Virtual Tumor Mapping: A New Standard for Surgeon–Pathologist Collaboration in Treating Oral Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
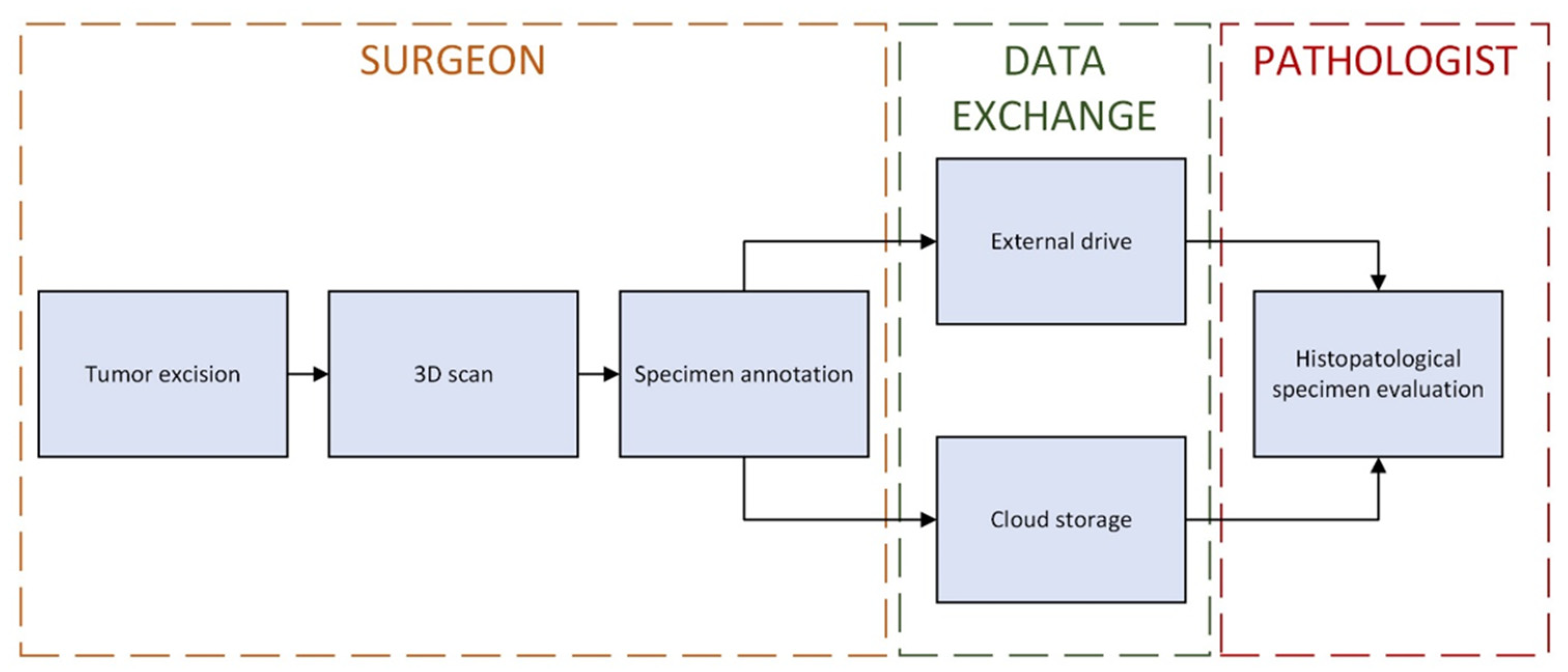
2. Materials and Methods
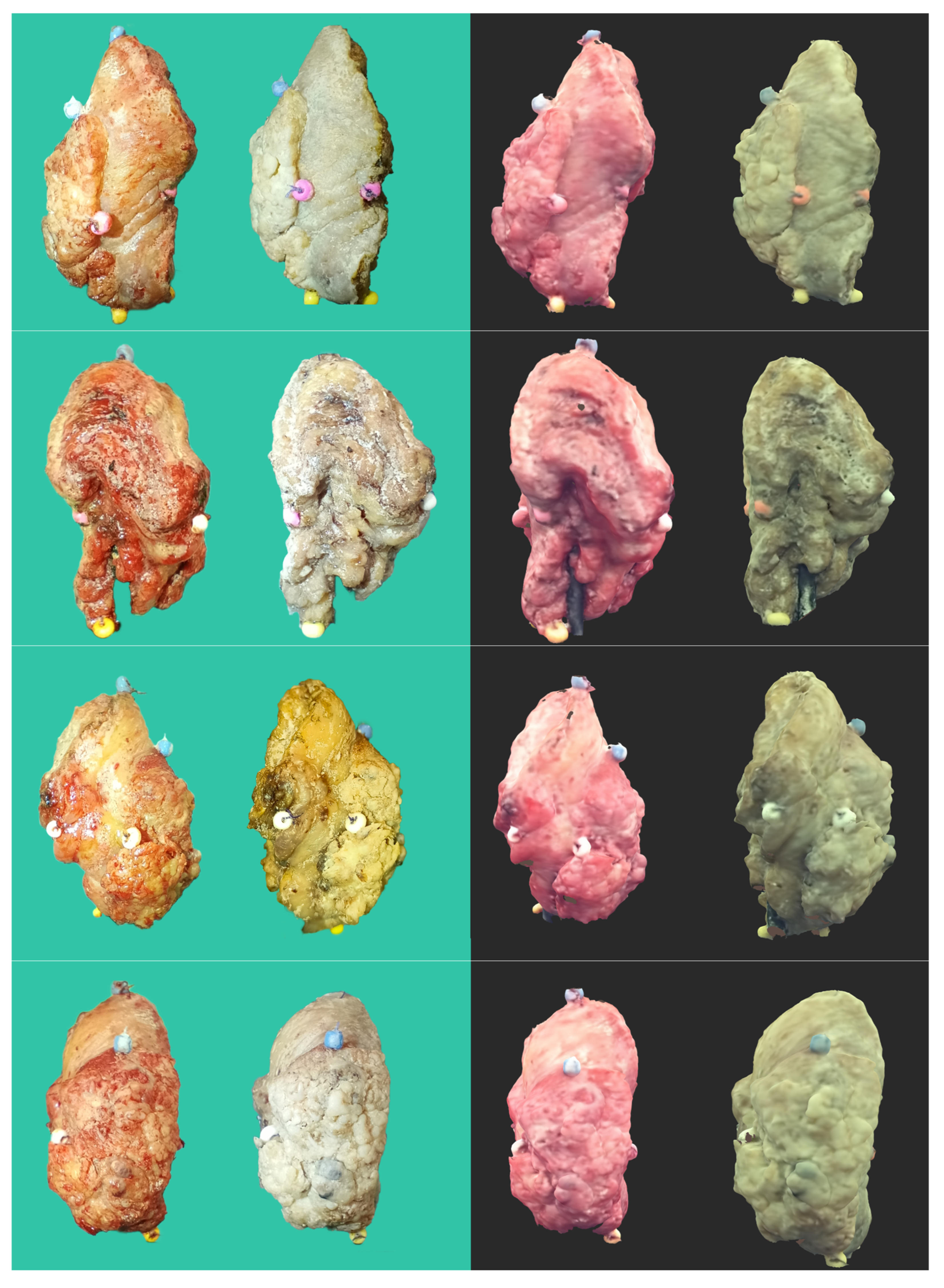
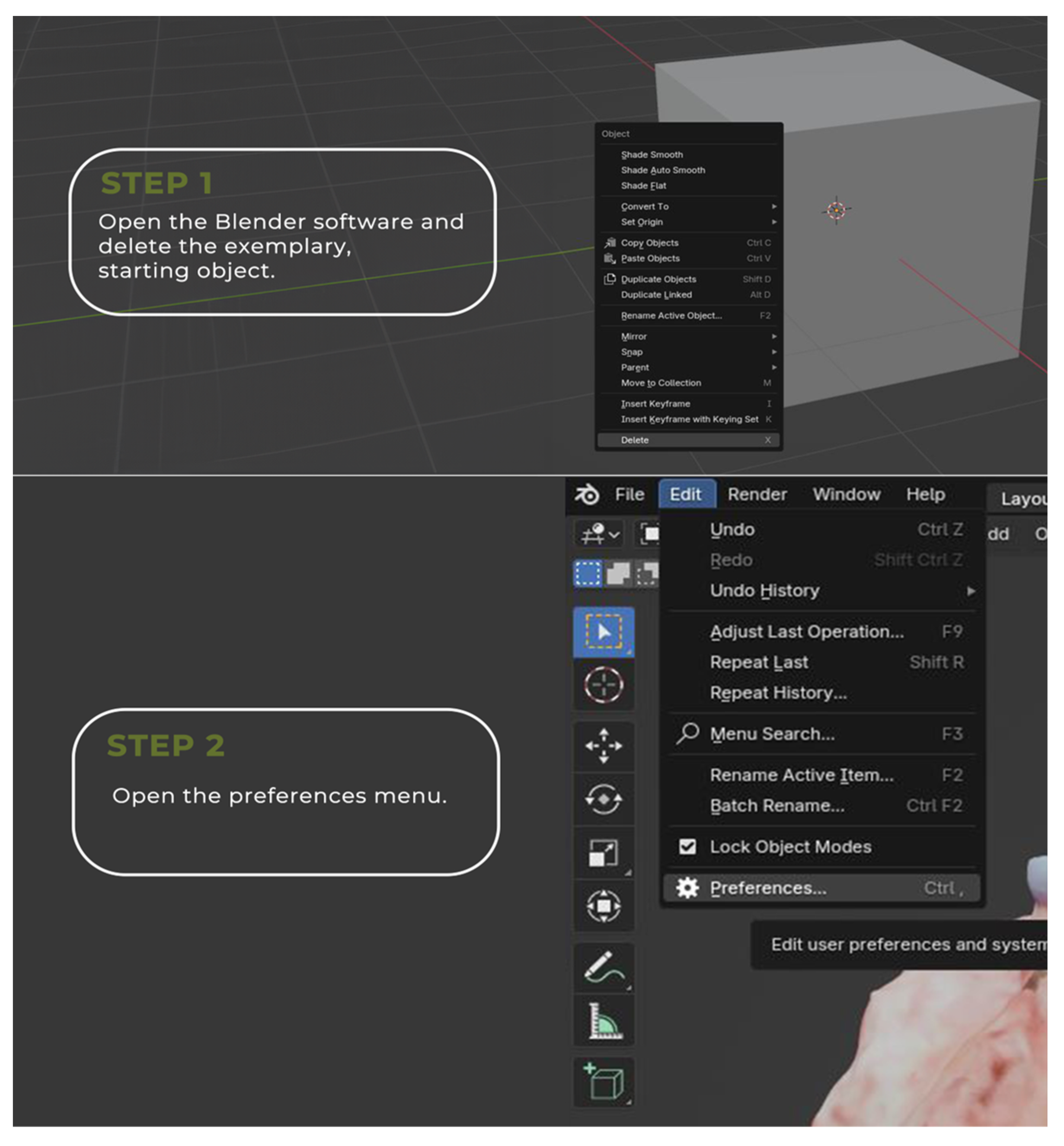
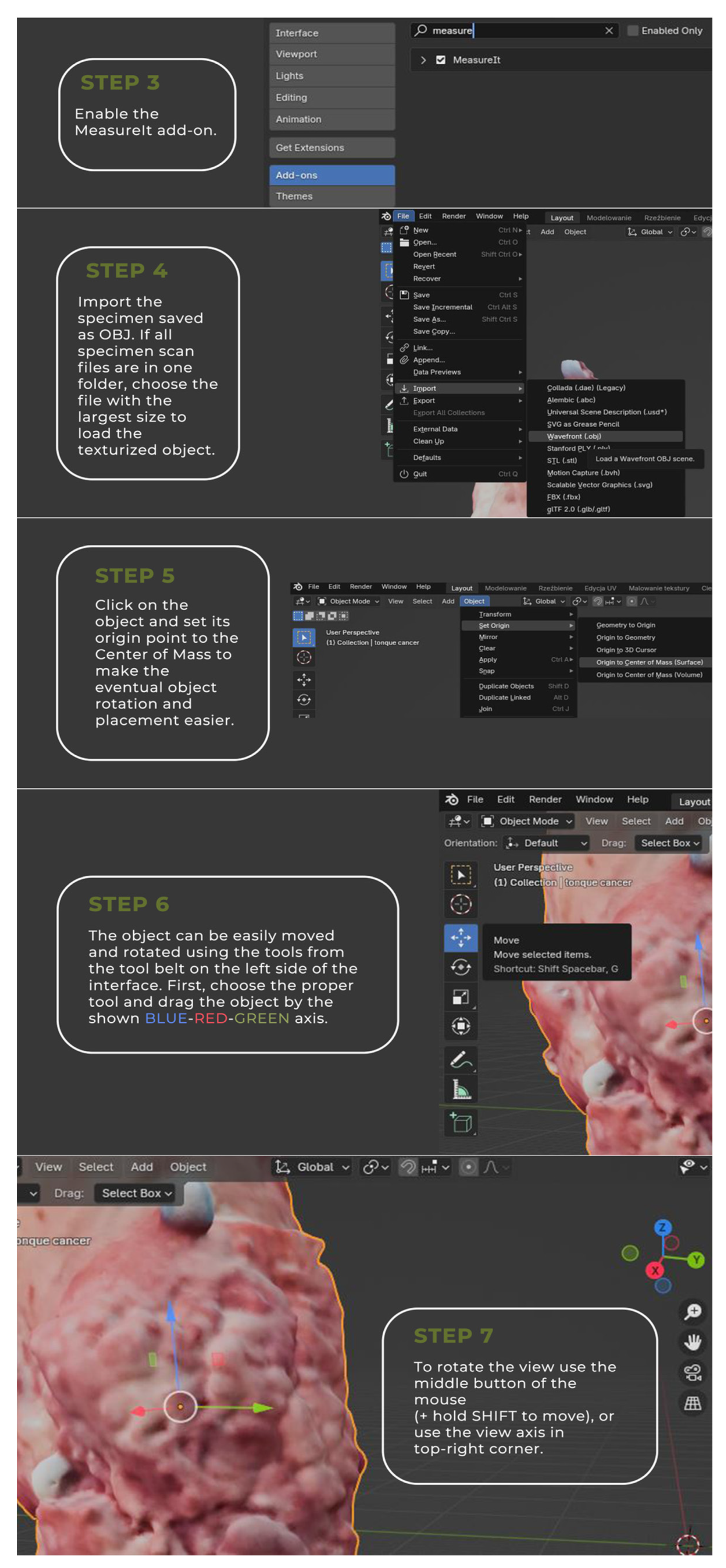
2.1. Tumor 3D Sample Acquisition
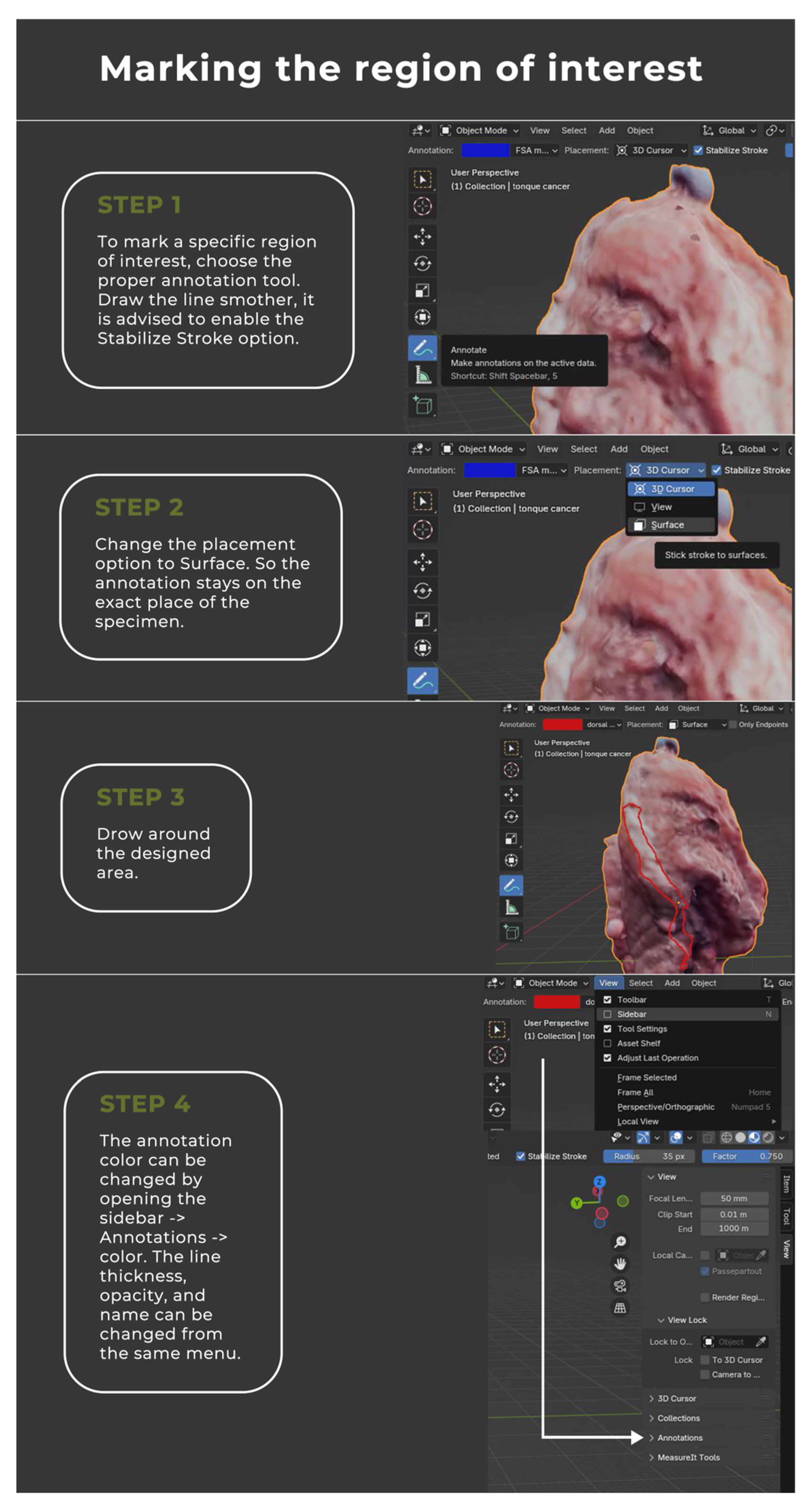
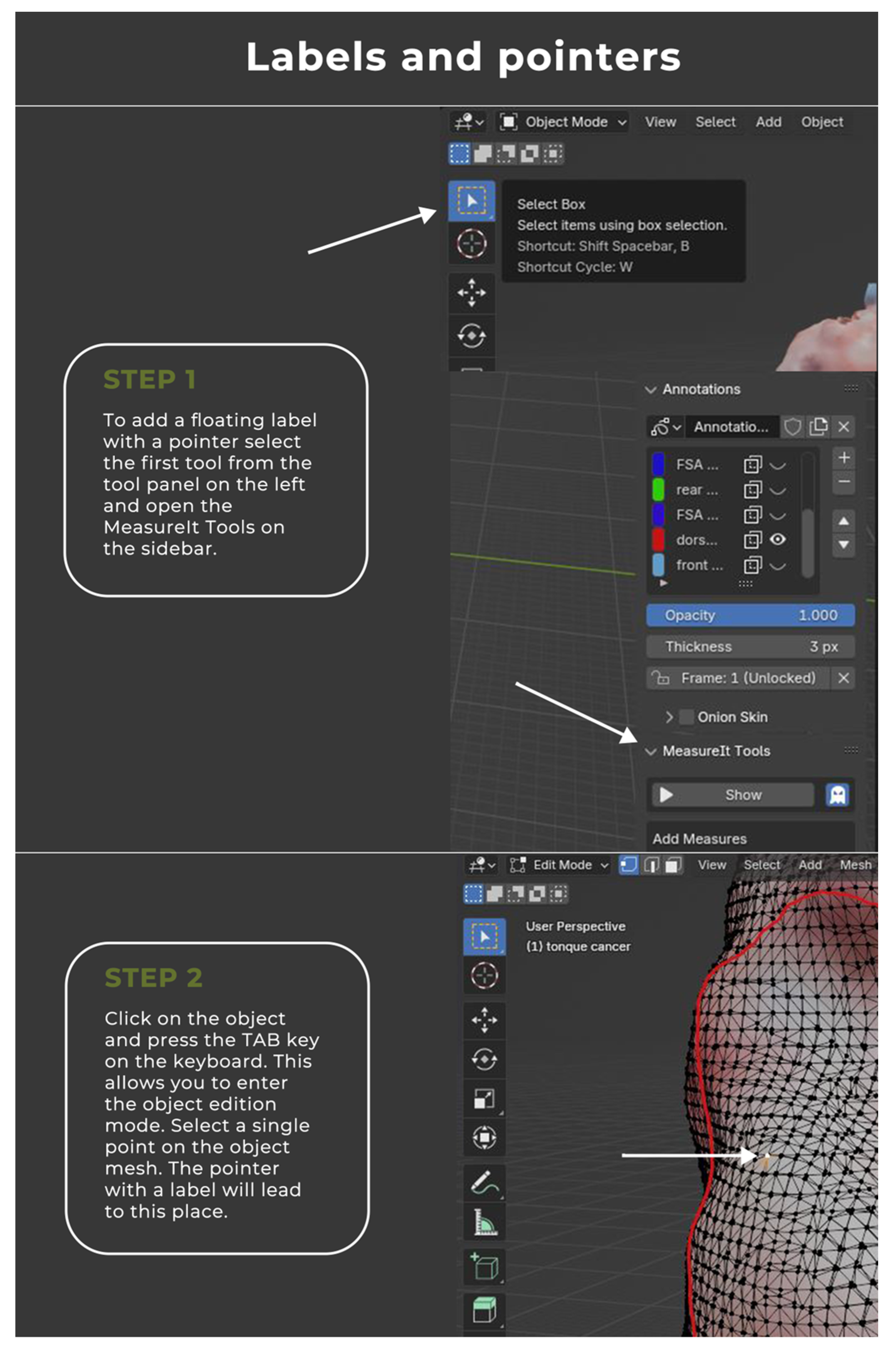
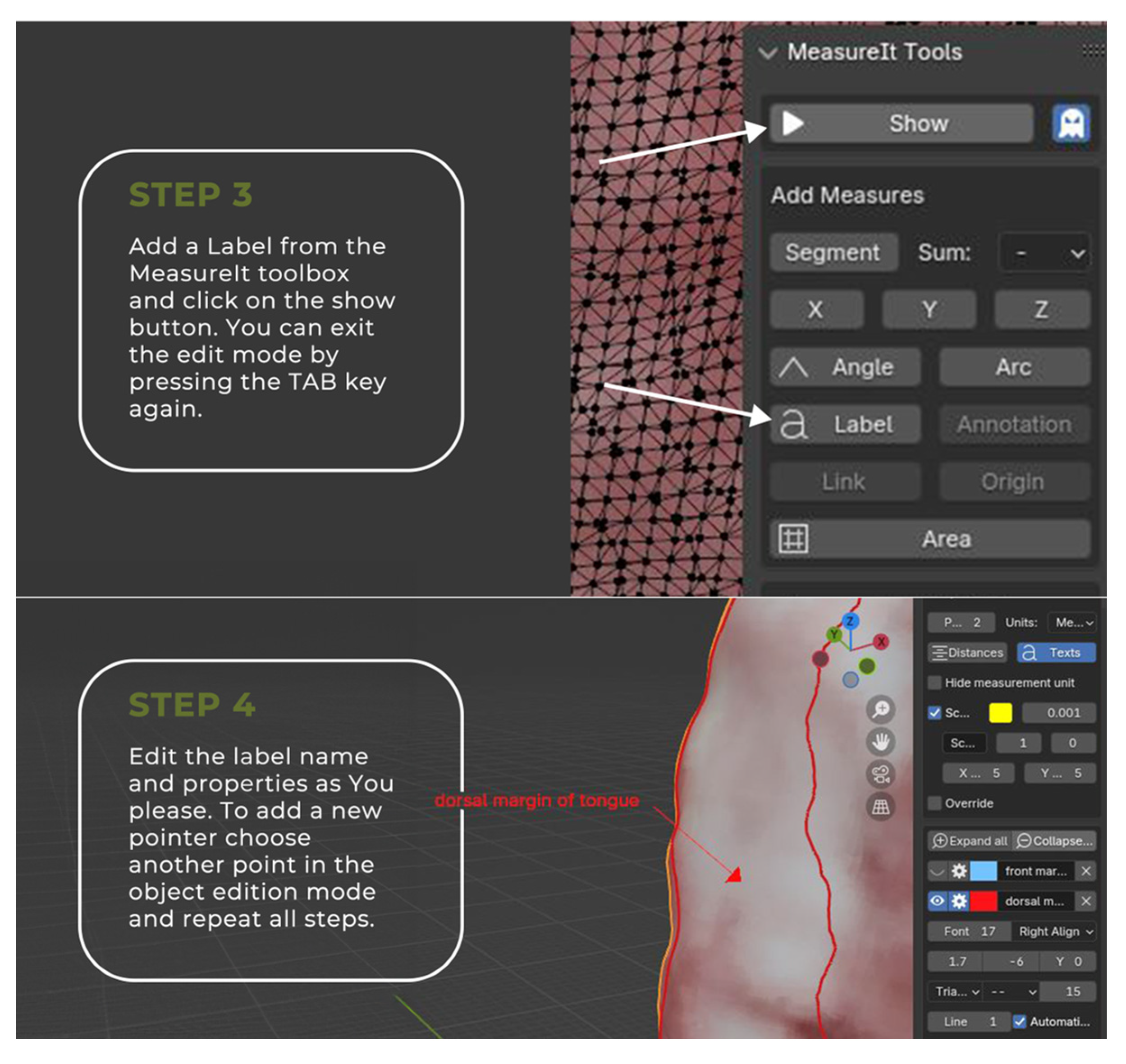
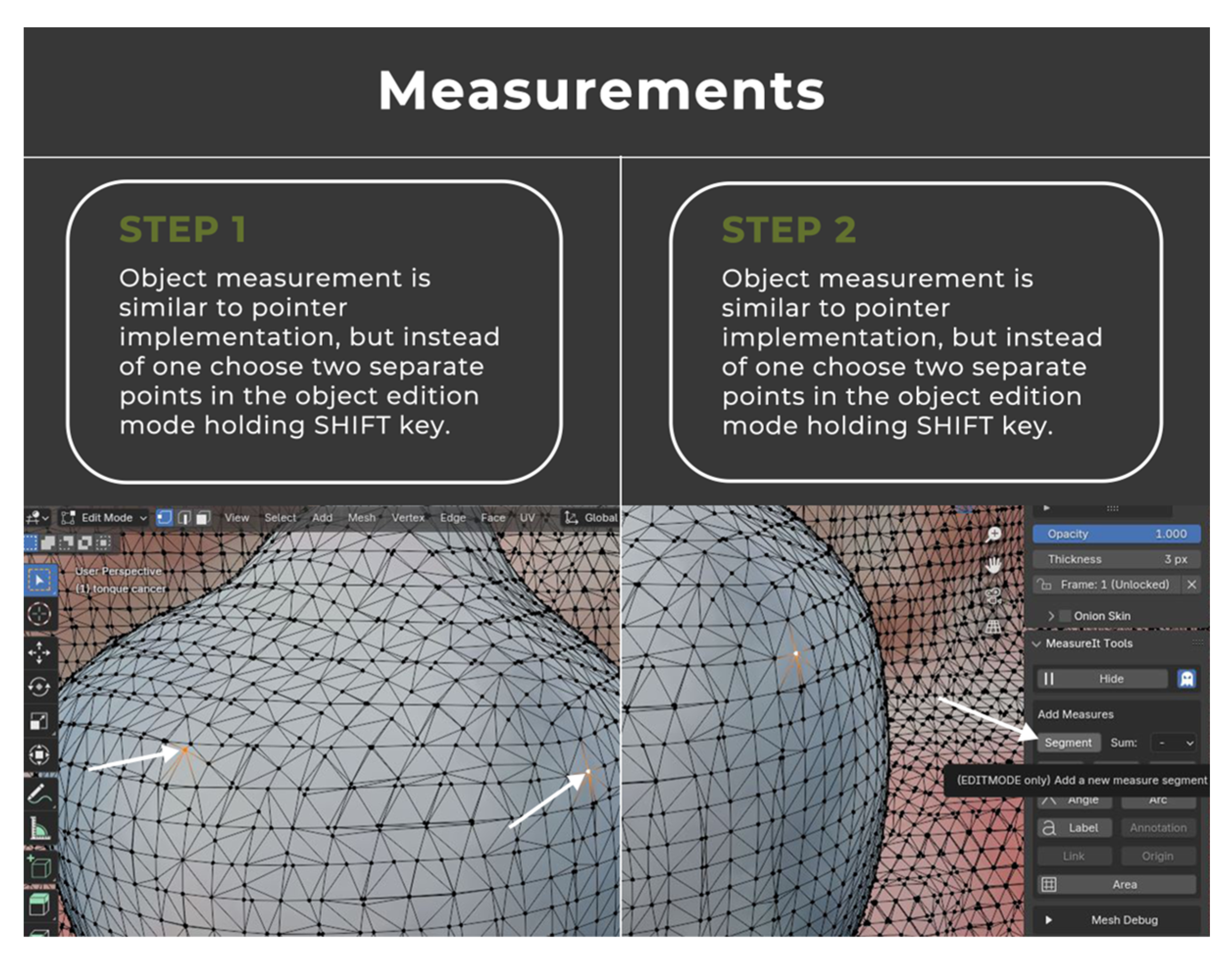
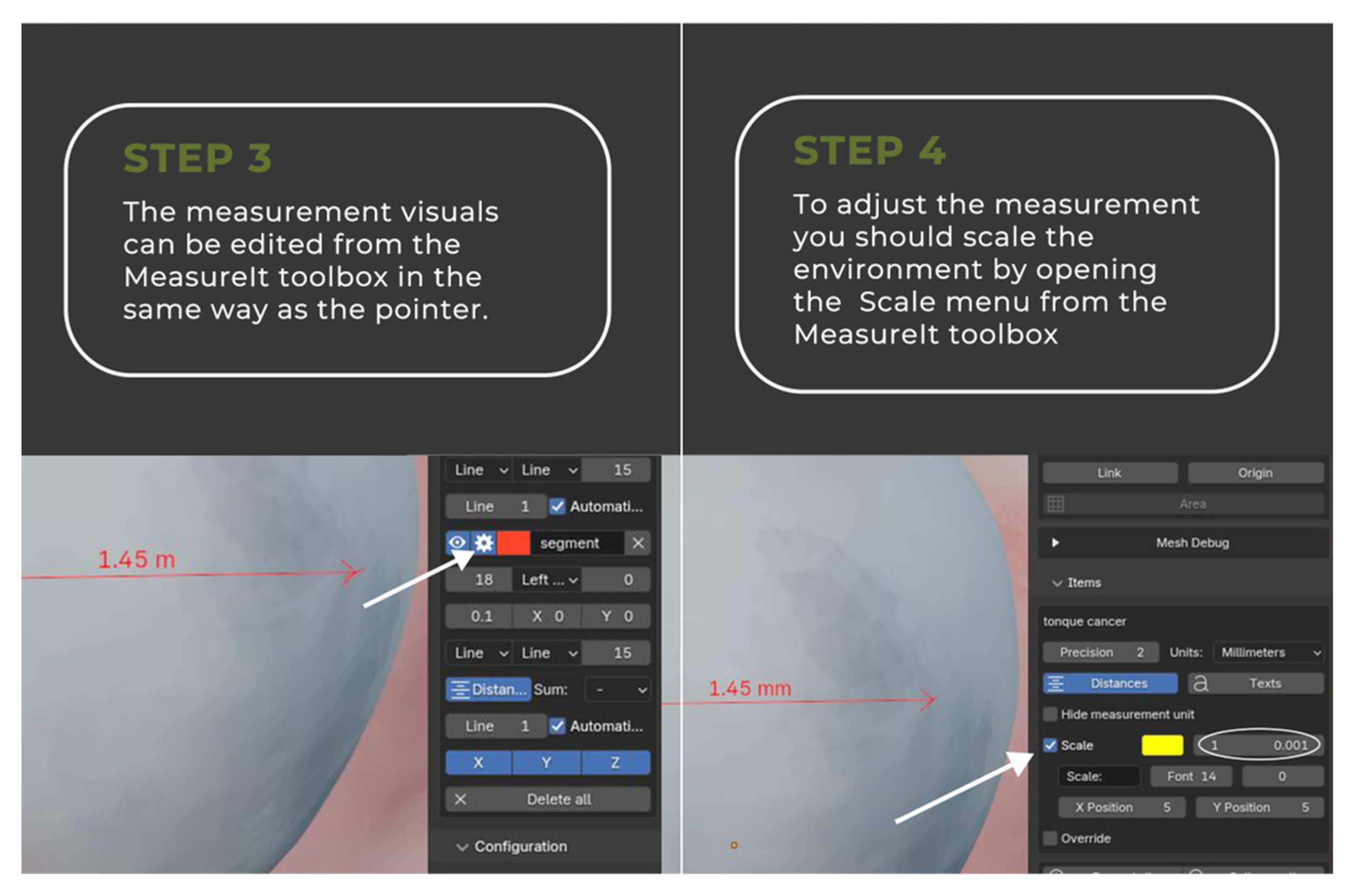
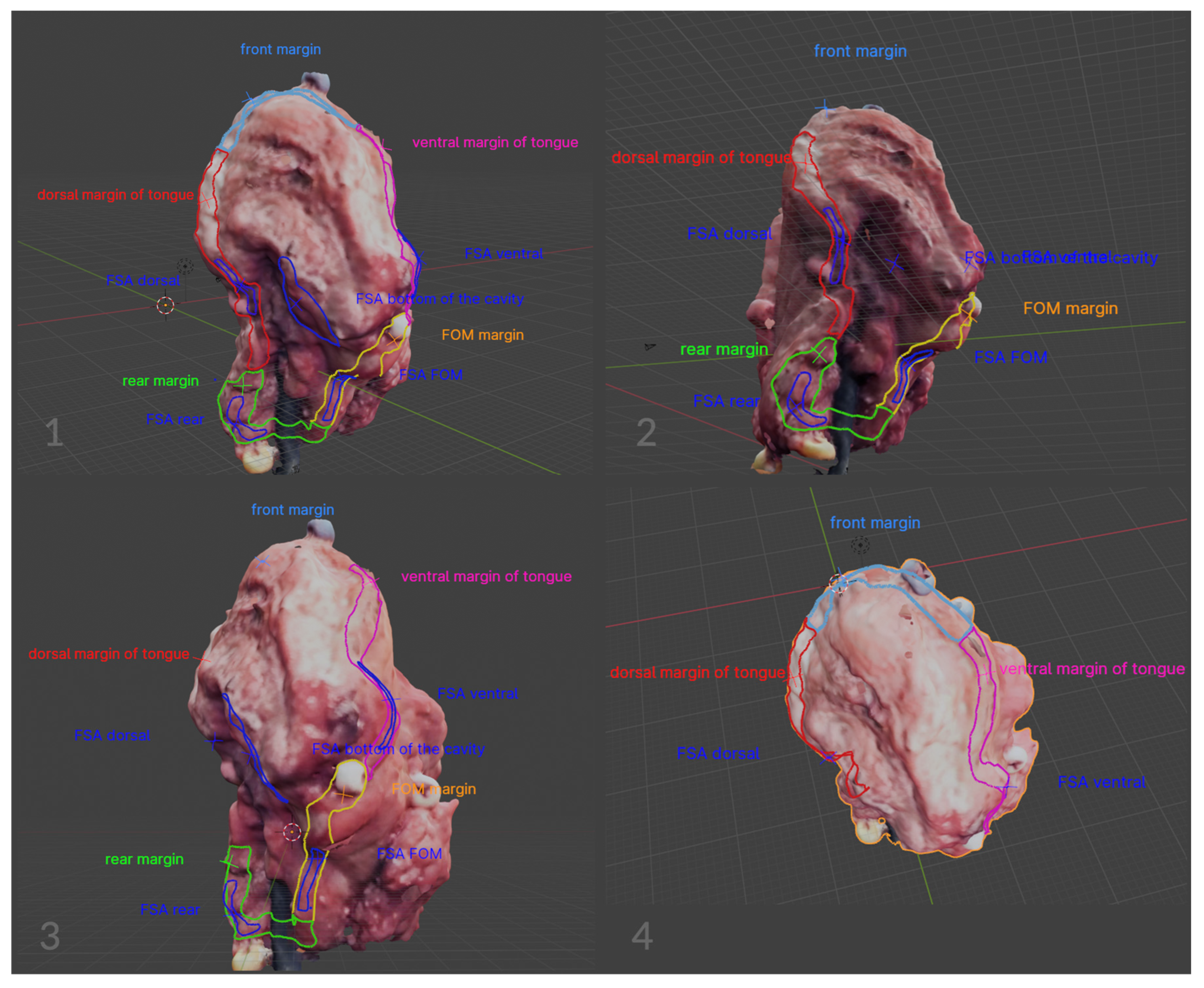
2.2. Image Annotation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Michcik, A.; Polcyn, A.; Sikora, M.; Wach, T.; Garbacewicz, Ł.; Drogoszewska, B. Oral squamous cell carcinoma—Do we always need elective neck dissection? Evaluation of clinicopathological factors of greatest prognostic significance: A cross-sectional observational study. Front. Oncol. 2023, 13, 1203439. [Google Scholar] [CrossRef] [PubMed]
