Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. AML NPM1mut and Prognosis
3. AML NPM1mut Treatment Strategies
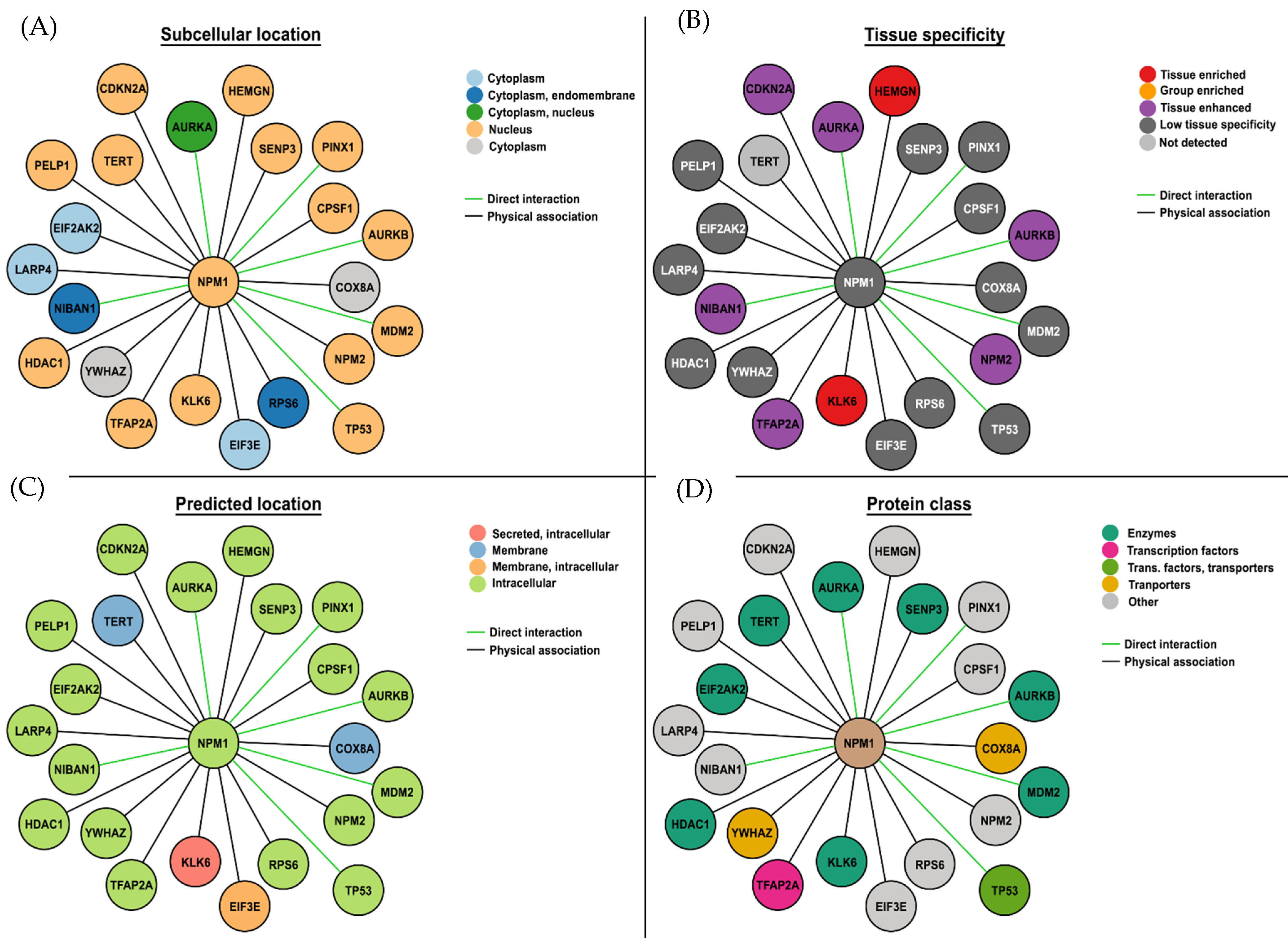
4. Immunogenic Mutation-Related Targets
5. Monoclonal Antibody Therapies
5.1. αCD33
5.2. αCD123
5.3. The Immune Checkpoint Inhibitors—Antibodies That Bind Programmed Cell Death-1 (αPD-1) Protein and Its Ligand (αPD-L1)
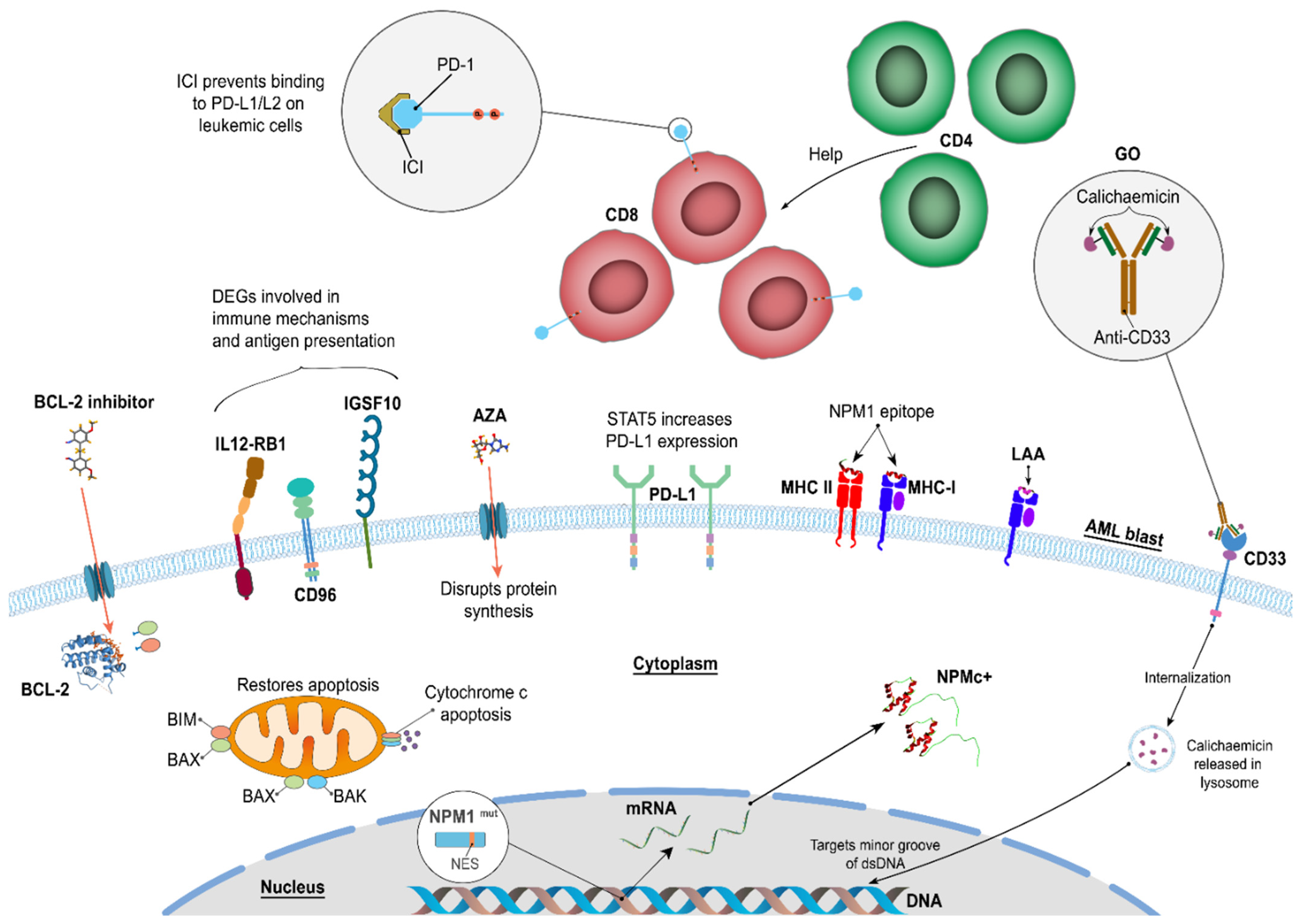
6. Venetoclax and Hypomethylating Agents
7. Discussion
8. Conclusions and Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| alloHSCT | Allogeneic hematopoietic stem cell transplant | MDS | Myelodysplastic syndrome |
| AML | Acute myeloid leukemia | MLL | Mixed lineage leukemia |
| APC | Antigen-presenting cell | MRD | Minimal residual disease |
| NADP | Nicotinamide adenine dinucleotide phosphate | ||
| AZA | Azacitidine | NPM1WT | Wild-type Nucleophosmin 1 |
| BCL-2 | B cell leukemia/lymphoma-2 | mut | Mutated |
| CEBPA | CCAAT/enhancer-binding protein-α | OS | Overall survival |
| CR | Complete remission | PD-1 | Programmed cell death-1 |
| CTL | Cytotoxic T-lymphocyte | ||
| DLI | Donor lymphocyte infusion | PD-L1 | Programmed cell death 1 ligand 1 |
| ELN | European LeukemiaNet | ||
| FAB | French American British | PDx | PD-1/PD-L1 axis |
| FDA | Food and Drug Administration | PFS | Progression-free survival |
| FLT3-ITD | Fms-related receptor tyrosine kinase 3-internal tandem duplication | PRAME | Preferentially expressed antigen in melanoma |
| GO | Gemtuzumab–ozogamicin | RHAMM | Receptor for hyaluronan-mediated motility |
| HMA | Hypomethylating agents | R/R | Relapsed/refractory |
| ICI | Immune checkpoint inhibitor | STAT5 | Signal transducer and activator of transcription |
| IDH | Isocitrate dehydrogenase | TET2 | tet methylcytosine dioxygenase 2 |
| LAA LDAC | Leukemia associated antigens Low-dose cytarabine | WHO NK | World Health Organization Natural killer |
| LPC/LSC | leukemic progenitor/stem cell |
References
- Grisendi, S.; Mecucci, C.; Falini, B.; Pandolfi, P.P. Nucleophosmin and cancer. Nat. Rev. Cancer 2006, 6, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Zarka, J.; Short, N.J.; Kanagal-Shamanna, R.; Issa, G.C. Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Nassiri, M. Acute Promyelocytic Leukemia: A Review and Discussion of Variant Translocations. Arch. Pathol. Lab. Med. 2015, 139, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.C.; Dube, I.D.; Valentine, M.B.; Mirro, J., Jr.; Watt, H.J.; Larson, R.A.; Bitter, M.A.; Le Beau, M.M.; Rowley, J.D. Clinicopathologic manifestations and breakpoints of the t(3;5) in patients with acute nonlymphocytic leukemia. Leukemia 1989, 3, 42–47. [Google Scholar] [PubMed]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Dumbar, T.S.; Gentry, G.A.; Olson, M.O. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry 1989, 28, 9495–9501. [Google Scholar] [CrossRef]
- Cordell, J.L.; Pulford, K.A.; Bigerna, B.; Roncador, G.; Banham, A.; Colombo, E.; Pelicci, P.G.; Mason, D.Y.; Falini, B. Detection of normal and chimeric nucleophosmin in human cells. Blood 1999, 93, 632–642. [Google Scholar] [CrossRef]
- Borer, R.A.; Lehner, C.F.; Eppenberger, H.M.; Nigg, E.A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989, 56, 379–390. [Google Scholar] [CrossRef]
- Falini, B.; Martelli, M.P.; Bolli, N.; Bonasso, R.; Ghia, E.; Pallotta, M.T.; Diverio, D.; Nicoletti, I.; Pacini, R.; Tabarrini, A.; et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood 2006, 108, 1999–2005. [Google Scholar] [CrossRef]
- Dillon, R.; Hills, R.; Freeman, S.; Potter, N.; Jovanovic, J.; Ivey, A.; Kanda, A.S.; Runglall, M.; Foot, N.; Valganon, M.; et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 2020, 135, 680–688. [Google Scholar] [CrossRef]
- Cela, I.; Di Matteo, A.; Federici, L. Nucleophosmin in Its Interaction with Ligands. Int. J. Mol. Sci. 2020, 21, 4885. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Baer, C.; Hutter, S.; Dicker, F.; Fuhrmann, I.; Meggendorfer, M.; Pohlkamp, C.; Kern, W.; Haferlach, T.; Haferlach, C.; et al. Risk assessment according to IPSS-M is superior to AML ELN risk classification in MDS/AML overlap patients defined by ICC. Leukemia 2023, 37, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Goudswaard, C.S.; van Putten, W.; Bijl, M.A.; Sanders, M.A.; Hugens, W.; Uitterlinden, A.G.; Erpelinck, C.A.; Delwel, R.; Lowenberg, B.; et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): Association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005, 106, 3747–3754. [Google Scholar] [CrossRef]
