HIPEC as Up-Front Treatment in Locally Advanced Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
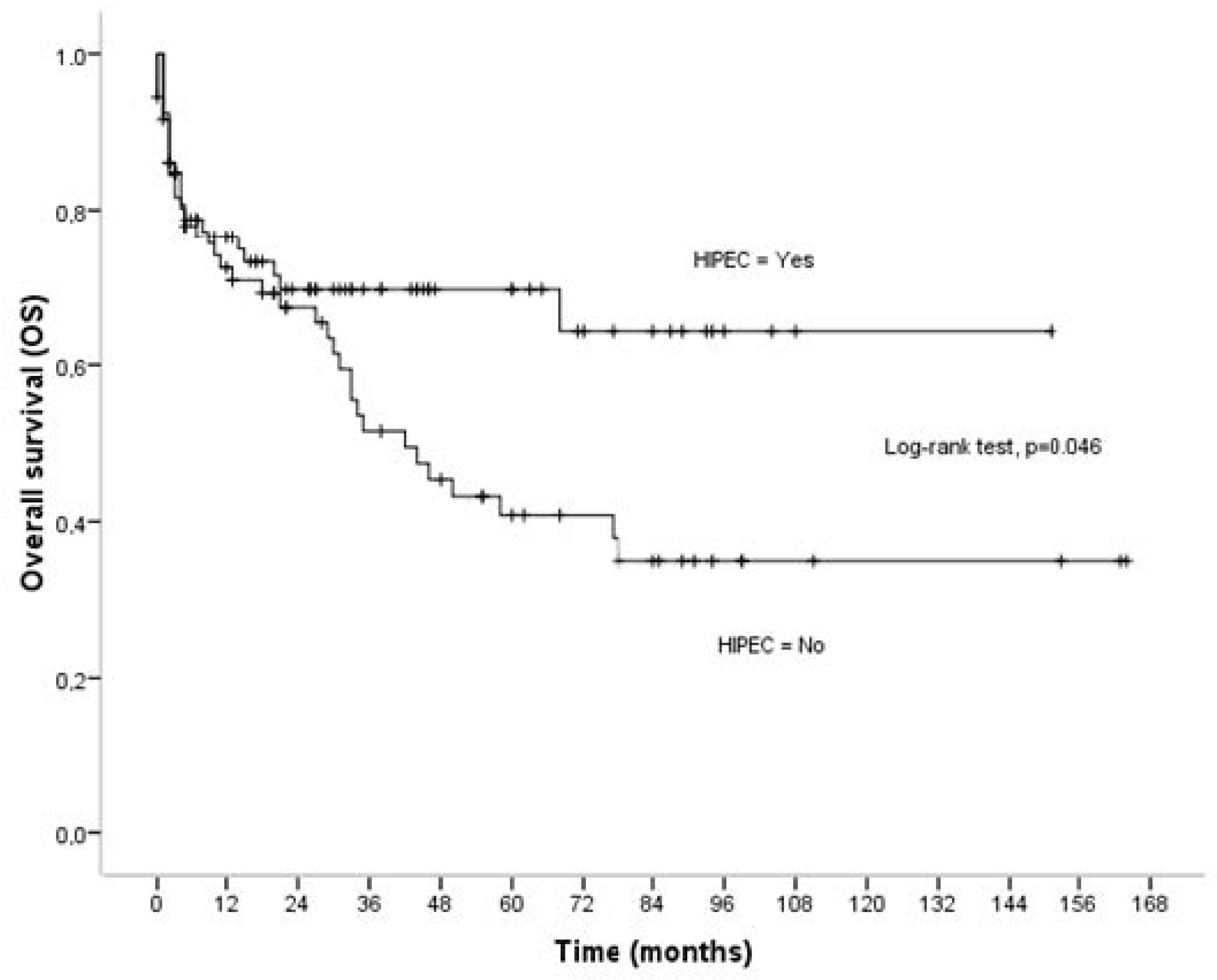
3.1. Overall Survival
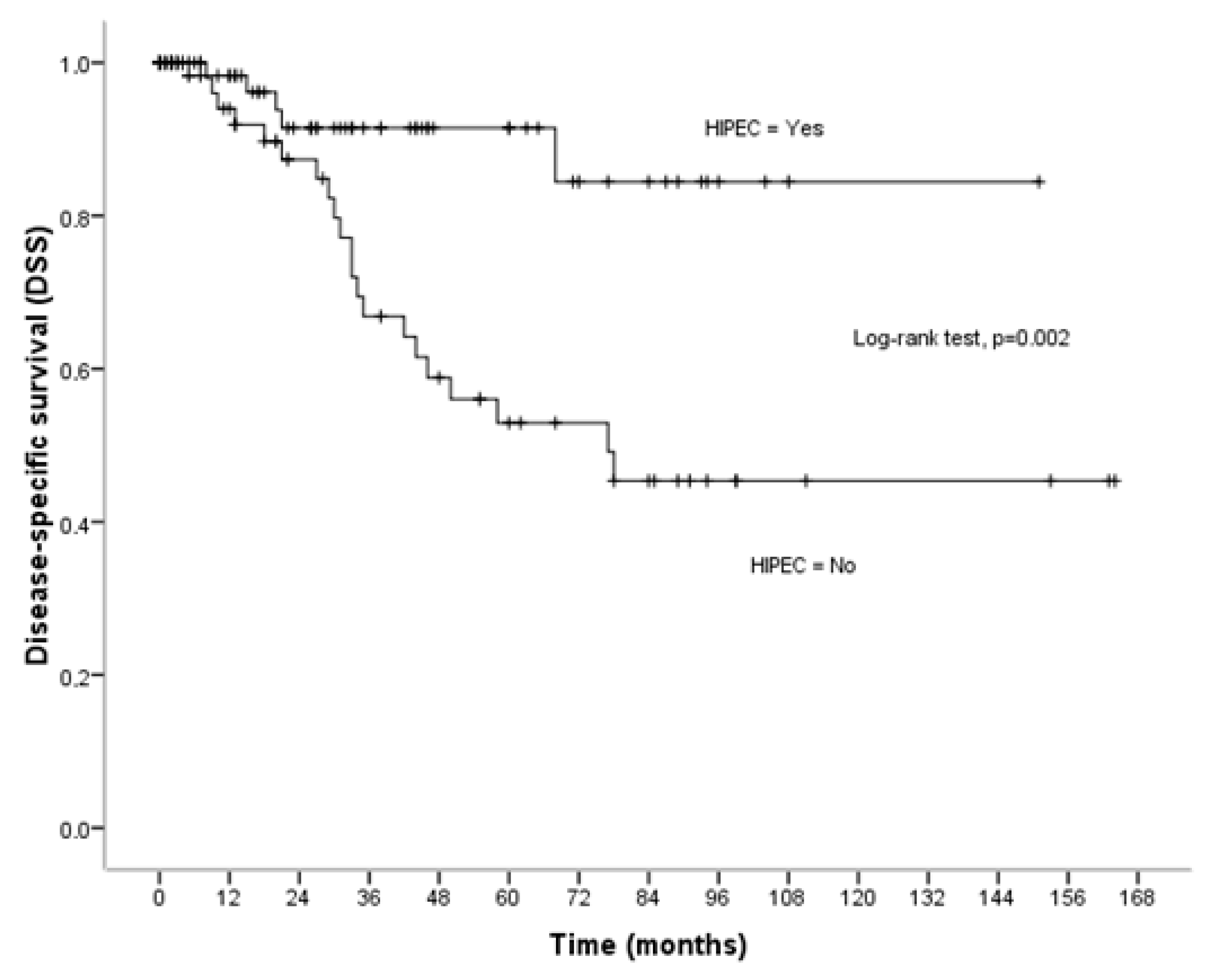
3.2. Disease-Specific Survival
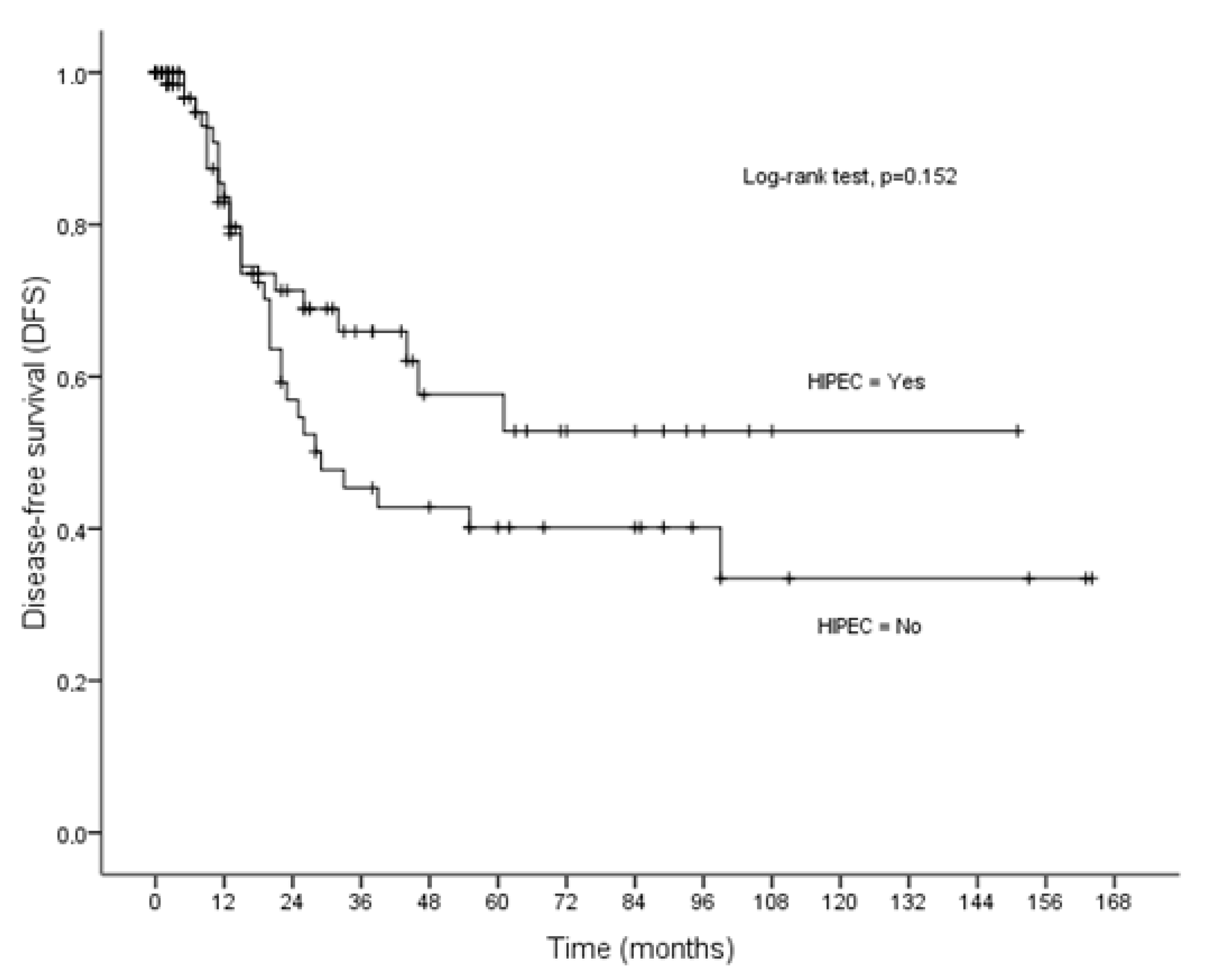
3.3. Disease-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Roviello, G.; Generali, D.; Marano, L.; Petrioli, R.; Marsili, S.; Caputo, E.; Marrelli, D.; Roviello, F. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for treatment of ovarian cancer. Int. J. Hyperthermia 2016, 32, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Ntatsis, K.; Papantoni, E.; Kyziridis, D.; Kalakonas, A.; Hristakis, C.; Tzavara, C.; Tentes, A.A. Ovarian cancer: 20-year experience with cytoreductive sirgery and perioperative intraperitoneal chemotherapy. JBUON 2021, 26, 1754–1761. [Google Scholar] [PubMed]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Le Saux, O.; Decullier, E.; Freyer, G.; Glehen, O.; Bakrin, N. Long-term survival in patients with epithelial ovarian cancer following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Int. J. Hyperthermia 2018, 35, 652–657. [Google Scholar] [CrossRef]
- Cheng, E.; Shamavonian, R.; Mui, J.; Hayler, R.; Karpes, J.; Wijayawardana, R.; Barat, S.; Ahmadi, N.; Morris, D. Overall survival and morbidity are not associated with advanced age for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A single centre experience. Pleura Peritoneum 2023, 8, 83–90. [Google Scholar] [CrossRef]
- Koole, S.N.; van Stein, R.M.; Sikorska, K.; Barton, D.P.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.E.; et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer 2020, 30, 888–892. [Google Scholar] [CrossRef]
