Prognostic Role of Inflammatory and Nutritional Biomarkers in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy as First-Line
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population and Data Collection on Disease and Treatment
2.2. Laboratory Tests and Biomarkers
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. ESMO Guidelines Committee. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Jaiyesimi, I.A.; Leighl, N.B.; Ismaila, N.; Alluri, K.; Florez, N.; Gadgeel, S.; Masters, G.; Schenk, E.L.; Schneider, B.J.; Sequist, L.; et al. Therapy for Stage IV Non-Small Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024, 42, e1–e22. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 75, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. POSEIDON investigators. Durvalumab with or Without Tremelimumab in Combination with Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2023, 41, 1213–1227. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.; Zhou, Q. The Resistance Mechanisms of Lung Cancer Immunotherapy. Front. Oncol. 2020, 10, 568059. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Sacchi de Camargo Correia, G.; Pai, T.; Li, S.; Connor, D.; Zhao, Y.; Lou, Y.; Manochakian, R. Immune-Related Adverse Events in Patients with Lung Cancer. Curr. Oncol. Rep. 2023, 25, 1259–1275. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Ning, J.; Zhang, M. Immune checkpoint inhibitors as first-line therapy for non-small cell lung cancer: A systematic evaluation and meta-analysis. Hum. Vaccines Immunother. 2023, 19, 2169531. [Google Scholar] [CrossRef]
- Brueckl, W.M.; Ficker, J.H.; Zeitler, G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020, 20, 1185. [Google Scholar] [CrossRef]
- Ishida, S.; Hashimoto, I.; Seike, T.; Abe, Y.; Nakaya, Y.; Nakanishi, H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: A retrospective study. J. Med. Investig. 2014, 61, 361–368. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Zaitsu, J.; Yamashita, Y.; Ishikawa, A.; Saito, A.; Kagimoto, A.; Mimura, T.; Hirakawa, T.; Mito, M.; Fukuhara, K.; Senoo, T.; et al. Systemic Inflammatory Score Predicts Response and Prognosis in Patients with Lung Cancer Treated with Immunotherapy. Anticancer Res. 2021, 41, 3673–3682. [Google Scholar] [CrossRef]
- Mountzios, G.; Samantas, E.; Senghas, K.; Zervas, E.; Krisam, J.; Samitas, K.; Bozorgmehr, F.; Kuon, J.; Agelaki, S.; Baka, S.; et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 2021, 6, 100254. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Uchino, J.; Yokoi, T.; Kijima, T.; Goto, Y.; Suga, Y.; Katayama, Y.; Nakamura, R.; Morimoto, K.; Nakao, A.; et al. Prognostic Nutritional Index and Lung Immune Prognostic Index as Prognostic Predictors for Combination Therapies of Immune Checkpoint Inhibitors and Cytotoxic Anticancer Chemotherapy for Patients with Advanced Non-Small Cell Lung Cancer. Diagnostics 2022, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 15 October 2024).
