Analysis of Predictive Factors Associated with Unsuccessful Sentinel Lymph Node Mapping in Endometrial Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. SLN Mapping Procedure
2.3. Pathologic Evaluation
2.4. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. SLN Mapping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endometrial Cancer Statistics|World Cancer Research Fund International. WCRF International. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 7 September 2024).
- Yang, L.; Yuan, Y.; Zhu, R.; Zhang, X. Time trend of global uterine cancer burden: An age-period-cohort analysis from 1990 to 2019 and predictions in a 25-year period. BMC Women’s Health 2023, 23, 384. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Cancer Observatory: Cancer Today. 2024. Available online: https://gco.iarc.who.int/today/ (accessed on 8 October 2024).
- Rabban, J.T.; Gilks, C.B.; Malpica, A.; Matias-Guiu, X.; Mittal, K.; Mutter, G.L.; Oliva, E.; Parkash, V.; Ronnett, B.M.; Staats, P.; et al. Issues in the Differential Diagnosis of Uterine Low-grade Endometrioid Carcinoma, Including Mixed Endometrial Carcinomas: Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S25–S39. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef]
- Kalampokas, E.; Giannis, G.; Kalampokas, T.; Papathanasiou, A.-A.; Mitsopoulou, D.; Tsironi, E.; Triantafyllidou, O.; Gurumurthy, M.; Parkin, D.E.; Cairns, M.; et al. Current Approaches to the Management of Patients with Endometrial Cancer. Cancers 2022, 14, 4500. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines Index Table of Contents Discussion; NCCN: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Suchetha, S.; Mathew, A.P.; Rema, P.; Thomas, S. Pattern of Lymph Node Metastasis in Endometrial Cancer: A Single Institution Experience. Indian J. Surg. Oncol. 2021, 12, 73–77. [Google Scholar] [CrossRef] [PubMed]
- ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma|International Journal of Gynecologic Cancer. Available online: https://ijgc.bmj.com/content/31/1/12 (accessed on 4 September 2024).
- Buda, A.; Crivellaro, C.; Elisei, F.; Di Martino, G.; Guerra, L.; De Ponti, E.; Cuzzocrea, M.; Giuliani, D.; Sina, F.; Magni, S.; et al. Impact of Indocyanine Green for Sentinel Lymph Node Mapping in Early Stage Endometrial and Cervical Cancer: Comparison with Conventional Radiotracer 99mTc and/or Blue Dye. Ann. Surg. Oncol. 2016, 23, 2183–2191. [Google Scholar] [CrossRef]
- Eriksson, A.G.Z.; Montovano, M.; Beavis, A.; Soslow, R.A.; Zhou, Q.; Abu-Rustum, N.R.; Gardner, G.J.; Zivanovic, O.; Barakat, R.R.; Brown, C.L.; et al. Impact of Obesity on Sentinel Lymph Node Mapping in Patients with Newly Diagnosed Uterine Cancer Undergoing Robotic Surgery. Ann. Surg. Oncol. 2016, 23, 2522–2528. [Google Scholar] [CrossRef]
- Nieweg, O.E.; Uren, R.F.; Thompson, J.F. The History of sentinel lymph node biopsy. Cancer J. 2015, 21, 3–6. [Google Scholar] [CrossRef]
- Garrett, A.A.; Wield, A.; Mumford, B.; Janmey, I.; Wang, L.; Grosse, P.; MacArthur, E.; Buckanovich, R.; Courtney-Brooks, M.; Sukumvanich, P.; et al. Clinical factors associated with failed sentinel lymph node mapping in endometrial cancer. Gynecol. Oncol. Rep. 2022, 44, 101080. [Google Scholar] [CrossRef]
- Taşkın, S.; Sarı, M.E.; Altın, D.; Ersöz, C.C.; Gökçe, A.; Yüksel, S.; Kankaya, D.; Ortaç, F. Risk factors for failure of sentinel lymph node mapping using indocyanine green/near-infrared fluorescent imaging in endometrial cancer. Arch. Gynecol. Obstet. 2019, 299, 1667–1672. [Google Scholar] [CrossRef]
- Tanner, E.J.; Sinno, A.K.; Stone, R.L.; Levinson, K.L.; Long, K.C.; Fader, A.N. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol. Oncol. 2015, 138, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The TCGA Molecular Classification of Endometrial Cancer and Its Possible Impact on Adjuvant Treatment Decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, G.; Fanfani, F.; Berretta, R.; Capozzi, V.A.; Uccella, S.; Buono, N.; Giallombardo, V.; Di Donna, M.C.; Monterossi, G.; Restaino, S.; et al. Laparoscopic sentinel node mapping with intracervical indocyanine green injection for endometrial cancer: The SENTIFAIL study—A multicentric analysis of predictors of failed mapping. Int. J. Gynecol. Cancer 2020, 30, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, L.; Casarin, J.; Multinu, F.; Cappuccio, S.; McGree, M.E.; Weaver, A.L.; Langstraat, C.L.; Keeney, G.L.; Kumar, A.; Melis, G.B.; et al. Sentinel lymph node biopsy with cervical injection of indocyanine green in apparent early-stage endometrial cancer: Predictors of unsuccessful mapping. Gynecol. Oncol. 2019, 155, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Andreika, L.; Vankevičienė, K.; Ramašauskaitė, D.; Rudaitis, V. Visualization Methods for Uterine Sentinel Lymph Nodes in Early-Stage Endometrial Carcinoma: A Comparative Analysis. Diagnostics 2024, 14, 552. [Google Scholar] [CrossRef]
- Bistas, E.; Sanghavi, D.K. Methylene Blue; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557593/ (accessed on 4 September 2024).