- Ghirardi, V.; Ronsini, C.; Trozzi, R. Hyperthermic intraperitoneal chemotherapy in interval debulking surgery for advanced epithelial ovarian cancer: A single-center real life experience. Cancer 2020, 126, 5256–5262. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.Y.; Cho, M.S. Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: A protocol-based pilot study. J. Gynecol. Oncol. 2019, 30, e3. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.W. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021, 6, 100149. [Google Scholar] [CrossRef] [PubMed]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rpcio, G.S.; Jeronimo, M.G.; Luis, A.R.J.; Anibal, N.D.; Francisco, B.V.; Jesus, G.R.A.; Pablo, R.R.; et al. Cytoreductive surgery with or without HIPEC after neoadjuvant chemotherapy in ovarian cancer: A phase 3 clinical trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sirorska, K.; Van Leewen, J.H.; Schreuder, H.; Hermans, R.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Charo, L.M.; Jou, J.; Binder, P. Current status of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer in the United States. Gynecol. Oncol. 2020, 159, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- O’toole, D.M.; Golden, A.M. Evaluating cancer patients for rehabilitation potential. West. J. Med. 1991, 155, 384–387. [Google Scholar] [PubMed]
- Lei, Z.; Wang, Y.; Wang, J.; Wang, K.; Tian, J.; Zhao, Y.; Chen, L.; Wang, J.; Luo, J.; Jua, M.; et al. Evaluation of cytoreducctive surgery with or without hyperthermic intraperitonea; chemotherapy for stage III epithelial ovarian cancer. JAMA Netw. Open 2020, 3, e2013940. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 79–100. [Google Scholar]
- Dedrick, R.L. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin. Oncol. 1985, 12 (Suppl. S4), 1–6. [Google Scholar]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO 2022, 7, 100586. [Google Scholar] [CrossRef]