- Tu, I.W.-H.; Shannon, N.B.; Thankappan, K.; Balasubramanian, D.; Pillai, V.; Shetty, V.; Rangappa, V.; Chandrasekhar, N.H.; Kekatpure, V.; Kuriakose, M.A.; et al. Risk Stratification in Oral Cancer: A Novel Approach. Front. Oncol. 2022, 12, 836803. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef]
- Maymone, M.B.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.-D.; Maymone, A.C.; Vashi, N.A. Premalignant and malignant oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 59–71. [Google Scholar] [CrossRef]
- Obermeier, K.T.; Wuersching, S.N.; Liokatis, P.; Smolka, W.; Poxleitner, P.; Kleye, C.; Ehrenfeld, M.; Kollmuss, M.; Otto, S. Metastases of OSCC Based on Oral Lichen Ruber Planus. Cancers 2023, 15, 4092. [Google Scholar] [CrossRef]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Cuffari, L.; Tesseroli de Siqueira, J.T.; Nemr, K.; Rapaport, A. Pain complaint as the first symptom of oral cancer: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 56–61. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, C.; Hu, S.; Liao, N.; Huang, Y.; Ding, H.; Li, R.; Li, Y. Research on neck dissection for oral squamous-cell carcinoma: A bibliometric analysis. Int. J. Oral Sci. 2021, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Valero, C.; McGill, M.R.; Montero, P.H.