- Holloway, R.W.; Ahmad, S.; Kendrick, J.E.; Bigsby, G.E.; Brudie, L.A.; Ghurani, G.B.; Stavitzski, N.M.; Gise, J.L.; Ingersoll, S.B.; Pepe, J.W. A Prospective Cohort Study Comparing Colorimetric and Fluorescent Imaging for Sentinel Lymph Node Mapping in Endometrial Cancer. Ann. Surg. Oncol. 2017, 24, 1972–1979. [Google Scholar] [CrossRef]
- Cianci, S.; Rosati, A.; Vargiu, V.; Capozzi, V.A.; Sozzi, G.; Gioè, A.; Alletti, S.G.; Ercoli, A.; Cosentino, F.; Berretta, R.; et al. Sentinel Lymph Node in Aged Endometrial Cancer Patients “The SAGE Study”: A Multicenter Experience. Front. Oncol. 2021, 11, 737096. [Google Scholar] [CrossRef]
- Raffone, A.; Fanfani, F.; Raimondo, D.; Rovero, G.; Renzulli, F.; Travaglino, A.; De Laurentiis, U.; Santoro, A.; Zannoni, G.F.; Casadio, P.; et al. Predictive factors of sentinel lymph node failed mapping in endometrial carcinoma patients: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 853–859. [Google Scholar] [CrossRef]
- Ianieri, M.M.; Puppo, A.; Novelli, A.; Campolo, F.; Staniscia, T.; Di Martino, G.; Piovano, E.; Bruni, F.; Roviglione, G.; Mautone, D.; et al. Sentinel Lymph Node Biopsy in the Treatment of Endometrial Cancer: Why We Fail? Results of a Prospective Multicenter Study on the Factors Associated with Failure of Node Mapping with Indocyanine Green. Gynecol. Obstet. Investig. 2019, 84, 383–389. [Google Scholar] [CrossRef]
- Vargiu, V.; Rosati, A.; Capozzi, V.A.; Sozzi, G.; Gioè, A.; Berretta, R.; Chiantera, V.; Scambia, G.; Fanfani, F.; Cosentino, F. Impact of Obesity on Sentinel Lymph Node Mapping in Patients with apparent Early-Stage Endometrial Cancer: The ObeLyX study. Gynecol. Oncol. 2022, 165, 215–222. [Google Scholar] [CrossRef]
- Goyal, A.; Douglas-Jones, A.; Newcombe, R.; Mansel, R.; ALMANAC Trialists Group. Predictors of non-sentinel lymph node metastasis in breast cancer patients. Eur. J. Cancer 2004, 40, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Body, N.; Grégoire, J.; Renaud, M.-C.; Sebastianelli, A.; Grondin, K.; Plante, M. Tips and tricks to improve sentinel lymph node mapping with Indocyanin green in endometrial cancer. Gynecol. Oncol. 