- Gonzalez Bayon, L.; Steiner, M.A.; Vasquez-Jimenez, W.; Asencio, J.M.; Alvarez de Sierra, P.; Atahualpa-Arenas, F.; Rodriguez del Campo, J.; Garcia Sabrido, J.L. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of advanced epithelial ovarian carcinoma: Upfront therapy, at first recurrence, or later? EJSO 2013, 39, 1109–1115. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.Y.; Seo, S.S.; Kang, S.; Yun, J.Y.; et al. Survival after Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.B.; Ma, R.; Ji, Z.H.; Li, Y. Long term survival of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in advanced epithelial ovarian cancer. Transl. Cancer Res. 2021, 10, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Casares, F.C.; Rufian, J.; Rubio, M.J.; Diaz, C.J.; Diaz, R.; Casado, A.; Arjona, A.; Munoz-Villanueva, M.C.; Muntane, J. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin. Transl. Oncol. 2009, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; De Iaco, P.; De Simone, M.; Garofalo, A.; Scambia, G.; Pinna, A.D.; Verdecchia, G.M.; Ansaloni, L.; Macri, A.; Cappellini, P.; et al. Cytoreduction (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: Retrospective Italian multicenter observational study of 511 cases. Ann. Surg. Oncol. 2017, 24, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Richards, A.; Liauw, W.; Morris, D.L. Hypethermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. EJSO 2015, 41, 1578–1589. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.H.; Lee, S.; Cho, H.; van Driel, W.J.; Sonke, G.S.; Bristow, R.E.; Park, S.Y.; Fotopoulou, C.; Lim, M.C. Hyperthermic intraperitoneal chemotherapy for epithelial ovarian cancer: A meta-analysis. Gynecol. Oncol. 2022, 167, 547–556. [Google Scholar] [CrossRef]