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Shi, R.; Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013, 13, 158. [Google Scholar] [CrossRef]
- Li, D.; Yuan, X.; Liu, J.; Li, C.; Li, W. Prognostic value of prognostic nutritional index in lung cancer: A meta-analysis. J. Thorac. Dis. 2018, 10, 5298–5307. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Li, L.; Lv, J. Prognostic Role of Pretreatment Prognostic Nutritional Index in Advanced Lung Cancer Patients Receiving First-Line Immunotherapy: A Meta-Analysis. Cureus 2024, 16, e52720. [Google Scholar] [CrossRef]
- Oku, Y.; Toyokawa, G.; Wakasu, S.; Kinoshita, F.; Takamori, S.; Watanabe, K.; Haratake, N.; Nagano, T.; Kosai, K.; Takada, K.; et al. Impact of the pretreatment prognostic nutritional index on the survival after first-line immunotherapy in non-small-cell lung cancer patients. Cancer Med. 2023, 12, 14327–14336. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, L.; Patel, S.H.; Lopez, G.; Grogan, M.; Li, M.; Haddad, T.; Johns, A.; Ganesan, L.P.; Yang, Y.; et al. Serum Albumin: Early Prognostic Marker of Benefit for Immune Checkpoint Inhibitor Monotherapy but Not Chemoimmunotherapy. Clin. Lung Cancer 2022, 23, 345–355. [Google Scholar] [CrossRef]