2018, 150, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers 2023, 15, 4101. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.S.; Al-Haddad, H.S.; McAllister, K.A.; Yasseen, A.A. The clinicopathology and survival characteristics of patients with POLE proofreading mutations in endometrial carcinoma: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263585. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Glaser, G.E.; Zivanovic, O.; Sonoda, Y.; Levine, D.A.; Chi, D.S.; Gemignani, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol. Oncol. 2009, 115, 453–455. [Google Scholar] [CrossRef]
| Variable | Global Cohort (n = 120) | Successful Staining (n = 59) | Unsuccessful Staining (n = 61) | Univariate Analysis p Value | Multivariate Analysis p Value |
|---|---|---|---|---|---|
| Age (years) (mean (95% CI), range) | 62.5 ± 9.7 (33 to 83) | 58.4 ± 8.4 (33 to 75) | 66.1 ± 9.4 (35 to 83) | <0.001 | 0.007 |
| Menopause status | 0.001 | 0.27 | |||
| Yes | 102 (85.0%) | 41 (73.2%) | 61 (95.3%) | ||
| No | 18 (15.0%) | 15 (26.8%) | 3 (4.7%) | ||
| BMI (kg/m2) (mean (95% CI), range) | 32.0 ± 6.0 (18 to 50) | 30.4 ± 6.2 (18 to 50) | 32.4 ± 5.6 (20 to 43) | 0.13 | |
| BMI ≥ 30 kg/m2 | 0.06 | ||||
| Yes | 75 (62.5%) | 32 (54.2%) | 43 (70.5%) | ||
| No | 45 (37.5%) | 27 (45.8%) | 18 (29.5%) | ||
| Previous deliveries * | 0.22 | ||||
| Yes | 103 (88.8%) | 48 (85.7%) | 55 (91.7%) | ||
| No | 13 (11.2%) | 8 (14.3%) | 5 (8.3%) | ||
| Previous cesarean deliveries * | 0.11 | ||||
| Yes | 10 (9.4%) | 8 (14.3%) | 2 (3.3%) | ||
| No | 106 (90.6%) | 48 (85.7%) | 58 (96.7%) | ||
| Previous pelvic surgery | 0.11 | ||||
| Yes | 37 (30.8%) | 13 (23.2%) | 24 (35.3%) | ||
| No | 83 (69.2%) | 43 (76.8%) | 40 (64.7%) | ||
| Tracer agent used | 0.006 | 0.013 | |||
| MB | 40 (33.3.3%) | 11 (27.5%) | 29 (72.5%) | ||
| ICG | 40 (33.3.3%) | 21 (52.8%) | 19 (47.2%) | ||
| ICG + MB | 40 (33.3.3%) | 27 (67.5%) | 13 (32.5%) | ||
| Uterine volume (mL) (mean (95% CI), range) | 121 ± 122 (12 to 858) | 145.5 ± 155.1 (29 to 858) | 100.5 ± 79.3 (12 to 392) | 0.054 | |
| Tumor max. diameter (mm) (mean (95% CI), range) | 26.3 ± 17.1 (1 to 85) | 24.1 ± 16.8 (1 to 70) | 27.8 ± 17.5 (1 to 85) | 0.27 | |
