Metal Peroxide Nanoparticles for Modulating the Tumor Microenvironment: Current Status and Recent Prospects
Simple Summary
Abstract
1. Introduction
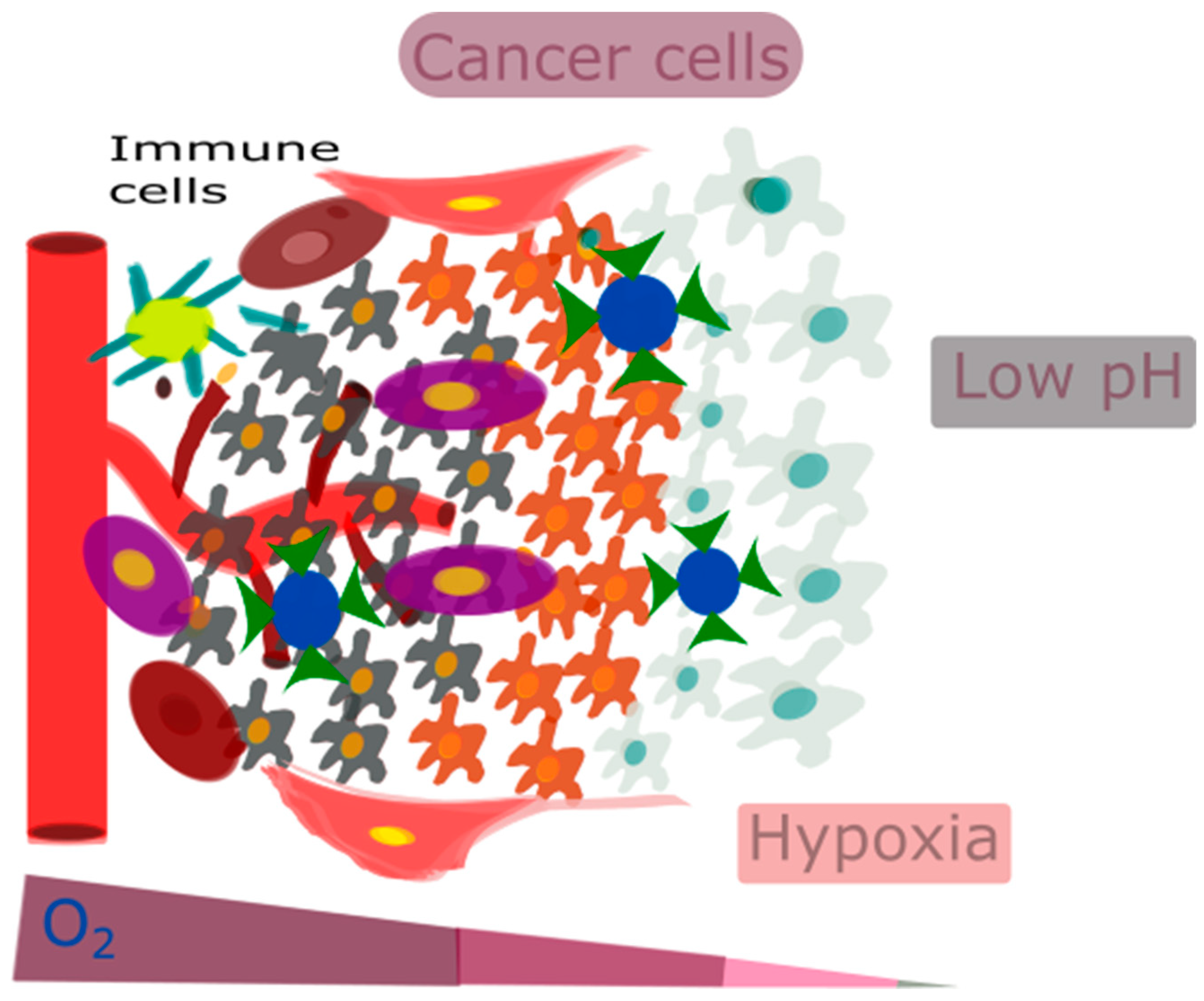
2. The Tumor Microenvironment
2.1. Factors Associated with the TME
2.2. Targeting the Tumor’s Microenvironment
2.3. The Strategy Involves the Surface Modification of Nanoparticles
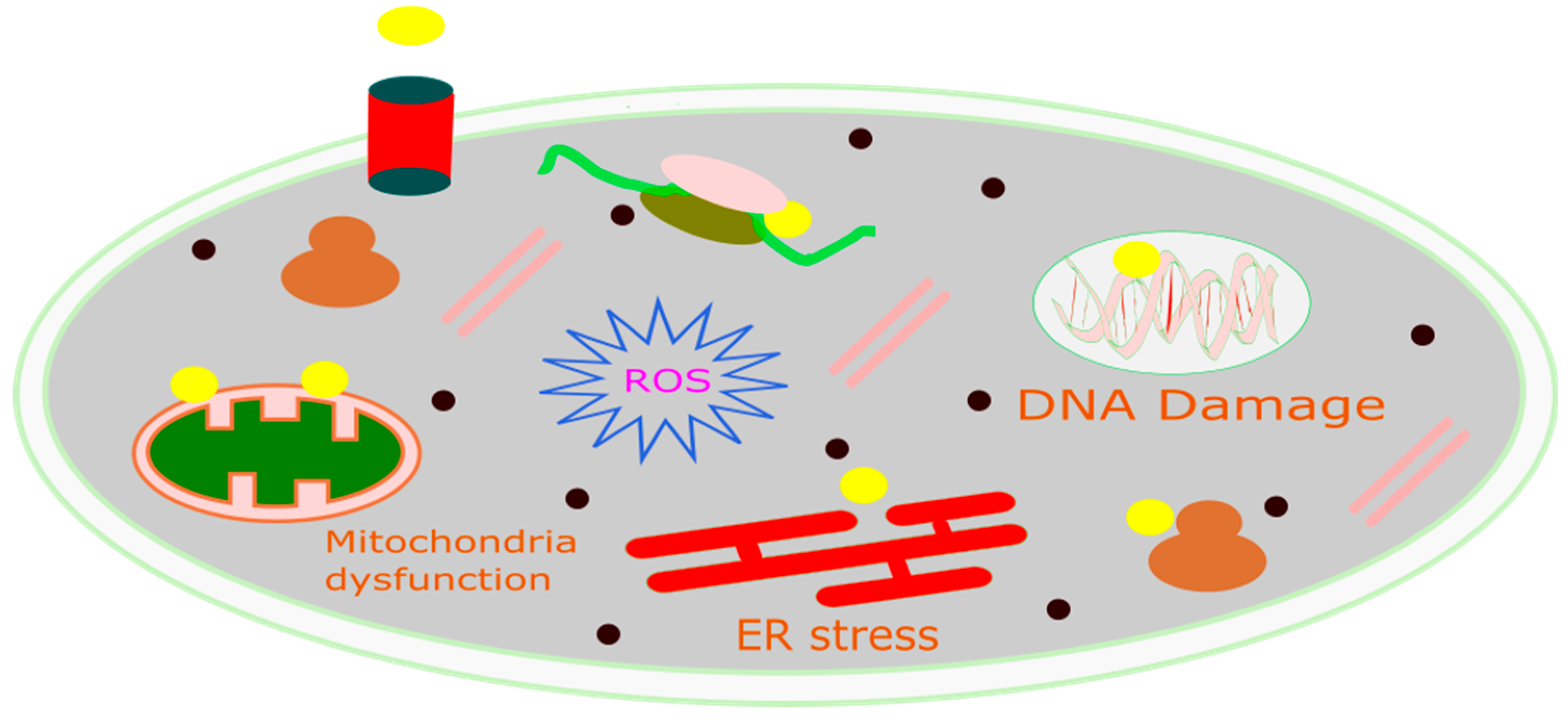
3. Fenton Reaction
Role of Chemodynamic Therapy in Cancer
4. Metal-Based Peroxides for Cancer Therapy
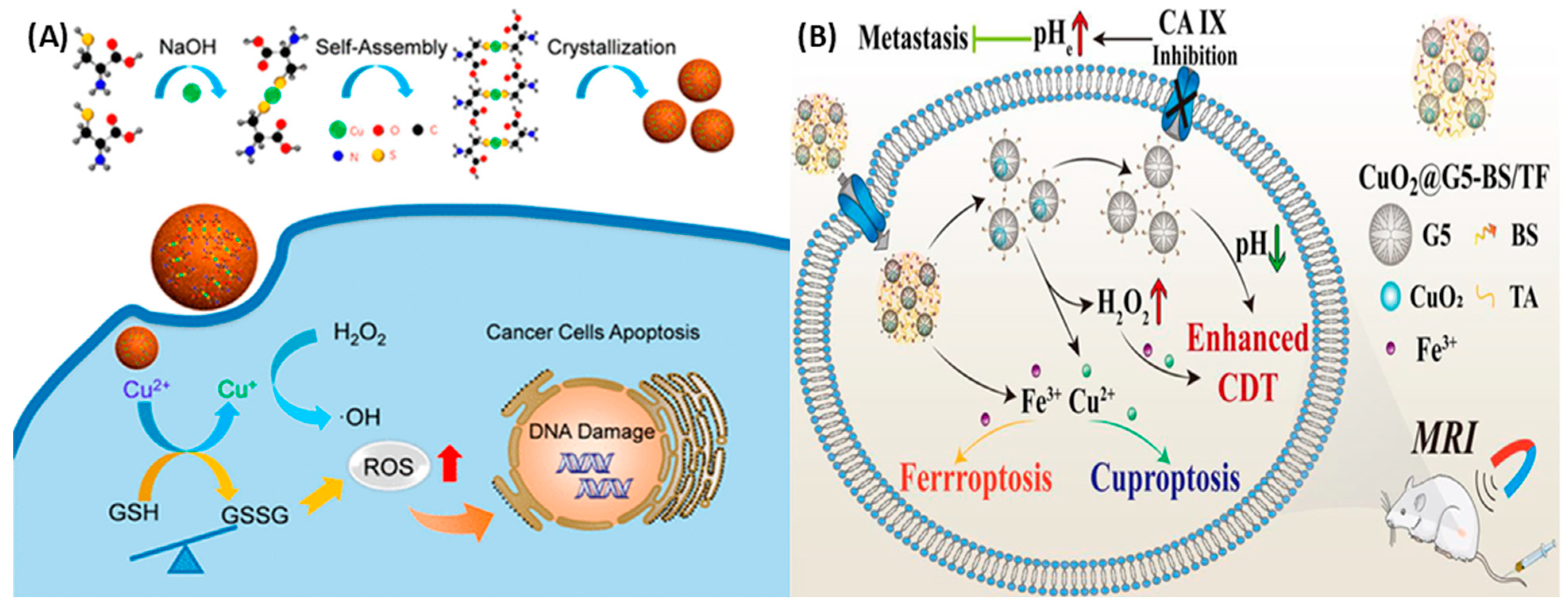
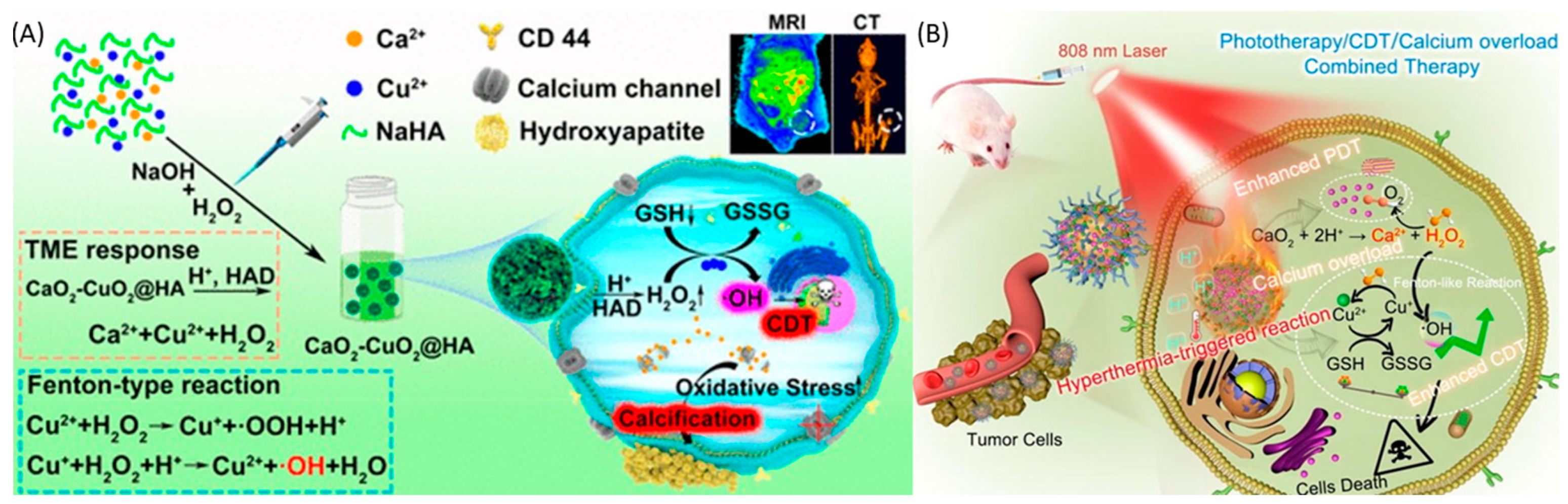
4.1. Copper Peroxide
4.2. Calcium Peroxide
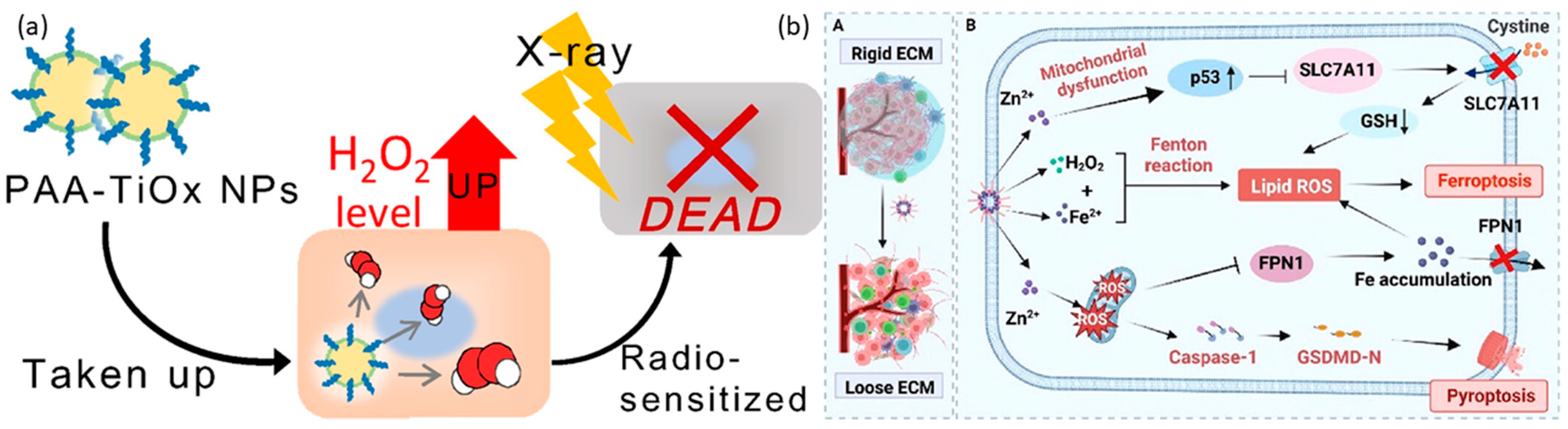
4.3. Other Metal Peroxide Nanoparticles
| MO2 | Surface Modifier | Application | Targeting Approach Passive or Active | Model (In Vivo and In Vitro) | Ref |
|---|---|---|---|---|---|
| CuO2 | PVP | CDT | Passive | Both | [132] |
| CuO2 | PLGA | PTT | Passive | Both | [133] |
| CuO2 | luminol | CDT/PDT | active | Both | [134] |
| CuO2 | CDT | Passive | In vitro | [135] | |
| CuO2 | PVP | CDT/ Starvation therapy | active | In vitro | [136] |
| CuO2 | PVP | CDT | Passive | In vitro | [137] |
| CuO2 | CDT | Passive | In vitro | [138] | |
| CuO2 | CMC | CDT/PDT | Passive | Both | [139] |
| CuO2 | CCM | CDT/PDT/PTT | Passive | Both | [140] |
| CuO2 | SiO2 | CDT/cuproptosis/ chemotherapy | Passive | Both | [142] |
| CuO2 | 5-poly(amidoamine) dendrimer | ferroptosis/cuproptosis/CDT | Active | Both | [142] |
| CaO2 | ferrocene | calcium overload/CDT | passive | Both | [143] |
| CaO2 | Hyaluronic acid | Calcification | Active | Both | [144] |
| CaO2 | ε-Poly Lysine and Hyaluronic Acid | PDT/calcium overload | Active | Both | [145] |
| CaO2 | PCM | calcium overload/calcification | Passive | Both | [146] |
| CaO2 | Sodium alginate | PTT/calcium overload | Passive | Both | [147] |
| CaO2 | liposomes | PDT | Passive | Both | [148] |
| CaO2 | sodium-hyaluronate | Calcium overload | Passive | Both | [150] |
| CaO2 | ZIF-67 | Chemo/CDT | Passive | Both | [153] |
| CaO2 | Lauric acid | PDT/CDT | Passive | Both | [154] |
| CaO2 | solid lipid monostearin | Chemo/CDT | Active | Both | [155] |
| CaO2 | Hyaluronic acid | calcicoptosis therapy/chemotherapy | Active | Both | [156] |
| CaO2 | phospholipid-coated liposomes | PDT/PTT | Passive | Both | [157] |
| TiOx | radiotherapy | Passive | Both | [158] | |
| TiOx | PAA | Radiotherapy | Passive | In vitro | [159] |
| TiOx | PAA | Radiotherapy | Passive | Both | [160] |
| TiOx | PAA | Radiotherapy | Passive | Both | [161] |
| Fe-ZnO2 | Hyaluronic acid | Ferroptosis/pyroptosis Immunotherapy | Passive | [162] | |
| Mn-ZnO2 | Immunotherapy | Passive | [163] | ||
| Mn-ZnO2 | dual ions and ROS | Passive | Both | [164] |
5. Biosafety
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| O2 | Oxygen |
| H2O2 | Hydrogen Peroxide |
| CuO2 | copper peroxide |
| CaO2 | calcium peroxide |
| MgO2 | magnesium peroxide |
| ZnO2 | zinc peroxide |
| BaO2 | barium peroxide |
| TiOx | titanium peroxide |
| TME | Tumor Microenvironment |
| •OH | hydroxyl radical |
| ROS | Reactive Oxygen Species |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| CDT | CDT |
| NIR | Near-Infrared Radiation |
| DNA | Deoxyribonucleic acid |
| HIF-1α | hypoxia-inducible factor 1α |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| LPO | Lipid Peroxidation |
| ICD | Immunogenic cell death |
| B1 | BODIPY dye |
| CSC | Cancer Stem Cells |
| PAA | Polyacrylic acid |
| MB | Methylene Blue |
| NH4HCO3 | Ammonium Bicarbonate |
| HMCPN | Hollow Mesoporous Calcium Peroxide Nanoparticles |
| P-gp | P-glycoprotein |
| ECM | Extracellular matrix |
| DCs | Dendritic cells |
| CAFs | Cancer-associated fibroblasts |
| MDSCs | Myeloid-derived suppressor cells |
| PC | Pancreatic cancer |
| CRC | Colorectal cancer |
| NPs | Nanoparticles |
| VEGF | Vascular endothelial growth factor |
| TGF | Transforming growth factor |
| EPR | Enhanced permeability and retention effect |
| FDA | Food and Drug Administration |
| TAM | Tumor-associated macrophage |
| GOx | Glucose oxidase |
| SOD | Superoxide dismutase |
| PVP | Polyvinylpyrrolidone |
| UCN | Upconversion nanoparticles |
| SRF | Sorafenib |
| Cys | Cysteine |
| MSN | Manganese Silicate Nanospheres |
| CFN | Copper Ferrite Nanospheres |
| MRP2 | Multidrug Resistance-associated Protein 2 |
| Fe3O4 | Iron Oxide Nanoparticles |
| HA | Hyaluronic acid |
| ICG | Indocyanine Green |
| LA | lauric acid |
References