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Distant metastasis in oral squamous cell carcinoma: Does the neutrophil-to-lymphocyte ratio act as a surrogate of the host immune status? Oral Oncol. 2022, 124, 105641. [Google Scholar] [CrossRef] [PubMed]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.-M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef] [PubMed]
- Amirchaghmaghi, M.; Mohtasham, N.; Delavarian, Z.; Shakeri, M.T.; Taghizadeh, A.; Khazaeni, K.; Hatami, M. Analyzing the relationship between tissue color observed in VELscope examination and histopathological factors in OSCC patients. Photodiagn. Photodyn. Ther. 2023, 41, 103248. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Chu, L.; Fishman, E. 3D CT cinematic rendering of the spleen: Potential role in problem solving. Diagn. Interv. Imaging. 2019, 100, 477–483. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Kim, Y.; Kim, H.-J.; Kim, H.J.; Kim, E.-H.; Woo, S.-Y.; Chung, M.K.; Son, Y.-I. Imaging of Facial Nerve with 3D-DESS-WE-MRI Before Parotidectomy: Impact on Surgical Outcomes. Korean J. Radiol. 2023, 24, 860–870. [Google Scholar] [CrossRef]
- Makanjuola, J.K.; Aggoun, A.; Swash, M.; Grange, P.C.; Challacombe, B.; Dasgupta, P. 3D-holoscopic imaging: A new dimension to enhance imaging in minimally invasive therapy in urologic oncology. J. Endourol./Endourol. Soc. 2013, 27, 535–539. [Google Scholar] [CrossRef]
- D’onofrio, M.; Ciaravino, V.; Cardobi, N.; De Robertis, R.; Cingarlini, S.; Landoni, L.; Capelli, P.; Bassi, C.; Scarpa, A. CT Enhancement and 3D Texture Analysis of Pancreatic Neuroendocrine Neoplasms. Sci. Rep. 2019, 9, 2176. [Google Scholar] [CrossRef]
- Bhargava, A.; Popel, A.S.; Pathak, A.P. Vascular phenotyping of the invasive front in breast cancer using a 3D angiogenesis atlas. Microvasc. Res. 2023, 149, 104555. [Google Scholar] [CrossRef]
- Kraeima, J.; Dorgelo, B.; Gulbitti, H.A.; Steenbakkers, R.J.H.M.; Schepman, K.P.; Roodenburg, J.L.N.; Spijkervet, F.K.L.; Schepers, R.H.; Witjes, M.J.H. Multi-modality 3D mandibular resection planning in head and neck cancer using CT and MRI data fusion: A clinical series. Oral Oncol. 2018, 81, 22–28. [Google Scholar] [CrossRef]