- Falini, B.; Martelli, M.P.; Bolli, N.; Sportoletti, P.; Liso, A.; Tiacci, E.; Haferlach, T. Acute myeloid leukemia with mutated nucleophosmin (NPM1): Is it a distinct entity? Blood 2011, 117, 1109–1120. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Borrow, J.; Dyer, S.A.; Akiki, S.; Griffiths, M.J. Molecular roulette: Nucleophosmin mutations in AML are orchestrated through N-nucleotide addition by TdT. Blood 2019, 134, 2291–2303. [Google Scholar] [CrossRef]
- Heath, E.M.; Chan, S.M.; Minden, M.D.; Murphy, T.; Shlush, L.I.; Schimmer, A.D. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef]
- Juliusson, G.; Jadersten, M.; Deneberg, S.; Lehmann, S.; Mollgard, L.; Wennstrom, L.; Antunovic, P.; Cammenga, J.; Lorenz, F.; Olander, E.; et al. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020, 4, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ostronoff, F.; Othus, M.; Lazenby, M.; Estey, E.; Appelbaum, F.R.; Evans, A.; Godwin, J.; Gilkes, A.; Kopecky, K.J.; Burnett, A.; et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council report. J. Clin. Oncol. 2015, 33, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Döhner, K.; Krauter, J.; Fröhling, S.; Corbacioglu, A.; Bullinger, L.; Habdank, M.; Späth, D.; Morgan, M.; Benner, A. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Angenendt, L.; Rollig, C.; Montesinos, P.; Ravandi, F.; Juliusson, G.; Recher, C.; Itzykson, R.; Racil, Z.; Wei, A.H.; Schliemann, C. Revisiting coexisting chromosomal abnormalities in NPM1-mutated AML in light of the revised ELN 2022 classification. Blood 2023, 141, 433–435. [Google Scholar] [CrossRef]
- Shiah, H.S.; Kuo, Y.Y.; Tang, J.L.; Huang, S.Y.; Yao, M.; Tsay, W.; Chen, Y.C.; Wang, C.H.; Shen, M.C.; Lin, D.T.; et al. Clinical and biological implications of partial tandem duplication of the MLL gene in acute myeloid leukemia without chromosomal abnormalities at 11q23. Leukemia 2002, 16, 196–202. [Google Scholar] [CrossRef]
- Dohner, K.; Tobis, K.; Ulrich, R.; Frohling, S.; Benner, A.; Schlenk, R.F.; Dohner, H. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: A study of the Acute Myeloid Leukemia Study Group Ulm. J. Clin. Oncol. 2002, 20, 3254–3261. [Google Scholar] [CrossRef]
- Barjesteh van Waalwijk van Doorn-Khosrovani, S.; Erpelinck, C.; van Putten, W.L.; Valk, P.J.; van der Poel-van de Luytgaarde, S.; Hack, R.; Slater, R.; Smit, E.M.; Beverloo, H.B.; Verhoef, G.; et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: A study of 319 de novo AML patients. Blood 2003, 101, 837–845. [Google Scholar] [CrossRef]