| Tumor-to-resection-edge distance (mm) (mean (95% CI), range) | 38.9 ± 16.9 (3 to 77) | 40.9 ± 19.1 (3 to 77) | 37.3 ± 15.0 (4 to 60) | 0.38 | |
| Tumor-to-serosa distance (mm) (mean (95% CI), range) | 7.7 ± 8.2 (1 to 53) | 9.8 ± 10.5 (1 to 53) | 5.4 ± 3.9 (1 to 15) | 0.048 | 0.87 |
| Tumor histological type | 0.71 | ||||
| endometrioid | 108 (90.0%) | 51 (91.1%) | 57 (89.1%) | ||
| non-endometrioid | 12 (10.0%) | 5 (8.9%) | 7 (10.9%) | ||
| Tumor grading | 0.82 | ||||
| G1 | 90 (75.0%) | 43 (76.8%) | 47 (73.4%) | ||
| G2 | 17 (14.2%) | 8 (14.3%) | 9 (14.1%) | ||
| G3 | 13 (10.8%) | 5 (8.9%) | 8 (12.5%) | ||
| Molecular type | 0.68 | ||||
| Type 2 | 32 (26.7%) | 16 (28.6%) | 16 (25.0%) | ||
| Type 3 | 79 (65.8%) | 37 (66.1%) | 42 (65.6%) | ||
| Type 4 | 9 (7.5%) | 3 (5.4%) | 6 (9.4%) | ||
| Myometrial infiltration (50% and more) | 0.17 | ||||
| Yes | 42 (35.0%) | 16 (17.9%) | 26 (15.6%) | ||
| No | 78 (65.0%) | 40 (82.1%) | 38 (84.4%) | ||
| FIGO stage | 0.66 | ||||
| Early (I and II) | 110 (91.7%) | 52 (92.9%) | 58 (90.6%) | ||
| Advanced (III and IV) | 10 (8.3%) | 4 (7.1%) | 6 (9.4%) | ||
| LVSI | 0.74 | ||||
| Yes | 20 (16.7%) | 10 (17.9%) | 10 (15.6%) | ||
| No | 100 (83.3%) | 46 (82.1%) | 54 (84.4%) | ||
| Lymph nodes >2 cm | 0.018 | 0.013 | |||
| Yes | 41 (34.2%) | 13 (23.2%) | 28 (43.8%) | ||
| No | 79 (65.8%) | 43 (76.8%) | 36 (56.3%) | ||
| Lymph node involvement ** | 0.21 | ||||
| Yes | 9 (7.5%) | 3 (5.4%) | 6 (9.4%) | ||
| No | 96 (92.5%) | 53 (94.6%) | 43 (90.6%) | ||
| Myomatosis | 0.45 | ||||
| Yes | 62 (51.7%) | 31 (55.4%) | 31 (48.4%) | ||
| No | 58 (48.3%) | 25 (44.6%) | 33 (51.6%) | ||
| Adenomyosis | 0.9 | ||||
| Yes | 23 (19.2%) | 11 (19.6%) | 12 (18.8%) | ||
| No | 97 (80.8%) | 45 (80.4%) | 52 (81.2%) |
| Variables | N (%) |
|---|---|
| Sentinel node detection | |
| Absence | 32 (26.6) |
| Unilateral pelvic | 29 (24.2) |
| Left | 14 (11.7) |
| Right | 15 (12.5) |
| Bilateral pelvic | 56 (46.7) |
| Aortic | 3 (2.5) |
| Total successful | 59 (49.2) |
| Overall detection rate | 88 (73.4) |
| Pelvic lymphadenectomy | |
| Not performed | 76 (63.4) |
| Unilateral | 13 (10.8) |
| Bilateral | 31 (25.8) |
| Para-aortic lymphadenectomy | |