- Praiss, A.M.; Zhou, Q.; Iasonos, A.; Moukarzel, L.; Dessources, K.; Soldan, K.; Su, K.; Sonoda, Y.; Roche, K.L.; Gardner, G.J.; et al. Morbidity after secondary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for ovarian cancer: An analysis of a randomized phase II trial. Gynecol. Oncol. 2023, 171, 23–30. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Baghestani, A.R.; Vahedi, M. How to control confounding effects by statistical analysis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 79–83. [Google Scholar]
- Colombo, N.; Gadducci, A.; Landoni, F.; Lorusso, D.; Sabbatini, R.; Artioli, G.; Berardi, R.; Ceccherini, R.; Cecere, S.C.; Cormio, G.; et al. Consensus statements and treatment algorithm to guide clinicians in the selection of maintenance therapy for patients with newly diagnosed, advanced ovarian carcinoma: Results of a Delphi study. Gynecol. Oncol. 2023, 175, 182–189. [Google Scholar] [CrossRef]
| Total | CRS + HIPEC | CRS | p Value | |
|---|---|---|---|---|
| Number of patients | 151 | 79 | 72 | |
| Age | 0.033 | |||
| <65 | 89 (58.9) | 53 (67.1) | 36 (50.0) | |
| ≥65 | 62 (41.1) | 26 (32.9) | 36 (50.0) | |
| Performance status | 0.021 | |||
| 90–100% | 108 (71.5) | 64 (81.0) | 44 (61.1) | |
| 70–80% | 39 (25.8) | 13 (16.5) | 26 (36.1) | |
| 50–60% | 4 (2.6) | 2 (2.5) | 2 (2.8) | |
| ASA class | 0.067 | |||
| I | 104 (68.9) | 61 (77.2) | 43 (59.7) | |
| II | 44 (29.1) | 17 (21.5) | 27 (37.5) | |
| III | 3 (2.0) | 1 (1.3) | 2 (2.8) | |
| Tumor volume | 0.489 | |||
| Small | 30 (19.9) | 14 (17.7) | 16 (22.2) | |
| Large | 121 (80.1) | 65 (82.3) | 56 (77.8) | |
| CC-score | 0.435 | |||
| CC-0 | 140 (92.7) | 72 (91.1) | 68 (94.4) | |
| CC-1 | 11 (7.3) | 7 (8.9) | 4 (5.6) | |
| Histopathology | 0.635 | |||
| Serous | 119 (78.8) | 61 (77.2) | 58 (80.6) | |