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Domínguez, A.; Pastor, N.; Martínez-López, L.; Colón-Pérez, J.; Bermúdez, B.; Orta, M.L. The role of DNA damage response in dysbiosis-induced colorectal cancer. Cells 2021, 10, 1934. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhang, W.C.; Guo, Y.W.; Chen, X.Y. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022, 29, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Nie, S.; Wang, M.D. Nanotechnology applications in surgical oncology. Annu. Rev. Med. 2010, 61, 359–373. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-based drug delivery system: A patient-friendly chemotherapy for oncology. Dose-Response 2020, 18, 1559325820936161. [Google Scholar] [CrossRef]
- Çeşmeli, S.; Biray Avci, C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Avram, M.M.; Ortiz, A.E. The evolving story of laser therapeutics for basal cell carcinoma. Dermatol. Surg. 2020, 46, 1045–1053. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Kushwaha, K.; Gupta, J. Emerging nano-strategies against tumour microenvironment (TME): A review. OpenNano 2023, 9, 100112. [Google Scholar] [CrossRef]
- Sun, R.; Kong, X.; Qiu, X.; Huang, C.; Wong, P.-P. The emerging roles of pericytes in modulating tumor microenvironment. Front. Cell Dev. Biol. 2021, 9, 676342. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Biswas, S.; Wang, T.; Zhu, L.; Torchilin, V. Hypoxia-targeted siRNA delivery. Angew. Chem. 2014, 126, 3430–3434. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Kumar, H.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic nanoparticle: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar]
- Naletova, I.; Tomasello, B.; Attanasio, F.; Pleshkan, V.V. Prospects for the use of metal-based nanoparticles as adjuvants for local cancer immunotherapy. Pharmaceutics 2023, 15, 1346. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Ge, K.; Li, Z.; Liu, H.; Dai, X.; Zhang, Y.; Chen, S.; Liang, X.; Zhang, J. Self-supply of O2 and H2O2 by a nanocatalytic medicine to enhance combined chemo/chemodynamic therapy. Adv. Sci. 2019, 6, 1902137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, B.; Li, M.; Huang, Y.; Li, L. Heterostructures made of upconversion nanoparticles and metal–organic frameworks for biomedical applications. Adv. Sci. 2022, 9, 2103911. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, G.; Ding, X.; Liu, Y.; Chen, K.; Shi, P.; Zhang, S. A DNA functionalized metal–organic framework combined with magnesium peroxide nanoparticles: Targeted and enhanced photodynamic therapy. Mater. Chem. Front. 2022, 6, 956–965. [Google Scholar] [CrossRef]
- Bi, X.; Bai, Q.; Liang, M.; Yang, D.; Li, S.; Wang, L.; Liu, J.; Yu, W.W.; Sui, N.; Zhu, Z. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small 2022, 18, 2104160. [Google Scholar] [CrossRef]
- Rastinfard, A.; Dalisson, B.; Barralet, J. Aqueous decomposition behavior of solid peroxides: Effect of pH and buffer composition on oxygen and hydrogen peroxide formation. Acta Biomater. 2022, 145, 390–402. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Liu, X.; Zheng, X.; Zhou, M.; Wang, W.; Su, G.; Liu, T.; Wang, L.; Xie, Z. Polymer-metal-organic framework hybrids for bioimaging and cancer therapy. Coord. Chem. Rev. 2022, 456, 214393. [Google Scholar] [CrossRef]
- Gera, A.K.; Burra, R.K. The rise of polymeric Microneedles: Recent developments, advances, challenges, and applications with regard to Transdermal drug delivery. J. Funct. Biomater. 2022, 13, 81. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, S.; Nice, E.C.; Huang, C.; He, W.; Zou, B.; Lin, J. A cascaded copper-based nanocatalyst by modulating glutathione and cyclooxygenase-2 for hepatocellular carcinoma therapy. J. Colloid Interface Sci. 2022, 607, 1516–1526. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Yang, J.; Chen, C.; Shi, J. Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment. Nat. Commun. 2022, 13, 1988. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Liu, C. Development of a horseradish peroxidase-Fenton-like system for the degradation of sulfamethazine under weak acid condition. Environ. Sci. Pollut. Res. 2022, 29, 12065–12074. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-Y.; Wang, P.-T.; Su, W.-C.; Teng, H.; Huang, W.-L. Nanomedicine-based strategies assisting photodynamic therapy for hypoxic tumors: State-of-the-art approaches and emerging trends. Biomedicines 2021, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, N.; Zhang, L.; Wang, D.; Zhang, P. Novel design of multifunctional nanozymes based on tumor microenvironment for diagnosis and therapy. Eur. J. Med. Chem. 2022, 238, 114456. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-C.; Lu, C.-X.; Cho, E.-C.; Lee, P.-W.; Chi, N.-W.; Lin, P.-Y.; Jheng, P.-R.; Chen, H.-L.; Mansel, B.W.; Chen, Y.-M. Calcium peroxide aids tyramine-alginate gel to crosslink with tyrosinase for efficient cartilage repair. Int. J. Biol. Macromol. 2022, 208, 299–313. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, Y.; Yao, H.; Yan, L.; Yin, W.; Gu, Z. A copper peroxide Fenton nanoagent-hydrogel as an in situ pH-responsive wound dressing for effectively trapping and eliminating bacteria. ACS Appl. Bio. Mater. 2022, 5, 1779–1793. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, F.; Li, X.; Niu, G.; Yang, Y.; Li, H.; Jiang, Y. Tumor microenvironment-responsive fenton nanocatalysts for intensified anticancer treatment. J. Nanobiotechnol. 2022, 20, 69. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, K.; Liang, Y.; Wei, Y.; An, J.; Wang, Y.; Yang, J.; Zhang, H.; Zhang, Z.; Liu, J. Nano-enabled tumor Systematic energy exhaustion via zinc (II) Interference mediated glycolysis inhibition and specific GLUT1 depletion. Adv. Sci. 2022, 9, 2103534. [Google Scholar] [CrossRef]