- Lopez, R.; Gantet, P.; Julian, A.; Hitzel, A.; Herbault-Barres, B.; Alshehri, S.; Payoux, P. Value of PET/CT 3D visualization of head and neck squamous cell carcinoma extended to mandible. J. Cranio-Maxillofac. Surg. 2018, 46, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, J.H.; Kwon, O.; Min Park, J.; Wu, H.G. Correlation between 3D scanner image and MRI for tracking volume changes in head and neck cancer patients. J. Appl. Clin. Med. Phys. 2021, 22, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.; Bajaj, N.; Burrows, H.; Mathew, R.; Dai, A.; Wilke, C.T.; Palasi, S.; Hergenrother, R.; Chung, C.; Fuller, C.D.; et al. Creating customized oral stents for head and neck radiotherapy using 3D scanning and printing. Radiat. Oncol. 2019, 14, 148. [Google Scholar] [CrossRef]
- Herpel, C.; Schwindling, F.S.; Held, T.; Christ, L.; Lang, K.; Schwindling, M.; Moratin, J.; Zaoui, K.; Moutsis, T.; Plinkert, P.; et al. Individualized 3D-Printed Tissue Retraction Devices for Head and Neck Radiotherapy. Front. Oncol. 2021, 11, 628743. [Google Scholar] [CrossRef]