| Endometrioid | 22 (14.6) | 11 (13.9) | 11 (15.3) | |
| Mucinous | 4 (2.6) | 2 (2.5) | 2 (2.8) | |
| Border-line | 5 (3.3) | 4 (5.1) | 1 (1.4) | |
| Yolk-sac | 1 (0.7) | 1 (1.3) | 0 (0.0) | |
| Degree of differentiation | 0.087 | |||
| G1 | 29 (19.2) | 16 (20.3) | 13 (18.1) | |
| G2 | 13 (8.6) | 3 (3.8) | 10 (13.9) | |
| G3 | 109 (72.2) | 60 (75.9) | 49 (68.0) | |
| RLND | 31 (20.5) | 29 (36.7) | 2 (2.8) | <0.001 |
| Relapse | 49 (32.5) | 20 (25.3) | 29 (40.3) | 0.050 |
| PCI | 0.078 | |||
| 0–13 | 82 (63.9) | 36 (45.6) | 46 (63.9) | |
| 14–20 | 40 (26.5) | 25 (31.6) | 15 (20.8) | |
| 21–39 | 29 (19.2) | 18 (22.8) | 11 (15.3) | |
| Morbidity | 47 (31.1) | 34 (43.0) | 13 (18.1) | 0.001 |
| Overall Survival (OS) | Disease-Specific Survival (DSS) | Disease-Free Survival (DFS) | ||||
|---|---|---|---|---|---|---|
| Mean ± SE | p Value | Mean ± SE | p Value | Mean ± SE | p Value | |
| Age | <0.001 | 0.043 | 0.215 | |||
| <65 | 109.73 ± 8.80 | 126.71 ± 8.73 | 93.67 ± 9.37 | |||
| ≥65 | 41.16 ± 5.92 | 64.61 ± 7.08 | 50.04 ± 7.91 | |||
| Performance status | <0.001 | <0.001 | 0.003 | |||
| 90–100% | 111.35 ± 7.89 | 130.65 ± 7.68 | 93.99 ± 8.85 | |||
| <90% | 24.30 ± 4.72 | 42.87 ± 6.42 | 26.57 ± 4.81 | |||
| ASA class | <0.001 | 0.001 | 0.034 | |||
| I | 108.30 ± 8.10 | 129.22 ± 7.96 | 92.96 ± 9.00 | |||
| II–III | 31.89 ± 5.66 | 51.16 ± 7.16 | 34.96 ± 6.76 | |||
| Tumor volume | 0.013 | 0.226 | 0.049 | |||
| Small | 76.78 ± 6.90 | 82.19 ± 6.24 | 71.68 ± 7.73 | |||
| Large | 81.21 ± 7.79 | 110.99 ± 8.78 | 77.42 ± 8.70 | |||
| CCC-score | 0.092 | 0.181 | 0.928 | |||
| CC-0 | 92.81 ± 7.47 | 118.55 ± 7.97 | 85.29 ± 7.87 | |||
| CC-1 | 38.09 ± 11.94 | 59.40 ± 13.49 | 52.67 ± 16.47 | |||
| Histopathology | <0.001 | 0.005 | <0.001 | |||
| Serous | 74.07 ± 7.84 | 99.67 ± 9.22 | 67.23 ± 8.72 | |||