- Zhuang, Y.; Han, S.; Fang, Y.; Huang, H.; Wu, J. Multidimensional transitional metal-actuated nanoplatforms for cancer chemodynamic modulation. Coord. Chem. Rev. 2022, 455, 214360. [Google Scholar] [CrossRef]
- Barnestein, R.; Galland, L.; Kalfeist, L.; Ghiringhelli, F.; Ladoire, S.; Limagne, E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. OncoImmunology 2022, 11, 2120676. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Zhang, X.; Fu, J.; Xing, X.; Wang, C.; Gao, L.; Liu, Y.; Shi, L. Potential applications of nanoparticles for tumor microenvironment remodeling to ameliorate cancer immunotherapy. Int. J. Pharm. 2019, 570, 118636. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor microenvironment and immunotherapy of oral cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef]
- Chang, R.B.; Beatty, G.L. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J. Leukoc. Biol. 2020, 108, 363–376. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S. Imaging the Tumor Microenvironment. Cancer J. 2015, 21, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef]
- Watnick, R.S. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harb. Perspect. Med. 2012, 2, a006676. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.; Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qiao, Z.; Li, Y.; Hu, S.; Ma, Y.; Wei, S.; Zhang, L. Persistent Luminescence Nanoplatform with Fenton-like Catalytic Activity for Tumor Multimodal Imaging and Photoenhanced Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 25572–25580. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qian, J.; Yu, A.; Pan, B. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Yue, D.; Guo, C.; Yan, X.; Wang, R.; Fang, M.; Wu, Y.; Qian, X.; Zhao, Y. Secondary battery inspired NiO nanosheets with rich Ni(III) defects for enhancing persulfates activation in phenolic waste water degradation. Chem. Eng. J. 2019, 360, 97–103. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Wang, J. Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole. J. Hazard. Mater. 2018, 342, 166–176. [Google Scholar] [CrossRef]
- Lutfi, W.; Talamonti, M.S.; Kantor, O.; Wang, C.H.; Liederbach, E.; Stocker, S.J.; Bentrem, D.J.; Roggin, K.K.; Winchester, D.J.; Marsh, R.; et al. Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery 2016, 160, 714–724. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.C.; Kang, M.K. Technical advances in external radiotherapy for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7311–7321. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Huang, L. Modulation of tumor microenvironment for immunotherapy: Focus on nanomaterial-based strategies. Theranostics 2020, 10, 3099–3117. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Janoniene, A.; Liu, Z.; Baranauskiene, L.; Mäkilä, E. A Versatile Carbonic Anhydrase IX Targeting Ligand-Functionalized Porous Silicon Nanoplatform for Dual Hypoxia Cancer Therapy and Imaging. ACS Appl. Mater. Interfaces 2017, 9, 13976–13987. [Google Scholar] [CrossRef]
- Mpekris, F.; Baish, J.W.; Stylianopoulos, T. Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.C.; Wang, L.L.; Zhang, X.D.; Xu, J.L.; Li, P.F.; Liang, H.; Zhang, X.B.; Xie, L.; Zhou, Z.H.; Yang, J.; et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J. Hematol. Oncol. 2021, 14, 92. [Google Scholar] [CrossRef]
- Mayer, A.; Höckel, M.; Wree, A.; Leo, C.; Horn, L.C.; Vaupel, P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008, 68, 4719–4726. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Han, H.; Von Hoff, D.D. Orchestrating the Tumor Microenvironment to Improve Survival for Patients With Pancreatic Cancer: Normalization, Not Destruction. Cancer J. 2015, 21, 299–306. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Zub, K.A.; Sousa, M.M.; Sarno, A.; Sharma, A.; Demirovic, A.; Rao, S.; Young, C.; Aas, P.A.; Ericsson, I.; Sundan, A.; et al. Modulation of cell metabolic pathways and oxidative stress signaling contribute to acquired melphalan resistance in multiple myeloma cells. PLoS ONE 2015, 10, e0119857. [Google Scholar] [CrossRef] [PubMed]