- Banna, G.L.; Cantale, O.; Muthuramalingam, S.; Cave, J.; Comins, C.; Cortellini, A.; Addeo, A.; Signori, A.; McKenzie, H.; Escriu, C.; et al. Efficacy outcomes and prognostic factors from real-world patients with advanced non-small-cell lung cancer treated with first-line chemoimmunotherapy: The Spinnaker retrospective study. Int. Immunopharmacol. 2022, 110, 108985. [Google Scholar] [CrossRef]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022, 7, 100445. [Google Scholar] [CrossRef]
- Lenci, E.; Cantini, L.; Pecci, F.; Cognigni, V.; Agostinelli, V.; Mentrasti, G.; Lupi, A.; Ranallo, N.; Paoloni, F.; Rinaldi, S.; et al. The Gustave Roussy Immune (GRIm)-Score Variation Is an Early-on-Treatment Biomarker of Outcome in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Pembrolizumab. J. Clin. Med. 2021, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Q.; Yan, T.; Guo, D.; Liu, J.; Wang, G.; Du, J. The Prognostic Value of Multiple Systemic Inflammatory Biomarkers in Preoperative Patients with Non-small Cell Lung Cancer. Front. Surg. 2022, 9, 830642. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Ferrara, R.; Rebuzzi, S.E.; Proto, C.; Signorelli, D.; Galli, G.; De Toma, A.; Randon, G.; Pagani, F.; Viscardi, G.; et al. EPSILoN: A Prognostic Score for Immunotherapy in Advanced Non-Small-Cell Lung Cancer: A Validation Cohort. Cancers 2019, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, M.; Igawa, S.; Manaka, H.; Yamada, K.; Akazawa, Y.; Manabe, H.; Yagami, Y.; Yamamoto, H.; Ito, H.; Kaizuka, N.; et