Molecular Genetic Factors of Risk Stratification of Lymph Node Metastasis in Endometrial Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. The Role of Lymph Node Status and Lymphovascular Space Involvement in the Appropriateness of Performing Lymphadenectomy
4. Laboratory and Diagnostic Methods for Assessing Lymph Node Status
5. Medical–Genetic Factors of Metastatic Lymph Node Involvement and Lymphovascular Space Invasion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 2020. Available online: https://gco.iarc.fr/today (accessed on 20 December 2023).
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhu, T.; Zhao, L.; Che, F.; Chen, Y.; Shou, H.; Yu, A. Metabolic syndrome is an independent prognostic factor for endometrial adenocarcinoma. Clin. Transl. Oncol. 2015, 17, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Stewart, C.J.R.; Clarke, E.M.; Lose, F.; Davies, C.; Armes, J.; Obermair, A.; Brennan, D.; Webb, P.M.; Nagle, C.M.; et al. ER and PR expression and survival after endometrial cancer. Gynecol. Oncol. 2018, 148, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J. Low. Genit. Tract Dis. 2012, 16, 205–242, Erratum in J. Low. Genit. Tract Dis. 2013, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Mills, A.M.; Modesitt, S.C. Endometrial Hyperplasia. Obstet. Gynecol. 2022, 140, 1061–1075. [Google Scholar] [CrossRef]
- Najioui, Y.; Karich, N.; Haloui, A.; Miry, A.; Bennani, A. Serous endometrial intraepithelial carcinoma: A case report. Pan. Afr. Med. J. 2023, 44, 122. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.; Ngan, H.; Pecorelli, S. Carcinoma of the Corpus Uteri. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. S1), S105–S143. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Modesitt, S.C. Hereditary Cancers in Gynecology: What Physicians Should Know About Genetic Testing, Screening, and Risk Reduction. Obstet. Gynecol. Clin. N. Am. 2018, 45, 155–173. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309, Erratum in Lancet Oncol. 2018, 19, e184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegenthaler, F.; Epstein, E.; Büchi, C.A.; Gmür, A.; Saner, F.A.C.M.; Rau, T.T.; Carlson, J.W.; Mueller, M.D.; Imboden, S. Prognostic value of lymphovascular space invasion according to the molecular subgroups in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 1702–1707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.Y.; Kim, E.Y.; Jung, K.W.; Shin, A.; Chan, K.K.; Aoki, D.; Kim, J.W.; Low, J.J.; Won, Y.J. Trends in gynecologic cancer mortality in East Asian regions. J. Gynecol. Oncol. 2014, 25, 174–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751, Erratum in Gynecol. Oncol. 2004, 94, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Briët, J.M.; Hollema, H.; Reesink, N.; Aalders, J.G.; Mourits, M.J.; ten Hoor, K.A.; Pras, E.; Boezen, H.M.; van der Zee, A.G.; Nijman, H.W. Lymphvascular space involvement: An independent prognostic factor in endometrial cancer. Gynecol. Oncol. 2005, 96, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60 (Suppl. S8), 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Lutman, C.V.; Havrilesky, L.J.; Cragun, J.M.; Secord, A.A.; Calingaert, B.; Berchuck, A.; Clarke-Pearson, D.L.; Soper, J.T. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol. Oncol. 