- Urken, M.L.; Yun, J.; Saturno, M.P.; Greenberg, L.A.; Chai, R.L.; Sharif, K.; Brandwein-Weber, M. Frozen Section Analysis in Head and Neck Surgical Pathology: A Narrative Review of the Past, Present, and Future of Intraoperative Pathologic Consultation. Oral Oncol. 2023, 143, 106445. [Google Scholar] [CrossRef]
- Sharif, K.F.; Lewis, J.S.; Ely, K.A.; Mehrad, M.; Pruthi, S.; Netterville, J.L.; Rohde, S.L.; Langerman, A.; Mannion, K.; Sinard, R.J.; et al. The computer-aided design margin: Ex vivo 3D specimen mapping to improve communication between surgeons and pathologists. Head. Neck 2023, 45, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Koivuholma, A.; Aro, K.; Mäkitie, A.; Salmi, M.; Mirtti, T.; Hagström, J.; Atula, T. Three-dimensional presentation of tumor histopathology: A model using tongue squamous cell carcinoma. Diagnostics 2021, 11, 109. [Google Scholar] [CrossRef]
- Franke, J.; Koutecký, T.; Koutný, D. Comparison of Sublimation 3D Scanning Sprays in Terms of Their Effect on the Resulting 3D Scan, Thickness, and Sublimation Time. Materials 2023, 16, 6165. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Ow, T.J.; Lo, Y.; Gersten, A.; Schiff, B.A.; Tassler, A.B.; Smith, R.V. Refining the utility and role of Frozen section in head and neck squamous cell carcinoma resection. Laryngoscope 2016, 126, 1768–1775. [Google Scholar] [CrossRef]