al. The Glasgow Prognostic Score Predicts Outcomes of Pembrolizumab or Atezolizumab Monotherapy in Patients with Pretreated Non-Small Cell Lung Cancer. Oncology 2023, 101, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hashimoto, K.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Kobayashi, K.; Imai, H.; Kuji, I.; et al. Clinical impact of inflammatory and nutrition index based on metabolic tumor activity in non-small cell lung cancer treated with immunotherapy. Oncol. Lett. 2024, 27, 110. [Google Scholar] [CrossRef]
- Li, Y.; Jin, G.; Liu, N.; Guo, H.; Xu, F. The post-chemotherapy changes of tumor physical microenvironment: Targeting extracellular matrix to address chemoresistance. Cancer Lett. 2024, 582, 216583. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Li, Y.; Zhang, F.; Cai, M.; Zhang, X.; Chen, J.; Ji, C.; Ma, J.; Xu, F. Targeting Tumor Physical Microenvironment for Improved Radiotherapy. Small Methods 2022, 6, e2200570. [Google Scholar] [CrossRef]
| All | ICI | ICI + chemo | p Value | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| 191 (100) | 93 (48.7) | 98 (51.3) | |||
| Age at diagnosis, m (IQr) | 68.98 (64.75, 74.70) | 70.41 (63.92, 76.46) | 69.04 (64.96, 73.45) | 0.075 | |
| Sex, n (%) | M | 125 (65.4) | 59 (63.4) | 66 (67.3) | 0.648 |