- Triner, D.; Shah, Y.M. Hypoxia-inducible factors: A central link between inflammation and cancer. J. Clin. Investig. 2016, 126, 3689–3698. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Guo, S.; Deng, C.X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef]
- Murata, T.; Mekada, E.; Hoffman, R.M. Reconstitution of a metastatic-resistant tumor microenvironment with cancer-associated fibroblasts enables metastasis. Cell Cycle 2017, 16, 533–535. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zha, H.; Yang, F.; Hu, C.; Chen, L.; Guo, B.; Zhu, B. Stroma-derived Fibrinogen-like Protein 2 Activates Cancer-associated Fibroblasts to Promote Tumor Growth in Lung Cancer. Int. J. Biol. Sci. 2017, 13, 804–814. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, T.; Liu, C.; Zhao, M.; Xie, M.; Li, G.; Liu, S.; Huang, W.; Zhao, Q. Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy. Chem. Sci. 2019, 10, 9091–9098. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, L.; Wu, S.; Zheng, H.; Chen, H.; Zhang, H.; Wang, Z.; Lin, Q. Microsatellite stability and mismatch repair proficiency in nasopharyngeal carcinoma may not predict programmed death-1 blockade resistance. Oncotarget 2017, 8, 113287–113293. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.; Wang, Q.; Wang, Y.; Chu, Y.; Luo, Y.; You, H.; Su, B.; Li, C.; Guo, Q.; Sun, T. Penetrating micelle for reversing immunosuppression and drug resistance in pancreatic cancer treatment. Small 2022, 18, 2107712. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef] [PubMed]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Hu, A.; Zhang, D.; Yang, H.; Mao, Y. Understanding the immunosuppressive microenvironment of glioma: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 31. [Google Scholar]
- Li, M.; Zhang, F.; Su, Y.; Zhou, J.; Wang, W. Nanoparticles designed to regulate tumor microenvironment for cancer therapy. Life Sci. 2018, 201, 37–44. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef]
- Sun, Q.; Bai, X.; Sofias, A.M.; van der Meel, R.; Ruiz-Hernandez, E.; Storm, G.; Hennink, W.E.; De Geest, B.; Kiessling, F.; Yu, H.J.; et al. Cancer nanomedicine meets immunotherapy: Opportunities and challenges. Acta Pharmacol. Sin. 2020, 41, 954–958. [Google Scholar] [CrossRef]
- Maddela, N.R.; Chakraborty, S.; Prasad, R. Nanotechnology for Advances in Medical Microbiology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Mozafari, M.; Torkaman, S.; Karamouzian, F.M.; Rasti, B.; Baral, B. Antimicrobial applications of nanoliposome encapsulated silver nanoparticles: A potential strategy to overcome bacterial resistance. Curr. Nanosci. 2021, 17, 26–40. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Kar Mahapatra, D. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. South Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2021, 12, 803304. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin-RGD interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Qutub, A.A.; Popel, A.S. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol. Cell. Biol. 2008, 28, 5106–5119. [Google Scholar] [CrossRef]
- Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. [Google Scholar] [CrossRef]
- George, B.P.; Chota, A.; Sarbadhikary, P.; Abrahamse, H. Fundamentals and applications of metal nanoparticle- enhanced singlet oxygen generation for improved cancer photodynamic therapy. Front. Chem. 2022, 10, 964674. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, Y. Development of chemical synthesis methods based on fusion of inorganic and organic chemistry for ceramic powder preparation and surface modification methods of nanoparticles and nanosheets. J. Jpn. Soc. Powder Powder Metall. 2022, 69, 13–21. [Google Scholar] [CrossRef]
- Talebzadeh, S.; Queffélec, C.; Knight, D.A. Surface modification of plasmonic noble metal–metal oxide core–shell nanoparticles. Nanoscale Adv. 2019, 1, 4578–4591. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Qiao, R.; Yan, S.; Yuan, D.; Zhao, Q.; Yun, G.; Davis, T.P.; Li, W. Microfluidic Mass Production of Stabilized and Stealthy Liquid Metal Nanoparticles. Small 2018, 14, e1800118. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, H.; Zhang, W.; Wang, M. Mussel-Inspired Surface Coating to Stabilize and Functionalize Supramolecular J-Aggregate Nanotubes Composed of Amphiphilic Cyanine Dyes. Langmuir 2022, 38, 8160–8168. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, L.; Lyu, P.; Wang, Y.; Li, C.; Xiao, X.; Dai, F.; Xu, W.; Liu, X.; Deng, B. Is It Possible To Fabricate a Nanocomposite with Excellent Mechanical Property Using Unmodified Inorganic Nanoparticles Directly? ACS Appl. Mater. Interfaces 2018, 10, 15357–15363. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Gan, L. Exsolved metallic iron nanoparticles in perovskite cathode to enhance CO2 electrolysis. J. Solid State Electrochem. 2022, 26, 409–417. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Hushmandi, K.; Rahmani Moghadam, E.; Zarrin, V.; Hosseinzadeh Kashani, S.; Bokaie, S.; Najafi, M.; Tavakol, S.; Mohammadinejad, R.; Nabavi, N. Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer. Bioengineering 2020, 7, 91. [Google Scholar] [CrossRef]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; Lopez de Cerain, A. Genotoxicity of silver nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef]
