and Therapeutic Considerations for Older Adults with Head and Neck Cancer. Clin. Interv. Aging 2023, 18, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, R.; Zumsteg, Z.S.; BrintzenhofeSzoc, K.; Trevino, K.M.; Gajra, A.; Korc-Grodzicki, B.; Epstein, J.B.; Bond, S.M.; Parker, I.; Kish, J.A.; et al. The Older Adult with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: Knowledge Gaps and Future Direction in Assessment and Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 868–883. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, N.A.; Deal, A.M.; Comitz, E.; Stravers, L.; Muss, H.; Reeve, B.B.; Basch, E.; Tepper, J.; Chera, B. Geriatric Assessment as a Predictor of Tolerance, Quality of Life, and Outcomes in Older Patients With Head and Neck Cancers and Lung Cancers Receiving Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 850–857. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Van Den Noortgate, N.; De Neve, W.; Rottey, S.; Geldhof, K.; et al. Serial Comprehensive Geriatric Assessment in Elderly Head and Neck Cancer Patients Undergoing Curative Radiotherapy Identifies Evolution of Multidimensional Health Problems and Is Indicative of Quality of Life. Eur. J. Cancer Care 2014, 23, 401–412. [Google Scholar] [CrossRef]
- Bossi, P.; Esposito, A.; Vecchio, S.; Nicolai, P.; Tarsitano, A.; Mirabile, A.; Ursino, S.; Cau, M.C.; Bonomo, P.; Marengoni, A.; et al. 864MO Role of Geriatric Assessment in Tailoring Treatment of Locally Advanced Head and Neck Cancer: The ELDERLY Study. Ann. Oncol. 2021, 32, S789. [Google Scholar] [CrossRef]
- Restifo, D.; Raab, G.; McBride, S.M.; Pfister, D.G.; Wong, R.J.; Lee, N.Y.; Shahrokni, A.; Zakeri, K. Correlation of an Electronic Geriatric Assessment With Receipt of Adjuvant Radiation and Chemotherapy in Older Adults with Head and Neck Cancer. Adv. Radiat. Oncol. 2023, 8, 101096. [Google Scholar] [CrossRef]
- Brugel, L.; Laurent, M.; Caillet, P.; Radenne, A.; Durand-Zaleski, I.; Martin, M.; Baron, M.; de Kermadec, H.; Bastuji-Garin, S.; Canouï-Poitrine, F.; et al. Impact of Comprehensive Geriatric Assessment on Survival, Function, and Nutritional Status in Elderly Patients with Head and Neck Cancer: Protocol for a Multicentre Randomised Controlled Trial (EGeSOR). BMC Cancer 2014, 14, 427. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Pushkar Gold, D.; Cohen, C.A.; Zucchero, C.A. Clock-drawing and Dementia in the Community: A Longitudinal Study. Int. J. Geriatr. Psychiatry 1993, 8, 487–496. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B. The Mini Nutritional Assessment (MNA) for Grading the Nutritional State of Elderly Patients: Presentation of the MNA, History and Validation. In Nestlé Nutrition Workshop Series: Clinical & Performance Program; Vellas, B., Garry, P.J., Guigoz, Y., Eds.; KARGER: Basel, Switzerland, 1999; Volume 1, pp. 3–12. ISBN 978-3-8055-6803-6. [Google Scholar]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A New Facility for the Measurement of Health-Related Quality of Life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Abete, P.; Basile, C.; Bulli, G.; Curcio, F.; Liguori, I.; Della-Morte, D.; Gargiulo, G.; Langellotto, A.; Testa, G.; Galizia, G.; et al. The Italian Version of the “Frailty Index” Based on Deficits in Health: A Validation Study. Aging Clin. Exp. Res. 2017, 29, 913–926. [Google Scholar] [CrossRef]
- Pottel, L.; Boterberg, T.; Pottel, H.; Goethals, L.; Van Den Noortgate, N.; Duprez, F.; De Neve, W.; Rottey, S.; Geldhof, K.; Van Eygen, K.; et al. Determination of an Adequate Screening Tool for Identification of Vulnerable Elderly Head and Neck Cancer Patients Treated with Radio(Chemo)Therapy. J. Geriatr. Oncol. 2012, 3, 24–32. [Google Scholar] [CrossRef]