| F | 66 (34.6) | 34 (36.6) | 32 (32.7) | ||
| ECOG PS, n (%) | 0 | 139 (72.8) | 68 (73.1) | 71 (72.4) | 0.924 |
| 1 | 44 (22.5) | 20 (21.5) | 23 (23.5) | ||
| 2 | 8 (4.2) | 4 (4.3) | 4 (4.1) | ||
| 3 | 1 (0.5) | 1 (1.1) | 0 (0) | ||
| Smoking, n (%) | never | 18 (9.4) | 5 (5.4) | 13 (13.3) | 0.107 |
| former | 92 (48.2) | 49 (52.7) | 43 (43.9) | ||
| smoker | 70 (36.6) | 36 (38.7) | 34 (34.7) | ||
| unknown | 11 (5.8) | 3 (3.2) | 8 (8.2) | ||
| Comorbidities, m (IQr) | 2 (1.3) | 2 (1.3) | 2 (1.3) | 0.130 | |
| Albumin, m (IQr) | 3.50 (3.00, 3.80) | 3.50 (3.20, 3.90) | 3.40 (2.92, 3.77) | 0.154 | |
| Histology, n (%) | adenocarcinoma | 140 (75.4) | 66 (71.0) | 78 (79.6) | 0.085 |
| squamous carcinoma | 44 (20.9) | 25 (26.9) | 15 (15.3) | ||
| others | 7 (3.7) | 2 (2.2) | 5 (5.1) | ||
| PDL1, n (%) | ≤1% | 63 (33.0) | 0 (0) | 63 (64.3) | <0.001 |
| 1–49 | 34 (17.8) | 0 (0) | 34 (34.7) | ||
| ≥50% | 93 (48.7) | 93 (100.0) | 0 (0) | ||
| not determinable | 1 (0.5) | 0 (0) | 1 (1.0) | ||
| Stage, n (%) | IV | 191 (100) | 93 (100) | 98 (100) | |
| Brain mets, n (%) | no | 150 (78.5) | 72 (77.4) | 78 (79.6) | 0.728 |
| yes | 41 (21.5) | 21 (22.6) | 20 (20.4) | ||
| Liver, n (%) | no | 164 (85.9) | 78 (83.9) | 86 (87.8) | 0.534 |
| yes | 27 (14.1) | 15 (16.1) | 12 (12.2) | ||
| Line, n (%) | I | 191 (100) | 93 (100) | 98 (100) | |