- Busatto, S.; Pham, A.; Suh, A.; Shapiro, S.; Wolfram, J. Organotropic drug delivery: Synthetic nanoparticles and extracellular vesicles. Biomed. Microdevices 2019, 21, 46. [Google Scholar] [CrossRef]
- Colby, A.H.; Liu, R.; Doyle, R.P.; Merting, A.; Zhang, H.; Savage, N.; Chu, N.-Q.; Hollister, B.A.; McCulloch, W.; Burdette, J.E. Pilot-scale production of expansile nanoparticles: Practical methods for clinical scale-up. J. Control. Release 2021, 337, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Nesbitt, H.; Callan, B.; Taylor, M.A.; Love, M.; McHale, A.P.; Callan, J.F. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release 2017, 264, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Wang, J.; Lu, S.-Y.; Yang, Q.; Chen, C.; Yang, H.; Hong, F.; Wu, C.; Zhao, Q. Polypyrrole-iron phosphate-glucose oxidase-based nanocomposite with cascade catalytic capacity for tumor synergistic apoptosis-ferroptosis therapy. Chem. Eng. J. 2022, 427, 131671. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Mu, Y.; Foda, M.F.; Han, H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials 2022, 284, 121520. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Z.; Liu, B.; He, F.; Gai, S.; Yang, P.; Yang, D.; Li, C.; Lin, J. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coord. Chem. Rev. 2022, 451, 214267. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, G.; Guo, Z.; Lu, J.; Ye, J.; Xu, W.; Gao, X.; Yue, K.; Wei, Y.; Zhao, L. Ultra-sensitive iron-doped Palladium nanocrystals with enhanced hydroxyl radical generation for chemo-/chemodynamic Nanotherapy. Adv. Funct. Mater. 2022, 32, 2107518. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, L.; Liu, J.; Wang, Y.; Zhang, T.; Liu, Y.; Zhao, Y.; Yin, N.; Niu, R.; Xue, D. Novel FeF2/Fe1–xS Nanoreactor-Mediated Mitochondrial Dysfunction via Oxidative Stress and Fluoride Ions Overloaded for Synergistic Chemodynamic Therapy and Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2113397. [Google Scholar] [CrossRef]
- Nie, X.; Xia, L.; Wang, H.-L.; Chen, G.; Wu, B.; Zeng, T.-Y.; Hong, C.-Y.; Wang, L.-H.; You, Y.-Z. Photothermal Therapy Nanomaterials Boosting Transformation of Fe(III) into Fe(II) in Tumor Cells for Highly Improving Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31735–31742. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, M.; Zhao, P.; Song, R.; Gong, T.; Liu, Y.; He, X.; Zhao, K.; Bu, W. Amorphous Fe-Based Nanoagents for Self-Enhanced Chemodynamic Therapy by Re-Establishing Tumor Acidosis. Adv. Funct. Mater. 2020, 30, 1908365. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Ding, Y.; Wu, J.; Hu, Y.; Yuan, A. Novel copper-based and pH-sensitive nanomedicine for enhanced chemodynamic therapy. Chem. Commun. 2020, 56, 7753–7756. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yu, J.; Lu, H.; Wei, Z.; Chao, Z.; Wang, Z.; Wu, W.; Jiang, H.; Tian, L. Mn–DNA coordination of nanoparticles for efficient chemodynamic therapy. Chem. Commun. 2021, 57, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, B.; Xu, L.; Yang, J.; Li, J. A CaO2@Tannic Acid-FeIII Nanoconjugate for Enhanced Chemodynamic Tumor Therapy. ChemMedChem 2021, 16, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Yang, N.; Cao, C.; Li, H.; Hong, Y.; Cai, Y.; Song, X.; Wang, W.; Mou, X.; Dong, X. Polymer-Based Therapeutic Nanoagents for Photothermal-Enhanced Combination Cancer Therapy. Small Struct. 2021, 2, 2100110. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Dong, S.; Zhang, F.; Dong, Y.; Tian, B.; He, F.; Gai, S.; Yang, P. An all-in-one theranostic nanoplatform based on upconversion dendritic mesoporous silica nanocomposites for synergistic chemodynamic/photodynamic/gas therapy. Nanoscale 2020, 12, 24146–24161. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Li, C.; Zhang, Y.; Yu, T.; Wu, R.; Zhao, J.; Liu, Z.; Liu, J.; Yu, H. Reactive Oxygen Species–Activatable Liposomes Regulating Hypoxic Tumor Microenvironment for Synergistic Photo/Chemodynamic Therapies. Adv. Funct. Mater. 2019, 29, 1905013. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Wang, J.; Wei, T.; Dai, Z. Mild Hyperthermia-Enhanced Enzyme-Mediated Tumor Cell Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23065–23071. [Google Scholar] [CrossRef]