- Bras, L.; Peters, T.T.A.; Wedman, J.; Plaat, B.E.C.; Witjes, M.J.H.; van Leeuwen, B.L.; van der Laan, B.F.A.M.; Halmos, G.B. Predictive Value of the Groningen Frailty Indicator for Treatment Outcomes in Elderly Patients after Head and Neck, or Skin Cancer Surgery in a Retrospective Cohort. Clin. Otolaryngol. 2015, 40, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Siwakoti, K.; Giri, S.; Nabell, L.; VanderWalde, N.A.; McDonald, A.; Williams, G.R. Prevalence and Impact of Frailty and Geriatric Assessment-Identified Impairments among Older Adults Diagnosed with Head and Neck Cancers. J. Geriatr. Oncol. 2024, 15, 101749. [Google Scholar] [CrossRef] [PubMed]
- Nieman, C.L.; Pitman, K.T.; Tufaro, A.P.; Eisele, D.W.; Frick, K.D.; Gourin, C.G. The Effect of Frailty on Short-Term Outcomes after Head and Neck Cancer Surgery. Laryngoscope 2018, 128, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kim, S.-A.; Roh, J.-L.; Lee, S.-W.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. An Introduction to a Head and Neck Cancer-Specific Frailty Index and Its Clinical Implications in Elderly Patients: A Prospective Observational Study Focusing on Respiratory and Swallowing Functions. Oncologist 2016, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Bras, L.; Sidorenkov, G.; Festen, S.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Witjes, M.J.H.; van der Laan, B.F.A.M.; de Bock, G.H.; Halmos, G.B. Frailty Is Associated with Decline in Health-Related Quality of Life of Patients Treated for Head and Neck Cancer. Oral Oncol. 2020, 111, 105020. [Google Scholar] [CrossRef]
- Padovan, B.V.; Bijl, M.A.J.; Langendijk, J.A.; van der Laan, H.P.; Van Dijk, B.A.C.; Festen, S.; Halmos, G.B. Evaluation of a New Two-Step Frailty Assessment of Head and Neck Patients in a Prospective Cohort. Eur. Arch. Otorhinolaryngol. 2024, 281, 4291–4304. [Google Scholar] [CrossRef]
- Mighali, P.; D’Angelo, E. Non Elective Vulnerable Elderly Radiotherapy. Identifier NCT04832555. 2021. Available online: https://clinicaltrials.gov/study/NCT04832555?term=NEVER&rank=3#study-overview (accessed on 19 November 2024).
- O’Donovan, A.; Leech, M.; Gillham, C. Assessment and Management of Radiotherapy Induced Toxicity in Older Patients. J. Geriatr. Oncol. 2017, 8, 421–427. [Google Scholar] [CrossRef]
- Lu, D.J.; Luu, M.; Mita, A.; Scher, K.; Shiao, S.L.; Yoshida, E.P.; Sittig, M.P.; Mallen-St Clair, J.; Ho, A.S.; Zumsteg, Z.S. Human Papillomavirus-Associated Oropharyngeal Cancer among Patients Aged 70 and Older: Dramatically Increased Prevalence and Clinical Implications. Eur. J. Cancer 2018, 103, 195–204. [Google Scholar] [CrossRef]
- Dave, E.; Su, W.; Gupta, V.; Miles, B.; Demicco, E.; Soriano, T.; Bakst, R.L. Human Papilloma Virus-Positive Oropharyngeal Squamous Cell Carcinoma in the Elderly. Anticancer Res. 2017, 37, 1847–1851. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet Lond. Engl. 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty, fitness, and the mathematics of deficit accumulation. Rev. Clin. Gerontol. 2007, 17, 1–12. [Google Scholar] [CrossRef]
- Mitnitski, A.; Song, X.; Skoog, I.; Broe, G.A.; Cox, J.L.; Grunfeld, E.; Rockwood, K. Relative Fitness and Frailty of Elderly Men and Women in Developed Countries and Their Relationship with Mortality. J. Am. Geriatr. Soc. 2005, 53, 2184–2189. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value n = 117 | IQR (0.25–0.75) |
|---|---|---|
| Sex (male) | 87 (74.4%) | |
| Age at diagnosis, y (median) | 76 | 72–81 |
| Smoking (yes vs. no) Smokers, PY (median) | 87 (74.4%) 38 | 4.5–70 |
| Cancer type Oropharynx Oral cavity Hypopharynx Nasal cavity and paranasal sinus Larynx Salivary glands Unknown primary tumor | 60 (51.3%) 13 (11.1%) 9 (7.7%) 4 (3.4%) 22 (18.8%) 1 (0.9%) 8 (6.8%) | |
| TNM staging T (1–2) T (3–4) N (0–1) N (2–3) | 35 (29.9%) 82 (70.1%) 44 (37.6%) 73 (62.4%) | |
| Tumor stage 1 2 3 4 | 5 (4.3%) 5 (4.3%) 19 (16.2%) 88 (75.2%) | |