| Drug, n (%) | atezolizumab | 12 (6.3) | 12 (12.9) | 0 (0) | <0.001 |
| pembrolizumab | 178 (93.2) | 81 (87.1) | 97 (99.0) | ||
| IPI + NIVO | 1 (0.5) | 0 (0) | 1 (1.0) | ||
| ALI score, n (%) | ≤18 | 82 (42.9) | 41 (44.1) | 41 (41.8) | 0.772 |
| >18 | 109 (57.01) | 52 (55.9) | 57 (58.2) | ||
| LIPI, n (%) | 0 | 99 (51.8) | 53 (57.0) | 46 (46.9) | 0.367 |
| 1 | 65 (34.0) | 29 (31.2) | 36 (36.7) | ||
| 2 | 27 (14.1) | 11 (11.8) | 16 (16.3) | ||
| PNI, n (%) | <45 | 112 (58.6) | 48 (51.6) | 64 (65.3) | 0.058 |
| ≥45 | 79 (41.4) | 45 (48.4) | 34 (34.7) | ||
| SIS, n (%) | 0 | 71 (37.2) | 35 (37.6) | 36 (36.7) | 0.916 |
| 1 | 72 (37.7) | 36 (38.7) | 36 (36.7) | ||
| 2 | 48 (25.1) | 22 (23.7) | 26 (26.5) |
| PFS | OS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Pretreatment albumin | 0.93 (0.88–0.97) | 0.001 | 0.93 (0.89–0.98) | <0.001 |
| PS.ECOG | ||||
| 0 | 1 | 1 | ||
| 1 | 2.43 (1.63–3.62) | <0.0001 | 2.38 (1.58–3.59) | <0.0001 |
| 2 | 1.77 (0.76–4.08) | 0.179 | 1.77 (0.75–4.15) | 0.190 |
| 3 | 0 (0-inf) | 0.993 | 0 (0–inf) | 0.994 |
| Liver | ||||
| no | 1 | 1 | ||
| yes | 1.99 (1.21–3.29) | 0.006 | 1.79 (1.09–2.95) | 0.021 |
| LIPI | ||||
| 0 | 1 | 1 | ||
| 1 | 1.66 (1.11–2.48) | 0.012 | 1.77 (1.17–2.68) | 0.006 |
| 2 | 1.85 (1.06–3.20) | 0.027 | 1.61 (0.93–2.78) | 0.083 |
| SIS | ||||
| 0 | 1 | 1 | ||