- Gregory, T.K.; Wald, D.; Chen, Y.; Vermaat, J.M.; Xiong, Y.; Tse, W. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J. Hematol. Oncol. 2009, 2, 23. [Google Scholar] [CrossRef]
- Angenendt, L.; Rollig, C.; Montesinos, P.; Martinez-Cuadron, D.; Barragan, E.; Garcia, R.; Botella, C.; Martinez, P.; Ravandi, F.; Kadia, T.; et al. Chromosomal Abnormalities and Prognosis in NPM1-Mutated Acute Myeloid Leukemia: A Pooled Analysis of Individual Patient Data From Nine International Cohorts. J. Clin. Oncol. 2019, 37, 2632–2642. [Google Scholar] [CrossRef]
- Dohner, K.; Paschka, P. Intermediate-risk acute myeloid leukemia therapy: Current and future. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 34–43. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.N.; Bill, M.; Rausch, C.; Metzeler, K.; Spiekermann, K.; Stasik, S.; Sauer, T.; Scholl, S.; Hochhaus, A.; Crysandt, M.; et al. Secondary-type mutations do not impact outcome in NPM1-mutated acute myeloid leukemia—Implications for the European LeukemiaNet risk classification. Leukemia 2023, 37, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Preudhomme, C.; Sagot, C.; Boissel, N.; Cayuela, J.M.; Tigaud, I.; de Botton, S.; Thomas, X.; Raffoux, E.; Lamandin, C.; Castaigne, S.; et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: A study from the Acute Leukemia French Association (ALFA). Blood 2002, 100, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh van Waalwijk van Doorn-Khosrovani, S.; Erpelinck, C.; Meijer, J.; van Oosterhoud, S.; van Putten, W.L.; Valk, P.J.; Berna Beverloo, H.; Tenen, D.G.; Lowenberg, B.; Delwel, R. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol. J. 2003, 4, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kapp-Schwoerer, S.; Weber, D.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Kronke, J.; Theis, F.; Rucker, F.G.; Teleanu, M.V.; Panina, E.; et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: Results from the AMLSG 09-09 trial. Blood 2020, 136, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Paschka, P.; Krzykalla, J.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Leis, C.; Fiedler, W.; Kindler, T.; Schroeder, T.; et al. Gemtuzumab Ozogamicin in NPM1-Mutated Acute Myeloid Leukemia: Early Results From the Prospective Randomized AMLSG 09-09 Phase III Study. J. Clin. Oncol. 2020, 38, 623–632. [Google Scholar] [CrossRef]