| Other | 104.13 ± 4.71 | 107.48 ± 3.46 | 98.53 ± 6.72 | |||
| Degree of differentiation | <0.001 | <0.001 | <0.001 | |||
| G1/G2 | 146.82 ± 7.70 | 158.17 ± 4.75 | 132.83 ± 11.01 | |||
| G3 | 68.42 ± 8.11 | 95.74 ± 9.88 | 61.87 ± 9.21 | |||
| Lymph node dissection | 0.349 | 0.157 | 0.428 | |||
| Conventional | 85.19 ± 7.87 | 110.25 ± 8.65 | 81.75 ± 9.01 | |||
| RLNR | 104.90 ± 12.83 | 136.72 ± 9.57 | 88.24 ± 16.84 | |||
| PCI | <0.001 | 0.071 | 0.002 | |||
| 0–13 | 113.58 ± 8.99 | 126.14 ± 8.85 | 97.56 ± 10.17 | |||
| 14–20 | 72.43 ± 13.79 | 103.92 ± 16.72 | 80.66 ± 16.66 | |||
| 21–39 | 26.06 ± 7.45 | 49.97 ± 11.79 | 25.28 ± 6.91 | |||
| Morbidity | <0.001 | 0.694 | 0.213 | |||
| No | 104.32 ± 8.34 | 114.47 ± 8.48 | 87.80 ± 9.04 | |||
| Yes | 55.92 ± 11.44 | 120.43 ± 16.01 | 61.98 ± 17.14 | |||
| HIPEC | 0.046 | 0.002 | 0.152 | |||
| No | 73.90 ± 9.37 | 95.16 ± 10.53 | 73.49 ± 10.56 | |||
| Yes | 102.51 ± 8.71 | 133.66 ± 7.70 | 91.29 ± 10.50 | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| cHR (95% CI) | p Value | aHR (95% CI) | p Value | |
| Age | ||||
| <65 | REF. | |||
| ≥65 | 2.67 (1.59–4.47) | <0.001 | – | |
| Performance status | ||||
| 90–100% | REF. | REF. | ||
| <90% | 4.22 (2.50–7.13) | <0.001 | 2.96 (1.70–5.17) | <0.001 |
| ASA class | ||||
| I | REF. | |||
| II–III | 3.07 (1.83–5.14) | <0.001 | – | |
| Tumor volume | ||||
| Small | REF. | |||
| Large | 2.99 (1.20–7.47) | 0.019 | – | |
| CC-score | ||||
| CC-0 | REF. | |||
| CC-1 | 1.93 (0.88–4.26) | 0.103 | – | |
| Histopathology | ||||
| Serous | REF. | REF. | ||
| Other | 0.10 (0.03–0.42) | 0.002 | 0.14 (0.03–0.59) | 0.007 |
| Degree of differentiation | ||||
| G1/G2 | REF. | |||
| G3 | 6.89 (2.49–19.03) | <0.001 | – | |
| Lymph node dissection | ||||
| Conventional | REF. | |||
| RLNR | 0.72 (0.35–1.46) | 0.357 | – | |