| 1 | 1.39 (0.83–2.32) | 0.199 | 1.42 (0.85–2.38) | 0.178 |
| 2 | 0.63 (0.30–1.31) | 0.223 | 0.87 (0.42–1.81) | 0.714 |
| All | HR | p Value | ICI | HR | p Value | ICI + Chemo | HR | p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Month (95% CI) | 95% CI | Month (95% CI) | 95% CI | Month (95% CI) | 95% CI | |||||
| Prognostic score | ||||||||||
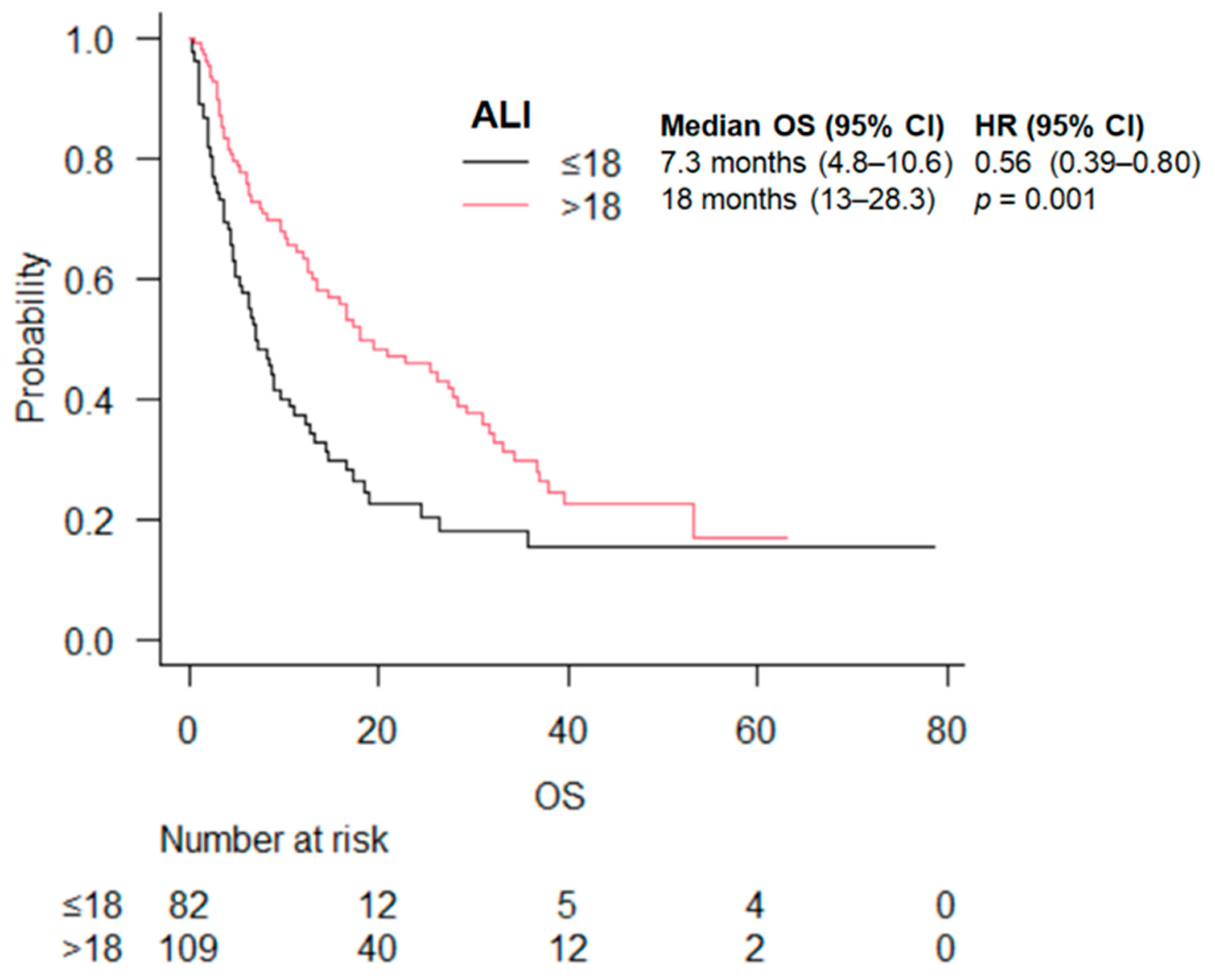
| ALI | ≤18 | 7.3 (4.8–10.6) | 0.56 (0.39–0.80) | 0.001 | 8.2 (4.5–14.6) | 0.69 (0.42–1.12) | 0.1 | 6.9 (4.2–9.7) | 0.40 (0.23–0.67) | 0.001 |
| >18 | 18.0 (13.0–28.3) | 20.8 (12.4–33.1) | 15.9 (11.4–28.3) | |||||||
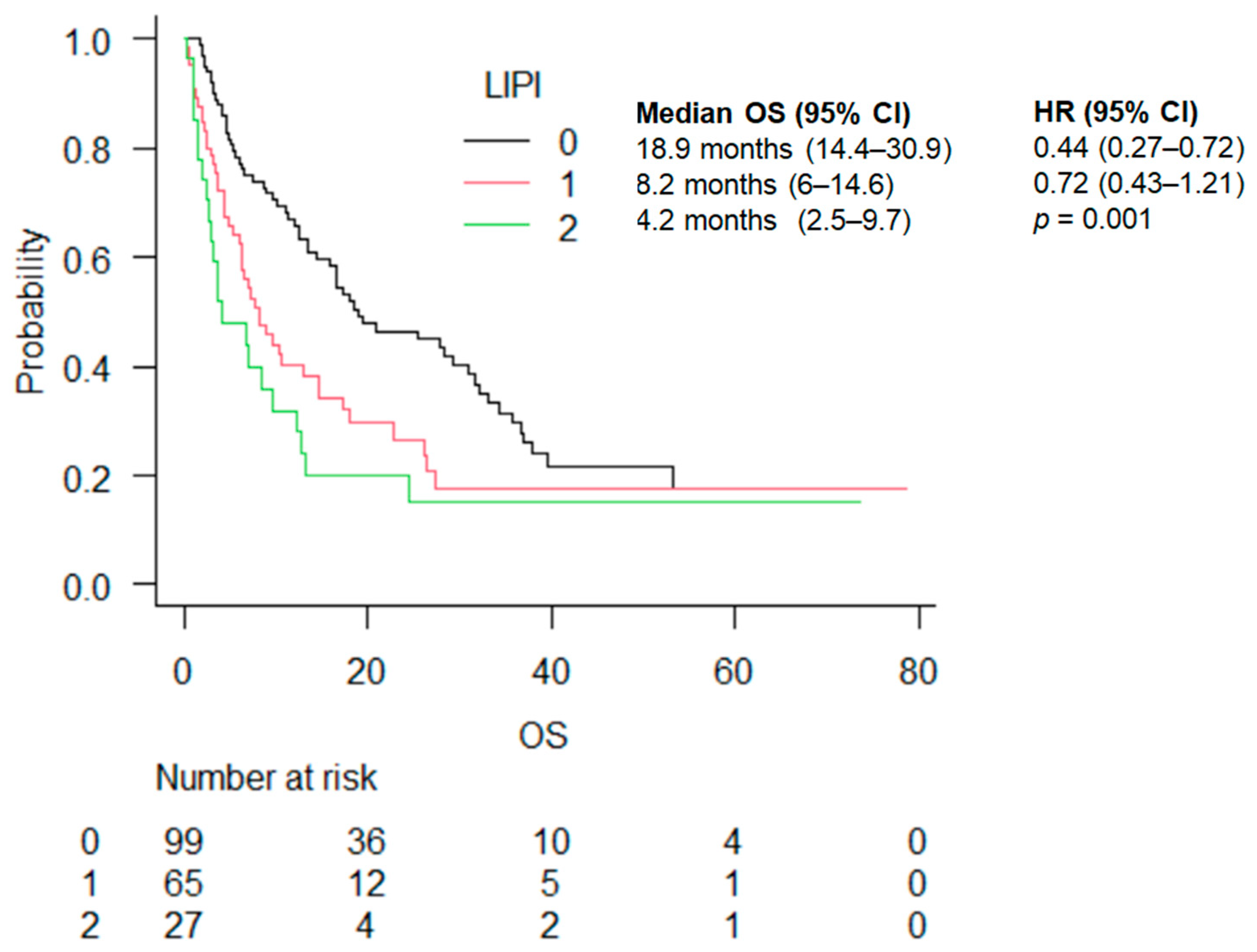
| LIPI | 0 | 18.9 (14.4–30.9) | 0.44 (0.27–0.72) | 0.001 | 18.9 (12.4–33.1) | 0.58 (0.28–1.20) | 0.2 | 16.7 (12–31.7) | 0.31 (0.16–0.62) | <0.001 |
| 1 | 8.2 (6–14.6) | 0.72 (0.43–1.21) | 8.2 (3.27–22.7) | 0.80 (0.36–1.75) | 7.7 (5.3–14.7) | 0.64 (0.33–1.27) | ||||
| 2 | 4.2 (2–5-9.7) | 6.8 (1.1–24.4) | 4 (2.5–12.3) | |||||||
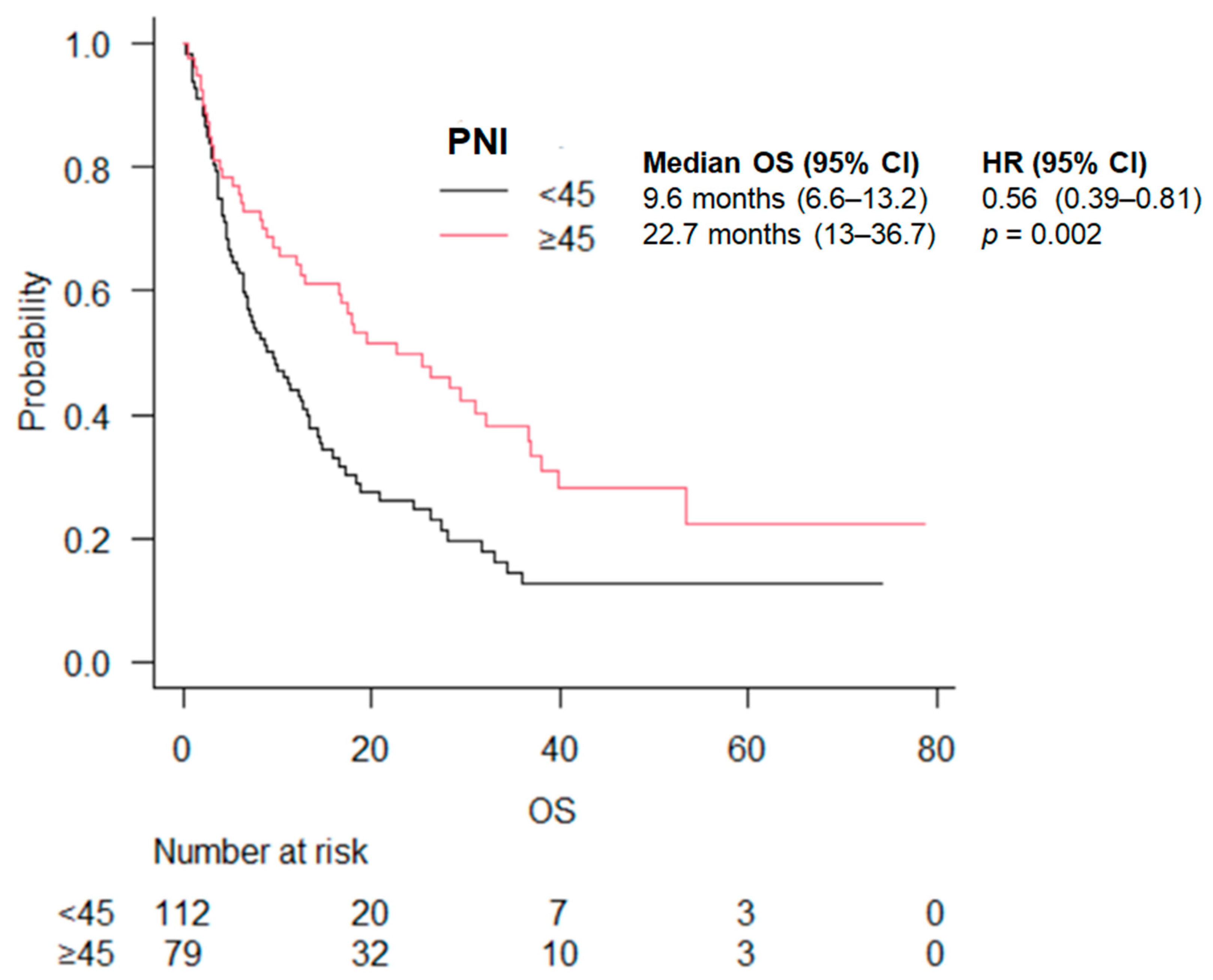
| PNI | <45 | 9.6 (6.6–13.2) | 0.56 (0.39–0.81) | 0.002 | 8.2 (4.6–17) | 0.62 (0.38–1.01) | 0.05 | 9.6 (6.3–13.5) | 0.51 (0.29–0.90) | 0.002 |
| ≥45 | 22.7 (13–36.7) | 22.7 (8.4–36.9) | 18.1 (10.3–NA) | |||||||
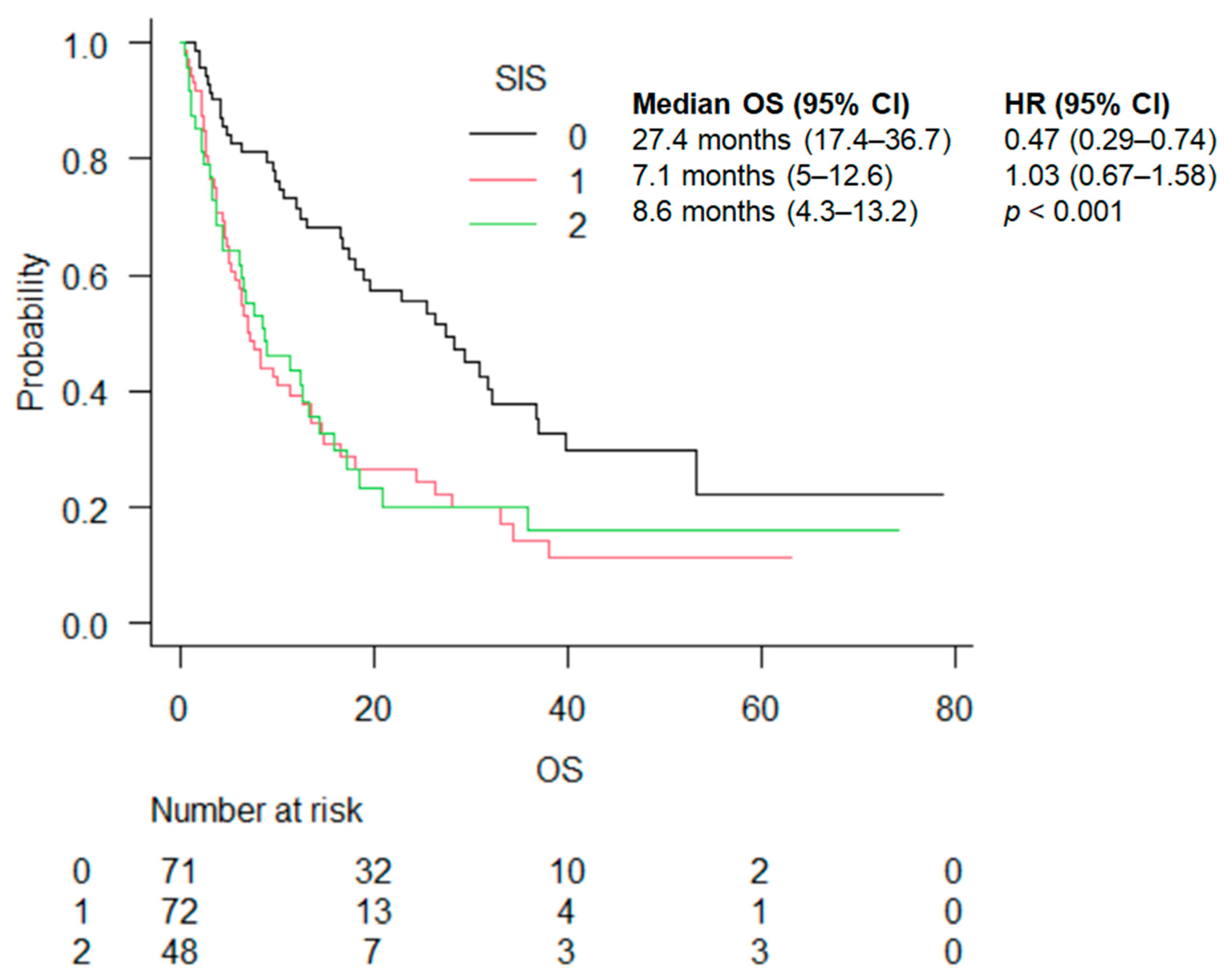
| SIS | 0 | 27.4 (17.4–36.7) | 0.47 (0.29–0.74) | <0.001 | 31 (16.6–39.7) | 0.79 (0.40–1.58) | 0.001 | 27.4 (12–31.7) | 0.24 (0.12–0.47) | <0.001 |