2006, 102, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172, Erratum in Lancet 2010, 376, 594. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Cheung, M.K.; Huh, W.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S. Therapeutic role of lymph node resection in endometrioid corpus cancer: A study of 12,333 patients. Cancer 2006, 107, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Honma, R.; Kojima, M.; Nomura, S.; Furukawa, S.; Soeda, S.; Watanabe, S.; Fujimori, K. Prediction of lymphovascular space invasion in endometrial cancer using the 55-gene signature selected by DNA microarray analysis. PLoS ONE 2019, 14, e0223178. [Google Scholar] [CrossRef] [PubMed]
- Jhang, H.; Chuang, L.; Visintainer, P.; Ramaswamy, G. CA 125 levels in the preoperative assessment of advanced-stage uterine cancer. Am. J. Obstet. Gynecol. 2003, 188, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Rockall, A.G.; Sohaib, S.A.; Harisinghani, M.G.; Babar, S.A.; Singh, N.; Jeyarajah, A.R.; Oram, D.H.; Jacobs, I.J.; Shepherd, J.H.; Reznek, R.H. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J. Clin. Oncol. 2005, 23, 2813–2821, Erratum in J. Clin. Oncol. 2005, 23, 4808. [Google Scholar] [CrossRef] [PubMed]
- Nogami, Y.; Banno, K.; Irie, H.; Iida, M.; Kisu, I.; Masugi, Y.; Tanaka, K.; Tominaga, E.; Okuda, S.; Murakami, K.; et al. The efficacy of preoperative positron emission tomography-computed tomography (PET-CT) for detection of lymph node metastasis in cervical and endometrial cancer: Clinical and pathological factors influencing it. Jpn. J. Clin. Oncol. 2015, 45, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ugaki, H.; Kimura, T.; Miyatake, T.; Ueda, Y.; Yoshino, K.; Matsuzaki, S.; Fujita, M.; Kimura, T.; Morii, E.; Enomoto, T. Intraoperative frozen section assessment of myometrial invasion and histology of endometrial cancer using the revised FIGO staging system. Int. J. Gynecol. Cancer 2011, 21, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.J.; Lester, S.C.; Tse, G.M.; Factor, R.E.; Allison, K.H.; Collins, L.C.; Chen, Y.Y.; Jensen, K.C.; Johnson, N.B.; Jeong, J.C.; et al. The molecular basis of breast cancer pathological phenotypes. J. Pathol. 2017, 241, 375–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshida, E.; Terao, Y.; Hayashi, N.; Mogushi, K.; Arakawa, A.; Tanaka, Y.; Ito, Y.; Ohmiya, H.; Hayashizaki, Y.; Takeda, S.; et al. Promoter-level transcriptome in primary lesions of endometrial cancer identified biomarkers associated with lymph node metastasis. Sci. Rep. 2017, 7, 14160. [Google Scholar] [CrossRef] [PubMed]
- Togami, S.; Kawamura, T.; Fukuda, M.; Yanazume, S.; Kamio, M.; Kobayashi, H. Quantitative RT-PCR Assay for Detecting Lymph Node Metastasis in Endometrial Cancer: A Preliminary Study. Oncology 2019, 96, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Liu, H.; Feng, Q.; Liu, J.; Ming, L. HOXB9 promotes endometrial cancer progression by targeting E2F3. Cell Death Dis. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, S.; Thompson, Z.; McClung, E.C.; Abdallah, R.; Lee, J.K.; Gonzalez-Bosquet, J.; Wenham, R.M.; Chon, H.S. Gene Expression Signature-Based Prediction of Lymph Node Metastasis in Patients With Endometrioid Endometrial Cancer. Int. J. Gynecol. Cancer 2018, 28, 260–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammad, M.O.; Elabbasy, L.M.; Abd Elghaffar, M.A.; Zaki, M.M.A.; Bazeed, F.B.; Zahran, M.A. Significance of CEP78 and WDR62 gene expressions in differentiated thyroid carcinoma: Possible predictors of lateral lymph node metastasis. Asia Pac. J. Clin. Oncol. 2019, 15, e154–e161. [Google Scholar] [CrossRef] [PubMed]