- Liang, R.; Chen, Y.; Huo, M.; Zhang, J.; Li, Y. Sequential catalytic nanomedicine augments synergistic chemodrug and chemodynamic cancer therapy. Nanoscale Horiz. 2019, 4, 890–901. [Google Scholar] [CrossRef]
- Ding, B.; Shao, S.; Jiang, F.; Dang, P.; Sun, C.; Huang, S.; Ma, P.A.; Jin, D.; Kheraif, A.A.A.; Lin, J. MnO2-Disguised Upconversion Hybrid Nanocomposite: An Ideal Architecture for Tumor Microenvironment-Triggered UCL/MR Bioimaging and Enhanced Chemodynamic Therapy. Chem. Mater. 2019, 31, 2651–2660. [Google Scholar] [CrossRef]
- Chen, F.; Cai, W. Tumor Vasculature Targeting: A Generally Applicable Approach for Functionalized Nanomaterials. Small 2014, 10, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, J.; Chen, Z.; Luo, Q.; Xu, J.; Song, G. Tumor-Specific Expansion of Oxidative Stress by Glutathione Depletion and Use of a Fenton Nanoagent for Enhanced Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 30551–30565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Han, Y.-H.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Ding, M.; Fan, Y.; Lv, Y.; Liu, J.; Yu, N.; Kong, D.; Sun, H.; Li, J. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022, 149, 334–346. [Google Scholar] [CrossRef]
- Avdonin, P.V.; Nadeev, A.D.; Mironova, G.Y. Enhancement by Hydrogen Peroxide of Calcium Signals in Endothelial Cells Induced by 5-HT1B and 5-HT2B Receptor Agonists. Oxidative Med. Cell. Longev. 2019, 2019, 1701478. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Chen, L.; Yu, H.; Chen, Y. Catalytic Biomaterials and Nanomedicines with Exogenous and Endogenous Activations. Adv. Healthc. Mater. 2023, 12, e2201607. [Google Scholar] [CrossRef]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, e2103868. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, T.; Gong, S.; Shen, M.; Ma, S.; Huang, X.; Li, L.; Wang, L.; Wu, Q.; Gong, C. A tumor pH-responsive autocatalytic nanoreactor as a H2O2 and O2 self-supplying depot for enhanced ROS-based chemo/photodynamic therapy. Acta Biomater. 2022, 154, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Lv, G.; Bu, W. Redox dyshomeostasis strategy for tumor therapy based on nanomaterials chemistry. Chem. Sci. 2022, 13, 2202–2217. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Salimi, A. Multienzymes activity of metals and metal oxide nanomaterials: Applications from biotechnology to medicine and environmental engineering. J. Nanobiotechnol. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.X.; Wan, S.S.; Zhang, X.Z. Nanocatalyst-mediated chemodynamic tumor therapy. Adv. Healthc. Mater. 2022, 11, 2101971. [Google Scholar] [CrossRef]
- Yang, L.; Jia, P.; Song, S.; Dong, Y.; Shen, R.; He, F.; Gai, S. On-demand triggered chemodynamic therapy by NIR-II light on oxidation-prevented bismuth nanodots. ACS Appl. Mater. Interfaces 2022, 14, 21787–21799. [Google Scholar] [CrossRef]
- Lin, L.S.; Huang, T.; Song, J. Synthesis of Copper Peroxide Nanodots for H2O2 Self-Supplying Chemodynamic Therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef]
- Yang, N.; Li, H.; Cao, C.; Zhao, L.; Song, X.; Wang, W.; Xu, W.; Zhang, Y.; Chen, P.; Dong, X. Tumor microenvironment-activated theranostic nanoreactor for NIR-II Photoacoustic imaging-guided tumor-specific photothermal therapy. Fundam. Res. 2024, 4, 178–187. [Google Scholar] [CrossRef]
- Cao, X.; Li, S.; Chen, W.; Lu, H.; Ye, L.; Min, Z.; Sun, S.; Teng, C.; Yin, H.; Zhang, Q.; et al. Multifunctional Hybrid Hydrogel System Enhanced the Therapeutic Efficacy of Treatments for Postoperative Glioma. ACS Appl. Mater. Interfaces 2022, 14, 27623–27633. [Google Scholar] [CrossRef]
- Deng, H.; Yang, Z.; Pang, X.; Zhao, C.; Tian, J.; Wang, Z.; Chen, X. Self-sufficient copper peroxide loaded pKa-tunable nanoparticles for lysosome-mediated chemodynamic therapy. Nano Today 2022, 42, 101337. [Google Scholar] [CrossRef]
- Hong, Y.; Tao, Q.; Liu, Y.-Y.; Wang, Z.; Wang, H.; Sun, L. Copper peroxide coated upconversion nanoparticle modified with glucose oxidase for H2O2 self-supplying starvation-enhanced chemodynamic therapy in vitro. Dalton Trans. 2022, 51, 11325–11334. [Google Scholar] [CrossRef]