| 1 | 7.1 (5–12.6) | 1.03 (0.67–1.58) | 5.1 (2.5–8.3) | 2.10 (1.01–4.02) | 10 (6–16.6) | 0.44 (0.24–0.81) | ||||
| 2 | 8.6 (4.3–13.2) | 18.4 (6.3–NA) | 6 (2.9–11.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veccia, A.; Dipasquale, M.; Kinspergher, S.; Caffo, O. Prognostic Role of Inflammatory and Nutritional Biomarkers in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy as First-Line. Cancers 2024, 16, 3871. https://doi.org/10.3390/cancers16223871
Veccia A, Dipasquale M, Kinspergher S, Caffo O. Prognostic Role of Inflammatory and Nutritional Biomarkers in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy as First-Line. Cancers. 2024; 16(22):3871. https://doi.org/10.3390/cancers16223871
Chicago/Turabian StyleVeccia, Antonello, Mariachiara Dipasquale, Stefania Kinspergher, and Orazio Caffo. 2024. "Prognostic Role of Inflammatory and Nutritional Biomarkers in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy as First-Line" Cancers 16, no. 22: 3871. https://doi.org/10.3390/cancers16223871
APA StyleVeccia, A., Dipasquale, M., Kinspergher, S., & Caffo, O. (2024). Prognostic Role of Inflammatory and Nutritional Biomarkers in Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy as First-Line. Cancers, 16(22), 3871. https://doi.org/10.3390/cancers16223871