- van den Heerik, A.S.V.M.; Horeweg, N.; de Boer, S.M.; Bosse, T.; Creutzberg, C.L. Adjuvant therapy for endometrial cancer in the era of molecular classification: Radiotherapy, chemoradiation and novel targets for therapy. Int. J. Gynecol. Cancer 2021, 31, 594–604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Yu, D.; Wang, J.; Zhu, N.; Tang, X.B.; Chen, X.; Su, X.M.; Huang, Y.G. Immune signatures of the POLE mutation in endometrial carcinomas: A systematic study based on TCGA data and clinical cohort validation. Front. Oncol. 2023, 13, 1250558. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Köbel, M.; Kang, E.Y. The Many Uses of p53 Immunohistochemistry in Gynecological Pathology: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual9 Meeting. Int. J. Gynecol. Pathol. 2021, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, C.J.; Brown, M.; Haight, P.J.; Backes, F.J.; Cohn, D.E.; Goodfellow, P.J.; Cosgrove, C.M. Epigenetic MMR defect identifies a risk group not accounted for through traditional risk stratification algorithms in endometrial cancer. Front. Oncol. 2023, 13, 1147657. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Mitsumoto, Y.; Kyushiki, H.; Kanemoto, N.; Watanabe, A.; Taniguchi, Y.; Nishino, N.; Okamoto, T.; Kondo, M.; Mori, T.; et al. Identification and characterization of TMEFF2, a novel survival factor for hippocampal and mesencephalic neurons. Genomics 2000, 67, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Robertson, K.D.; Talmadge, C.; Sumegi, J.; Jones, P.A. The gene for a novel transmembrane protein containing epidermal growth factor and follistatin domains is frequently hypermethylated in human tumor cells. Cancer Res. 2000, 60, 4907–4912. [Google Scholar] [PubMed]
- Clarke, M.A.; Luhn, P.; Gage, J.C.; Bodelon, C.; Dunn, S.T.; Walker, J.; Zuna, R.; Hewitt, S.; Killian, J.K.; Yan, L.; et al. Discovery and validation of candidate host DNA methylation markers for detection of cervical precancer and cancer. Int. J. Cancer 2017, 141, 701–710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.C.; Tsao, C.M.; Kuo, C.C.; Yu, M.H.; Lin, Y.W.; Yang, C.Y.; Li, H.J.; Yan, M.D.; Wang, T.J.; Chou, Y.C.; et al. Quantitative DNA methylation analysis of selected genes in endometrial carcinogenesis. Taiwan J. Obstet. Gynecol. 2015, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Nie, X.; Zheng, M.; Li, X.; Guo, Q.; Liu, J.; Liu, Q.; Hao, Y.; Lin, B. TMEFF2 is a novel prognosis signature and target for endometrial carcinoma. Life Sci. 2020, 243, 116910. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Liu, Z.; Wang, T.; Shi, F.; Zhang, Y.; Su, J.; Jia, Y. Upregulated delta-like protein 3 expression is a diagnostic and prognostic marker in endometrial cancer: A retrospective study. Medicine 2018, 97, e13442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chapman, G.; Sparrow, D.B.; Kremmer, E.; Dunwoodie, S.L. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum. Mol. Genet. 2011, 20, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. miR-107-5p promotes tumor proliferation and invasion by targeting estrogen receptor-α in endometrial carcinoma. Oncol. Rep. 2019, 41, 1575–1585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, E.A.; Rajendran, P.; Scherthan, H. The microRNA-202 as a Diagnostic Biomarker and a Potential Tumor Suppressor. Int. J. Mol. Sci. 2022, 23, 5870. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; He, X.; Wang, Y.; Li, J. Effect of microRNA-29b on proliferation, migration, and invasion of endometrial cancer cells. J. Int. Med. Res. 2019, 47, 3803–3817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.; Hou, L.; Qiu, C.; Kong, B. MiR-501 promotes tumor proliferation and metastasis by targeting HOXD10 in endometrial cancer. Cell Mol. Biol. Lett. 2021, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, N.; Chen, H.; Zhang, M.; Lin, Q. MicroRNA-199a/b-5p inhibits endometrial cancer cell metastasis and invasion by targeting FAM83B in the epithelial-to-mesenchymal transition signaling pathway. Mol. Med. Rep. 2021, 23, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Zhang, L.; Jiang, W.; Zhang, R.; Zhang, B.; Silayiding, A.; Duan, X. MicroRNA-135a promotes proliferation, migration, invasion and induces chemoresistance of endometrial cancer cells. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 5, 100103. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Xu, Y.; Wu, F.; Pan, Q.; Liu, Y. Identification of a five-gene signature for predicting the progression and prognosis of stage I endometrial carcinoma. Oncol. Lett. 2020, 20, 2396–2410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, T.; Yang, K.; Chen, J.; Qi, L.; Zhou, X.; Wang, P. Comprehensive Pan-Cancer Analysis of KIF18A as a Marker for Prognosis and Immunity. Biomolecules 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liao, M.; Liao, Y.; Chen, X.; Huang, C.; Fan, J.; Liao, W. The role of kinesin KIF18A in the invasion and metastasis of hepatocellular carcinoma. World J. Surg. Oncol. 2018, 16, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Huang, S.; Shi, K.; Yi, L.; Liu, Y.; Liu, W. Prognostic Role of Matrix Metalloproteinases in Cervical Cancer: A Meta-Analysis. Cancer Control 2021, 28, 10732748211033743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erdemoglu, E.; Serel, T.A.; Karacan, E.; Köksal, O.K.; Turan, İ.; Öztürk, V.; Bozkurt, K.K. Artificial intelligence for prediction of endometrial intraepithelial neoplasia and endometrial cancer risks in pre- and postmenopausal women. AJOG Glob. Rep. 2023, 3, 100154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

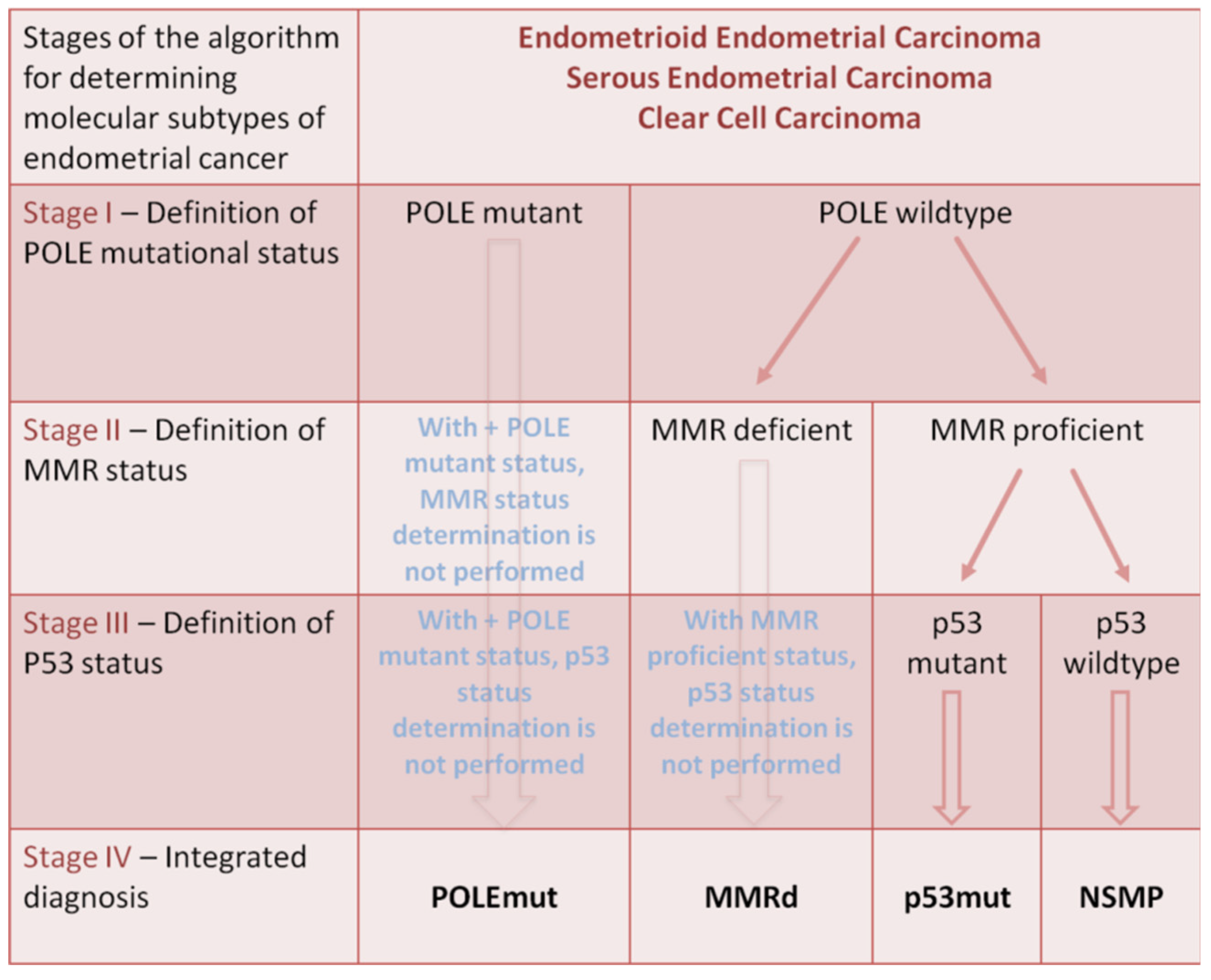

| Study | Biomarker | Expression | Results of Biomarker Expression Changes |
|---|---|---|---|
| Emiko Yoshida et al. (2017) [30] | mRNA SEMA3D | ↑ | inhibition of lymphangiogenesis, increased relapse-free survival |
| relative expression of SEMA3D compared to TACC2 isoform | ↑↑ | longer relapse-free survival elapse | |
| Shinichi Togami et al. (2018) [31] | Cytokeratin 19 mRNA (CK19) | ↑ | metastatic lymph nodes marker |
| Junhu Wan et al. (2018) [32] | HOXB9 | ↑ | higher risk of progression, metastasis, and shorter survival time |