- Chen, C.; Tan, Y.; Xu, T.; Sun, Y.; Zhao, S.; Ouyang, Y.; Chen, Y.; He, L.; Liu, X.; Liu, H. Sorafenib-Loaded Copper Peroxide Nanoparticles with Redox Balance Disrupting Capacity for Enhanced Chemodynamic Therapy against Tumor Cells. Langmuir 2022, 38, 12307–12315. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.; Liu, F.; Zhang, S.; Duan, J.; Li, Z.; Kong, Y.; Sang, Y.; Liu, H.; Bu, W.; et al. Self-Assembled Copper–Amino Acid Nanoparticles for in Situ Glutathione “AND” H2O2 Sequentially Triggered Chemodynamic Therapy. J. Am. Chem. Soc. 2019, 141, 849–857. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Zhang, S.; Cheng, Y.; Yang, F.; Xing, Y.; Xu, T.; Dong, H.; Zhang, X. Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief. ACS Nano 2019, 13, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhen, W.; Jin, L.; Zhang, S.; Sun, G.; Zhang, T.; Xu, X.; Song, S.; Wang, Y.; Liu, J.; et al. All-in-One Theranostic Nanoagent with Enhanced Reactive Oxygen Species Generation and Modulating Tumor Microenvironment Ability for Effective Tumor Eradication. ACS Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, M.; Fan, S.; Li, Y.; Fang, L.; Xiang, G.; Yang, T. Copper peroxide and cisplatin co-loaded silica nanoparticles-based trinity strategy for cooperative cuproptosis/chemo/chemodynamic cancer therapy. Chem. Eng. J. 2024, 481, 148522. [Google Scholar] [CrossRef]
- Huang, H.; Guo, H.; Liu, J.; Ni, C.; Xia, L.; Cao, X.; Xia, J.; Shi, X.; Guo, R. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO2 for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. 2024, 183, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Chu, Q.; Fang, C.; Cao, G.; Han, G.; Li, X. Cu–Ferrocene-Functionalized CaO2 Nanoparticles to Enable Tumor-Specific Synergistic Therapy with GSH Depletion and Calcium Overload. Adv. Sci. 2021, 8, 2100241. [Google Scholar] [CrossRef]
- Liu, B.; Bian, Y.; Liang, S.; Yuan, M.; Dong, S.; He, F. One-Step Integration of Tumor Microenvironment-Responsive Calcium and Copper Peroxides Nanocomposite for Enhanced Chemodynamic/Ion-Interference Therapy. ACS Nano 2022, 16, 617–630. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, W.; Wu, L.; Shao, K.; Wang, L.; Ding, M.; Shi, J.; Kong, X. Image-Guided TME-Improving Nano-Platform for Ca2+ Signal Disturbance and Enhanced Tumor PDT. Adv. Healthc. Mater. 2021, 10, e2100789. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, B.; Wang, Z.; Feng, L.; Zhao, R.; Dong, S.; Dong, Y.; Zhong, L.; Gai, S.; Yang, P. H2O2/O2 self-supplementing and GSH-depleting Ca2+ nanogenerator with hyperthermia-triggered, TME-responsive capacities for combination cancer therapy. Chem. Eng. J. 2021, 425, 131485. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Wang, Z.; Li, Y.; Liu, Y.; Xu, M.; Zhang, Z.; Deng, Y.; Cai, L.; Zhang, C.; et al. Nano-modulators with the function of disrupting mitochondrial Ca2+ homeostasis and photothermal conversion for synergistic breast cancer therapy. J. Nanobiotechnol. 2023, 21, 465. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Zhang, Y.H.; Qiu, W.X.; Zhang, L.; Gao, F.; Li, B.; Xu, L.; Fan, J.X.; Li, Z.H.; Zhang, X.Z. Dual-Stage Light Amplified Photodynamic Therapy against Hypoxic Tumor Based on an O2 Self-Sufficient Nanoplatform. Small 2017, 13, 1701621. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Metal peroxides for cancer treatment. Bioact. Mater. 2021, 6, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, R.; Liu, Y.; Yi, Z.; Meng, X.; Zhang, J.; Tang, Z.; Yao, Z.; Liu, Y.; Liu, X.; et al. Calcium-Overload-Mediated Tumor Therapy by Calcium Peroxide Nanoparticles. Chem 2019, 5, 2171–2182. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Pedersen, C.M. Vessel effects in organic chemical reactions; a century-old, overlooked phenomenon. Chem. Sci. 2022, 13, 6181–6196. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Cheng, Y.; Wang, D.; Xu, T. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat. Commun. 2020, 11, 1735. [Google Scholar] [CrossRef]
- He, C.; Zhang, X.; Chen, C.; Liu, X.; Chen, Y.; Yan, R.; Fan, T.; Gai, Y.; Lee, R.J.; Ma, X.; et al. A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 2021, 122, 354–364. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Li, Z.; Feng, J.; Duan, X.; Yan, C.; Wen, G.; Qiu, X.; Shen, Z. Hollow mesoporous calcium peroxide nanoparticles for drug-free tumor calcicoptosis therapy. Acta Biomater. 2024, 185, 456–466. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza-BODIPY for imaging-guided synergistic photodynamic and photothermal tumor therapy. Adv. Healthc. Mater. 2018, 7, 1800606. [Google Scholar] [CrossRef]
- Salah, M.; Akasaka, H.; Shimizu, Y.; Morita, K.; Nishimura, Y.; Kubota, H.; Kawaguchi, H.; Sogawa, T.; Mukumoto, N.; Ogino, C.; et al. Reactive oxygen species-inducing titanium peroxide nanoparticles as promising radiosensitizers for eliminating pancreatic cancer stem cells. J. Exp. Clin. Cancer Res. CR 2022, 41, 146. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Nishimura, Y.; Nakamura, S.; Arai, Y.; Numako, C.; Sato, K.; Nakayama, M.; Akasaka, H.; Sasaki, R.; Ogino, C.; et al. Titanium oxide nano-radiosensitizers for hydrogen peroxide delivery into cancer cells. Colloids Surf. B Biointerfaces 2021, 198, 111451. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Nakayama, M.; Salah, M.; Akasaka, H.; Kubota, H.; Nakahana, M.; Tagawa, T.; Morita, K. A Comparative Assessment of Mechanisms and Effectiveness of Radiosensitization by Titanium Peroxide and Gold Nanoparticles. Nanomaterials 2020, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Sasaki, R.; Ogino, C.; Tanaka, T.; Morita, K.; Umetsu, M.; Ohara, S.; Tan, Z.; Nishimura, Y.; Akasaka, H.; et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiat. Oncol. 2016, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.; Hou, G.; Lei, H.; Liu, L.; Huang, X.; Sun, S.; Liu, L.; Liu, X.; Na, J.; et al. Zinc-Iron Bimetallic Peroxides Modulate the Tumor Stromal Microenvironment and Enhance Cell Immunogenicity for Enhanced Breast Cancer Immunotherapy Therapy. ACS Nano 2024, 18, 10542–10556. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, S.; Liu, D.; Ma, K.; Yun, K.; Yao, J.; Peng, Y.; Hai, L.; Zhang, Q.; Wang, Z. Manganese-Enriched Zinc Peroxide Functional Nanoparticles for Potentiating Cancer Immunotherapy. Nano Lett. 2023, 23, 10350–10359. [Google Scholar] [CrossRef]
- Wang, J.; Qu, C.; Shao, X.; Song, G.; Sun, J.; Shi, D.; Jia, R.; An, H.; Wang, H. Carrier-free nanoprodrug for p53-mutated tumor therapy via concurrent delivery of zinc-manganese dual ions and ROS. Bioact. Mater. 2023, 20, 404–417. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Baun, A.; Andersen, H.R. Acute toxicity and risk evaluation of the CSO disinfectants performic acid, peracetic acid, chlorine dioxide and their by-products hydrogen peroxide and chlorite. Sci. Total Environ. 2019, 677, 1–8. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res. Rev. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Nurchi, V.M.; Szebesczyk, A.; Bilska, P.; Krzywoszynska, K.; Kozlowski, H. Chelating agents as tools for the treatment of metal overload. Z. Für Anorg. Und Allg. Chem. 2013, 639, 1321–1331. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Gao, S.; Lu, X.; Zhu, P.; Lin, H.; Yu, L.; Yao, H.; Wei, C.; Chen, Y.; Shi, J. Self-evolved hydrogen peroxide boosts photothermal-promoted tumor-specific nanocatalytic therapy. J. Mater. Chem. B 2019, 7, 3599–3609. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic hyperthermia–synergistic H2O2 self-sufficient catalytic suppression of osteosarcoma with enhanced bone-regeneration bioactivity by 3D-printing composite scaffolds. Adv. Funct. Mater. 2020, 30, 1907071. [Google Scholar] [CrossRef]
- Tang, Z.M.; Liu, Y.Y.; Ni, D.L.; Zhou, J.J.; Zhang, M.; Zhao, P.R.; Lv, B.; Wang, H.; Jin, D.Y.; Bu, W.B. Biodegradable Nanoprodrugs: “Delivering” ROS to Cancer Cells for Molecular Dynamic Therapy. Adv. Mater. 2020, 32, e1904011. [Google Scholar] [CrossRef]
- Koo, S.; Park, O.K.; Kim, J.; Han, S.I.; Yoo, T.Y.; Lee, N.; Kim, Y.G.; Kim, H.; Lim, C.; Bae, J.-S.; et al. Enhanced Chemodynamic Therapy by Cu–Fe Peroxide Nanoparticles: Tumor Microenvironment-Mediated Synergistic Fenton Reaction. ACS Nano 2022, 16, 2535–2545. [Google Scholar] [CrossRef]
- Chen, X.; Yin, X.; Zhan, L.; Zhang, J.; Zhang, Y.; Wu, Y.; Ju, J.; Li, Y.; Xue, Q.; Wang, X.; et al. Organelle-Specific Anchored Delivery System Stretching a Reversal of Tumor Hypoxia Microenvironment to a Combinational Chemo-Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2108603. [Google Scholar] [CrossRef]
- Dirersa, W.B.; Kan, T.-C.; Chang, J.; Getachew, G.; Ochirbat, S.; Kizhepat, S.; Wibrianto, A.; Rasal, A.; Chen, H.-A.; Ghule, A.V.; et al. Engineering H2O2 Self-Supplying Platform for Xdynamic Therapies via Ru–Cu Peroxide Nanocarrier: Tumor Microenvironment-Mediated Synergistic Therapy. ACS Appl. Mater. Interfaces 2024, 16, 24172–24190. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. (Int. Ed. Engl.) 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Z.; Zhao, K.; Zhang, P.; Zhong, Q.-Z.; Yu, Q.; Zhai, S.; Cui, J. Co-delivery of enzymes and photosensitizers via metal-phenolic network capsules for enhanced photodynamic therapy. Chin. Chem. Lett. 2022, 33, 1917–1922. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaram, J.; Kuthati, Y. Metal Peroxide Nanoparticles for Modulating the Tumor Microenvironment: Current Status and Recent Prospects. Cancers 2024, 16, 3581. https://doi.org/10.3390/cancers16213581
Rajaram J, Kuthati Y. Metal Peroxide Nanoparticles for Modulating the Tumor Microenvironment: Current Status and Recent Prospects. Cancers. 2024; 16(21):3581. https://doi.org/10.3390/cancers16213581
Chicago/Turabian StyleRajaram, Jagadeesh, and Yaswanth Kuthati. 2024. "Metal Peroxide Nanoparticles for Modulating the Tumor Microenvironment: Current Status and Recent Prospects" Cancers 16, no. 21: 3581. https://doi.org/10.3390/cancers16213581
APA StyleRajaram, J., & Kuthati, Y. (2024). Metal Peroxide Nanoparticles for Modulating the Tumor Microenvironment: Current Status and Recent Prospects. Cancers, 16(21), 3581. https://doi.org/10.3390/cancers16213581