| PCI | ||||
| 0–13 | REF. | |||
| 14–20 | 2.33 (1.26–4.31) | 0.007 | – | |
| 21–39 | 4.50 (2.40–8.44) | <0.001 | – | |
| Morbidity | ||||
| No | REF. | REF. | ||
| Yes | 3.53 (2.10–5.93) | <0.001 | 6.87 (3.49–13.52) | <0.001 |
| HIPEC | ||||
| No | REF. | REF. | ||
| Yes | 0.60 (0.35–1.00) | 0.051 | 0.33 (0.17–0.63) | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| cHR (95% CI) | p Value | aHR (95% CI) | p Value | |
| Age | ||||
| <65 | REF. | |||
| ≥65 | 2.18 (1.01–4.74) | 0.048 | – | |
| Performance status | ||||
| 90–100% | REF. | |||
| <90% | 5.29 (2.37–11.82) | <0.001 | – | |
| ASA class | ||||
| I | REF. | |||
| II–III | 3.64 (1.66–7.99) | 0.001 | – | |
| Tumor volume | ||||
| Small | REF. | |||
| Large | 2.07 (0.62–6.91) | 0.236 | – | |
| CC-score | ||||
| CC-0 | REF. | |||
| CC-1 | 2.23 (0.67–7.47) | 0.194 | – | |
| Histopathology | ||||
| Serous | REF. | |||
| Other | 0.10 (0.02–0.74) | 0.024 | – | |
| Degree of differentiation | ||||
| G1/G2 | REF. | REF. | ||
| G3 | 15.05 (2.04–111.19) | 0.008 | 8.64 (2.03–36.73) | 0.003 |
| Lymph node dissection | ||||
| Conventional | REF. | |||
| RLNR | 0.37 (0.09–1.56) | 0.174 | – | |
| PCI | ||||
| 0-13 | REF. | |||
| 14-20 | 1.59 (0.64–3.95) | 0.315 | – | |
| 21-39 | 3.14 (1.12–8.81) | 0.030 | – | |
| Morbidity | ||||
| No | REF. | |||
| Yes | 0.79 (0.24–2.63) | 0.695 | – | |
| HIPEC | ||||
| No | REF. | REF. | ||
| Yes | 0.25 (0.09–0.65) | 0.005 | 0.25 (0.10–0.63) | 0.003 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| cHR (95% CI) | p Value | aHR (95% CI) | p Value | |
| Age | ||||
| <65 | REF. | |||
| ≥65 | 1.44 (0.80–2.57) | 0.222 | – | |
| Performance status | ||||
| 90–100% | REF. | |||
| <90% | 2.52 (1.32–4.81) | 0.005 | – | |
| ASA class | ||||
| I | REF. | |||
| II–III | 1.94 (1.03–3.63) | 0.040 | – | |