| Sukbum Kang et al. (2019) [33] | GREM2, FMO2, TMEM212, ESR1, RPTN, PRR9, TCHHL1, CPB1, CLCN2, ITLN2, PKHD1L1 и SLC9C2 | ↑ | low risk of lymph node metastasis |
| van den Heerik et al. (2023) [35] | POLEmut | ↑ | decreased metastatic potential, low probability of recurrence |
| MMRd | ↑ | pronounced immune response with an intermediate prognosis | |
| p53abn | ↑ | non-endometrioid types and endometrioid adenocarcinoma G 3 (serous cancer, carcinosarcoma, about half of clear cell cancer cases), early metastasis, unfavorable prognosis | |
| Wang T et al. (2020) [36] | CD8, CD4, PD-L1 or Foxp3 mutants of POLE | ↑ | increased overall survival |
| Riedinger CJ et al. (2000) [38] | epigenetic defect of MMR | ↑ | predictor of metastasis and relapse to lymph nodes, regardless of tumor volume |
| Horie M et al. (2000) [39,40,41,42] Lingling Gao et al. (2018) [43] | TMEFF2 | ↑ | increased risk of progression, metastasis to lymph nodes, decreased overall relapse-free survival |
| Juan Wang et al. (2011) [44,45] | DDL3 | ↑ | deep myometrial invasion, metastasis to pelvic and paraaortic lymph nodes, higher tumor stage, lower grade of differentiation and shorter overall survival |
| Wei Bao et al. (2019) [46] | gene microRNA-107 (miR-107-5p) | ↑ | decreased histological differentiation, increased myometrial invasion and lymph node metastasis |
| Ahmed EA et al. (2022) [47] | miR-202 | ↓ | poor prognostic factor |
| Kong J et al. (2019) [48] | miR-29b | ↑ | inhibition of proliferation and invasion, increased sensitivity to chemotherapy |
| Sun X et al. (2021) [49] | miR-501 | ↑ | high risk of pelvic lymph node metastasis and shorter overall survival |
| Xiong H et al. (2021) [50] | miR-199a/b-5p | ↑ | inhibition of tumor cell migration and invasion |
| Wang J et al. (2019) [51] | miR-135a | ↑ | increased tumor invasion and proliferation |
| Jia Bian et al. (2020) [52] | BUB1B CCNB1 CDC20 NCAPG DLGAP5 | ↑ | higher tumor grade, higher risk of metastasis, and worse overall survival |
| Liu T et al. (2023) [53] Weiwei Luo et al. (2018) [54] Weiwei Chen et al. (2021) [55] | KIF18A | ↑ | accelerated division of tumor cells with invasion and metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilyadova, A.; Ishchenko, A.; Babayan, J.; Avin, M.; Sekacheva, M.; Reshetov, I. Molecular Genetic Factors of Risk Stratification of Lymph Node Metastasis in Endometrial Carcinoma. Cancers 2024, 16, 3560. https://doi.org/10.3390/cancers16213560
Gilyadova A, Ishchenko A, Babayan J, Avin M, Sekacheva M, Reshetov I. Molecular Genetic Factors of Risk Stratification of Lymph Node Metastasis in Endometrial Carcinoma. Cancers. 2024; 16(21):3560. https://doi.org/10.3390/cancers16213560
Chicago/Turabian StyleGilyadova, Aida, Anton Ishchenko, Julietta Babayan, Max Avin, Marina Sekacheva, and Igor Reshetov. 2024. "Molecular Genetic Factors of Risk Stratification of Lymph Node Metastasis in Endometrial Carcinoma" Cancers 16, no. 21: 3560. https://doi.org/10.3390/cancers16213560
APA StyleGilyadova, A., Ishchenko, A., Babayan, J., Avin, M., Sekacheva, M., & Reshetov, I. (2024). Molecular Genetic Factors of Risk Stratification of Lymph Node Metastasis in Endometrial Carcinoma. Cancers, 16(21), 3560. https://doi.org/10.3390/cancers16213560