- Aaboubout, Y.; Barroso, E.M.; Algoe, M.; Ewing-Graham, P.C.; Hove, I.T.; Mast, H.; Hardillo, J.A.; Sewnaik, A.; Monserez, D.A.; Keereweer, S.; et al. Intraoperative assessment of resection margins in oral cavity cancer: This is the way. J. Vis. Exp. 2021, 171, e62446. [Google Scholar]



| Characteristic | N | % | |
|---|---|---|---|
| Gender | male | 36 | 72 |
| female | 14 | 28 | |
| Age | 50th | 6 | 12 |
| 60th | 16 | 32 | |
| 70th | 18 | 36 | |
| 80th | 10 | 20 | |
| TPS | TC | 16 | 32 |
| FOM | 25 | 50 | |
| RMT | 5 | 10 | |
| MT | 4 | 8 | |
| G | G1 | 12 | 24 |
| G2 | 26 | 52 | |
| G3 | 12 | 24 | |
| pT | pT1 | 7 | 14 |
| pT2 | 12 | 24 | |
| pT3 | 21 | 42 | |
| pT4 | 10 | 20 | |
| DOI | ≤5 mm | 7 | 14 |
| 5–10 mm | 12 | 24 | |
| >10 mm | 31 | 62 |
| Traditional Method | Digitalized Method | |
|---|---|---|
| Data handling |
|
|
| Specimen annotations |
|
|
| Data storage |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michcik, A.; Jopek, M.; Pęksa, R.; Choma, P.; Garbacewicz, Ł.; Polcyn, A.; Wach, T.; Sikora, M.; Drogoszewska, B. Virtual