| Tumor volume | ||||
| Small | REF. | |||
| Large | 2.28 (0.97–5.37) | 0.059 | – | |
| CC-score | ||||
| CC-0 | REF. | |||
| CC-1 | 1.05 (0.33–3.40) | 0.929 | – | |
| Histopathology | ||||
| Serous | REF. | |||
| Other | 0.15 (0.05–0.49) | 0.002 | – | |
| Degree of differentiation | ||||
| G1/G2 | REF. | REF. | ||
| G3 | 4.58 (1.94–10.79) | 0.001 | 4.13 (1.69–10.13) | 0.002 |
| Lymph node dissection | ||||
| Conventional | REF. | |||
| RLNR | 0.74 (0.35–1.58) | 0.434 | – | |
| PCI | ||||
| 0–13 | REF. | REF. | ||
| 14–20 | 1.50 (0.75–2.97) | 0.250 | 1.17 (0.57–2.41) | 0.135 |
| 21–39 | 3.38 (1.65–6.91) | 0.001 | 2.32 (1.10–4.89) | <0.001 |
| Morbidity | ||||
| No | REF. | |||
| Yes | 1.55 (0.77–3.11) | 0.221 | – | |
| HIPEC | ||||
| No | REF. | REF. | ||
| Yes | 0.66 (0.38–1.17) | 0.152 | 0.54 (0.30–0.97) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanikas, M.; Kofina, K.; Kyziridis, D.; Trypsianis, G.; Kalakonas, A.; Tentes, A.-A. HIPEC as Up-Front Treatment in Locally Advanced Ovarian Cancer. Cancers 2024, 16, 3500. https://doi.org/10.3390/cancers16203500
Karanikas M, Kofina K, Kyziridis D, Trypsianis G, Kalakonas A, Tentes A-A. HIPEC as Up-Front Treatment in Locally Advanced Ovarian Cancer. Cancers. 2024; 16(20):3500. https://doi.org/10.3390/cancers16203500
Chicago/Turabian StyleKaranikas, Michail, Konstantinia Kofina, Dimitrios Kyziridis, Grigorios Trypsianis, Apostolos Kalakonas, and Antonios-Apostolos Tentes. 2024. "HIPEC as Up-Front Treatment in Locally Advanced Ovarian Cancer" Cancers 16, no. 20: 3500. https://doi.org/10.3390/cancers16203500
APA StyleKaranikas, M., Kofina, K., Kyziridis, D., Trypsianis, G., Kalakonas, A., & Tentes, A.-A. (2024). HIPEC as Up-Front Treatment in Locally Advanced Ovarian Cancer. Cancers, 16(20), 3500. https://doi.org/10.3390/cancers16203500