| Not performed | 108 (90) |
| Performed | 12 (10) |
| Complications | |
| Yes | 8 (6.7) |
| No | 112 (93.3) |
| Variable | Global Cohort | MB (a) | ICG (b) | ICG–MB (c) | p Value | a to b | b to c | a to c |
|---|---|---|---|---|---|---|---|---|
| Successful mapping (n (%)) | 59 (49.2) | 11 (27.5) | 21 (52.8) | 27 (67.5) | 0.006 | 0.056 | 0.391 | 0.001 |
| Variable | N |
|---|---|
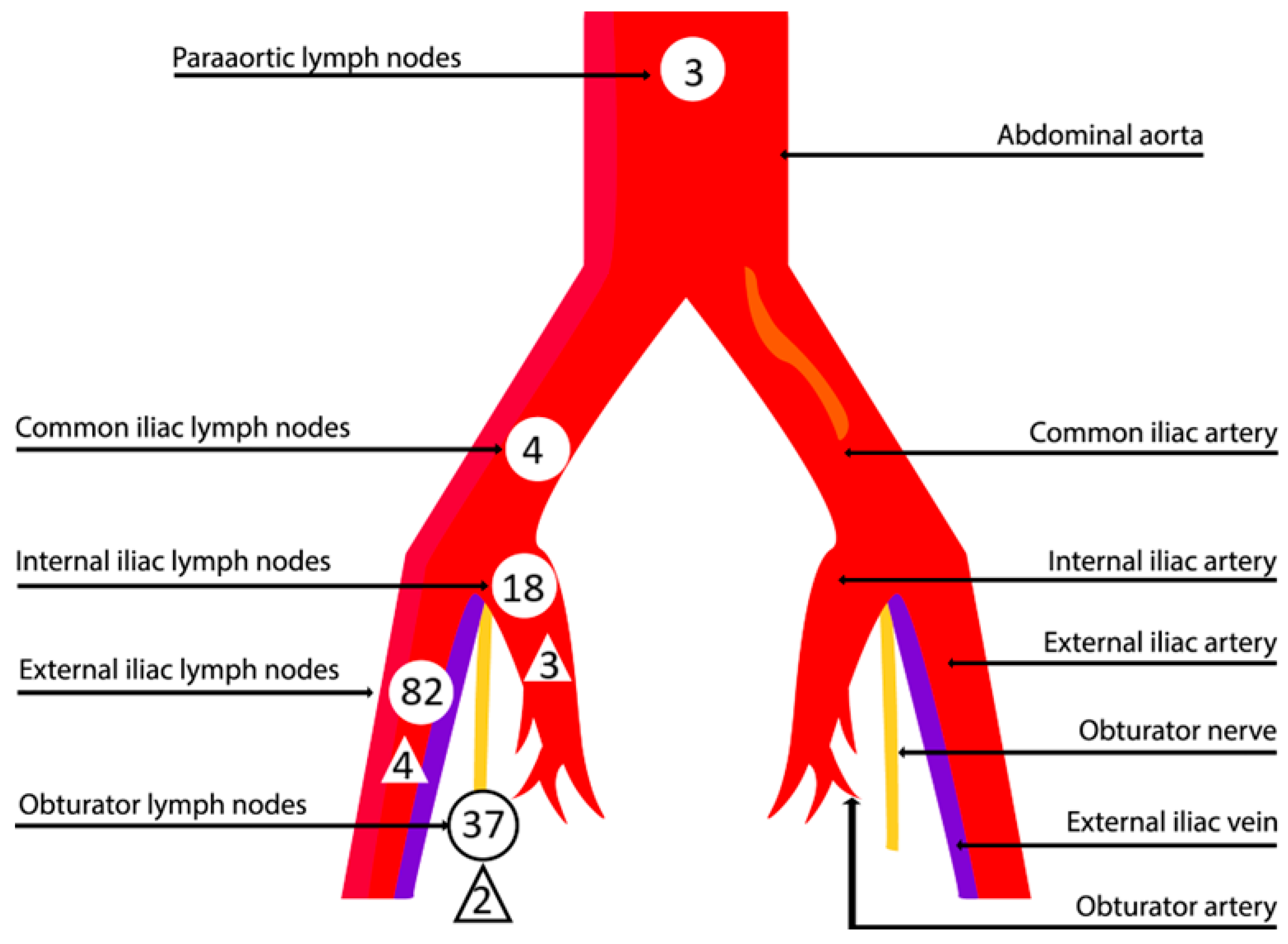
| Total number of sentinel nodes removed | 212 |
| Median number of sentinel nodes removed (range) | 2 (0–8) |
| Total sentinel node sites | 144 |
| External iliac | 82 (56.9%) |
| Obturator | 37 (25.7%) |
| Internal iliac | 18 (12.5%) |
| Common iliac | 4 (2.8%) |
| Para-aortic | 3 (2.1%) |
| Sentinel node number in sites | |
| External iliac | 110 (51.9%) |
| Obturator | 64 (30.2%) |
| Internal iliac | 29 (13.7%) |
| Common iliac | 4 (1.9%) |
| Para-aortic | 5 (2.3%) |
| Histological state of sentinel nodes | |
| Negative | 197 (92.9%) |
| 10 (4.7%) | |
| Positive | 5 (2.4%) |
| Empty nodes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreika, L.; Šiaudinytė, M.; Vankevičienė, K.; Ramašauskaitė, D.; Rudaitis, V. Analysis of Predictive Factors Associated with Unsuccessful Sentinel Lymph Node Mapping in Endometrial Carcinoma. Cancers 2024, 16, 3680. https://doi.org/10.3390/cancers16213680
Andreika L, Šiaudinytė M, Vankevičienė K, Ramašauskaitė D, Rudaitis V. Analysis of Predictive Factors Associated with Unsuccessful Sentinel Lymph Node Mapping in Endometrial Carcinoma. Cancers. 2024; 16(21):3680. https://doi.org/10.3390/cancers16213680
Chicago/Turabian StyleAndreika, Linas, Monika Šiaudinytė, Karolina Vankevičienė, Diana Ramašauskaitė, and Vilius Rudaitis. 2024. "Analysis of Predictive Factors Associated with Unsuccessful Sentinel Lymph Node Mapping in Endometrial Carcinoma" Cancers 16, no. 21: 3680. https://doi.org/10.3390/cancers16213680
APA StyleAndreika, L., Šiaudinytė, M., Vankevičienė, K., Ramašauskaitė, D., & Rudaitis, V. (2024). Analysis of Predictive Factors Associated with Unsuccessful Sentinel Lymph Node Mapping in Endometrial Carcinoma. Cancers, 16(21), 3680. https://doi.org/10.3390/cancers16213680