Tumor Mapping: A New Standard for Surgeon–Pathologist Collaboration in Treating Oral Squamous Cell Carcinoma. Cancers 2024, 16, 3761. https://doi.org/10.3390/cancers16223761
Michcik A, Jopek M, Pęksa R, Choma P, Garbacewicz Ł, Polcyn A, Wach T, Sikora M, Drogoszewska B. Virtual Tumor Mapping: A New Standard for Surgeon–Pathologist Collaboration in Treating Oral Squamous Cell Carcinoma. Cancers. 2024; 16(22):3761. https://doi.org/10.3390/cancers16223761
Chicago/Turabian StyleMichcik, Adam, Maksym Jopek, Rafał Pęksa, Piotr Choma, Łukasz Garbacewicz, Adam Polcyn, Tomasz Wach, Maciej Sikora, and Barbara Drogoszewska. 2024. "Virtual Tumor Mapping: A New Standard for Surgeon–Pathologist Collaboration in Treating Oral Squamous Cell Carcinoma" Cancers 16, no. 22: 3761. https://doi.org/10.3390/cancers16223761
APA StyleMichcik, A., Jopek, M., Pęksa, R., Choma, P., Garbacewicz, Ł., Polcyn, A., Wach, T., Sikora, M., & Drogoszewska, B. (2024). Virtual Tumor Mapping: A New Standard for Surgeon–Pathologist Collaboration in Treating Oral Squamous Cell Carcinoma. Cancers, 16(22), 3761. https://doi.org/10.3390/cancers16223761