| Concomitant chemotherapy | 43 (36.8%) | |
| Purpose of RT Curative Adjuvant | 96 (82%) 21 (18%) | |
| Geriatric assessment (median) | ||
| MMSE (n = 116) | 27.6 | 26–29 |
| CDT (n = 112) | 2 | 1–4 |
| MNA | 23.5 | 21–25 |
| IADL Lost IADL (≥1) | 8 34 (29.1%) | 5–8 |
| Barthel Index Barthel Index ≤ 90 | 100 19 (16.2%) | 95–100 |
| CIRS Severity Comorbidity | 1.9 4 | 1.7–2.1 3–5 |
| GDS (n = 113) GDS > 5 | 3 17 (15%) | 1–5 |
| TUG (n = 106) TUG ≥ 15” | 8.3 10 (9.4%) | 7–10 |
| HG (n = 107) M F | 27 30 18.3 | 21.9–35.6 25.4–37.4 16.1–20.9 |
| N of drugs | 5 | 2.8–7 |
| EuroQol (n = 106) | 0.78 | 0.68–0.85 |
| FI Fit (≤0.08) Pre-frail (0.09–0.24) Frail (≥0.25) | 0.16 20 (17.1%) 68 (58.1%) 29 (24.8%) | 0.10–0.25 |
| Univariate | Multivariate Model 1 | Multivariate Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age at diagnosis | 1.035 (0.992–1.080) | 0.116 | 1.013 (0.942–1.091) | 0.723 | 0.993 (0.939–1.049) | 0.792 |
| Smoking | 1.008 (1.002–1.015) | 0.012 | 1.002 (0.993–1.012) | 0.639 | ||
| T stage (ref T1-2) | 1.965 (0.979–3.940) | 0.057 | ||||
| N stage (ref 0) 1 | 1.801 (0.238–13.608) | 0.568 | ||||
| N 2-3 | 1.303 (0.717–2.367) | 0.385 | ||||
| Tumor stage | 1.557 (0.958–2.580) | 0.074 | 2.278 (1.225–4.236) | 0.009 | 1.728 (1.023–2.917) | 0.041 |
| Concomitant chemotherapy | 0.419 (0.214–0.820) | 0.011 | 0.883 (0.313–2.488) | 0.813 | 0.420 (0.176–0.999) | 0.050 |
| MMSE | 0.965 (0.882–1.055) | 0.435 | ||||
| CDT | 0.963 (0.786–1.179) | 0.712 | ||||
| MNA | 0.883 (0.829–0.940) | <0.001 | 0.958 (0.862–1.063) | 0.418 | ||
| Lost IADL | 1.482 (1.288–1.706) | <0.001 | 1.451 (1.087–1.936) | 0.012 | ||
| Barthel Index | 0.948 (0.926–0.971) | <0.001 | 0.919 (0.850–0.995) | 0.036 | ||
| CIRS severity | 2.570 (1.218–5.423) | 0.013 | 6.087 (0.720–51.448) | 0.097 | ||
| CIRS comorbidity | 1.206 (1.041–1.397) | 0.013 | 0.786 (0.529–1.167) | 0.233 | ||
| GDS | 1.116 (1.032–1.207) | 0.006 | 1.091 (0.937–1.272) | 0.261 | ||
| TUG | 1.063 (1.006–1.124) | 0.031 | 0.922 (0.834–1.019) | 0.113 | ||
| HG | 0.971 (0.939–1.004) | 0.084 | 0.994 (0.953–1.038) | 0.797 | ||
| N of drugs | 1.158 (1.054–1.271) | 0.002 | 0.953 (0.781–1.165) | 0.643 | ||
| EuroQoL | 0.113 (0.028–0.451) | 0.002 | 0.760 (0.055–10.507) | 0.838 | ||
| FI (+0.1) | 1.640 (1.33–2.022) | <0.001 | 1.478 (1.182–1.848) | <0.001 | ||
| Concordance: 0.742 | Concordance: 0.690 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannotti, C.; Ottaviani, S.; Muzyka, M.; Tagliafico, L.; Bacigalupo, A.; Belgioia, L.; Tominaj, C.; Vecchio, S.; Monacelli, F.; Nencioni, A. Frailty and Overall Survival of Older Patients Undergoing Radiotherapy for Head and Neck Cancer: A Prospective Analysis. Cancers 2024, 16, 3939. https://doi.org/10.3390/cancers16233939
Giannotti C, Ottaviani S, Muzyka M, Tagliafico L, Bacigalupo A, Belgioia L, Tominaj C, Vecchio S, Monacelli F, Nencioni A. Frailty and Overall Survival of Older Patients Undergoing Radiotherapy for Head and Neck Cancer: A Prospective Analysis. Cancers. 2024; 16(23):3939. https://doi.org/10.3390/cancers16233939
Chicago/Turabian StyleGiannotti, Chiara, Silvia Ottaviani, Mariya Muzyka, Luca Tagliafico, Almalina Bacigalupo, Liliana Belgioia, Celjeta Tominaj, Stefania Vecchio, Fiammetta Monacelli, and Alessio Nencioni. 2024. "Frailty and Overall Survival of Older Patients Undergoing Radiotherapy for Head and Neck Cancer: A Prospective Analysis" Cancers 16, no. 23: 3939. https://doi.org/10.3390/cancers16233939
APA StyleGiannotti, C., Ottaviani, S., Muzyka, M., Tagliafico, L., Bacigalupo, A., Belgioia, L., Tominaj, C., Vecchio, S., Monacelli, F., & Nencioni, A. (2024). Frailty and Overall Survival of Older Patients Undergoing Radiotherapy for Head and Neck Cancer: A Prospective Analysis. Cancers, 16(23), 3939. https://doi.org/10.3390/cancers16233939