- Hofmann, S.; Götz, M.; Schneider, V.; Guillaume, P.; Bunjes, D.; Döhner, H.; Wiesneth, M.; Greiner, J. Donor lymphocyte infusion induces polyspecific CD8+ T-cell responses with concurrent molecular remission in acute myeloid leukemia with NPM1 mutation. J. Clin. Oncol. 2013, 31, e44–e47. [Google Scholar] [CrossRef]
- Jäger, P.; Rautenberg, C.; Kaivers, J.; Kasprzak, A.; Geyh, S.; Baermann, B.-N.; Haas, R.; Germing, U.; Schroeder, T.; Kobbe, G. Allogeneic hematopoietic stem cell transplantation and pre-transplant strategies in patients with NPM1-mutated acute myeloid leukemia: A single center experience. Sci. Rep. 2023, 13, 10774. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Forghieri, F.; Riva, G.; Lagreca, I.; Barozzi, P.; Bettelli, F.; Paolini, A.; Nasillo, V.; Lusenti, B.; Pioli, V.; Giusti, D.; et al. Neoantigen-Specific T-Cell Immune Responses: The Paradigm of NPM1-Mutated Acute Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 9159. [Google Scholar] [CrossRef]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Bene, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Dempke, W.C.M.; Desole, M.; Chiusolo, P.; Sica, S.; Schmidt-Hieber, M. Targeting the undruggable: Menin inhibitors ante portas. J. Cancer Res. Clin. Oncol. 2023, 149, 9451–9459. [Google Scholar] [CrossRef] [PubMed]
- Liso, A.; Colau, D.; Benmaamar, R.; De Groot, A.; Martin, W.; Benedetti, R.; Specchia, G.; Martelli, M.P.; Coulie, P.; Falini, B. Nucleophosmin leukaemic mutants contain C-terminus peptides that bind HLA class I molecules. Leukemia 2008, 22, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Ono, Y.; Hofmann, S.; Schmitt, A.; Mehring, E.; Götz, M.; Guillaume, P.; Döhner, K.; Mytilineos, J.; Döhner, H. Mutated regions of nucleophosmin 1 elicit both CD4+ and CD8+ T-cell responses in patients with acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2012, 120, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Schneider, V.; Schmitt, M.; Götz, M.; Döhner, K.; Wiesneth, M.; Döhner, H.; Hofmann, S. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut). Blood 2013, 122, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.; Zhang, L.; Bullinger, L.; Rojewski, M.; Hofmann, S.; Wiesneth, M.; Schrezenmeier, H.; Götz, M.; Botzenhardt, U.; Barth, T.F.E.; et al. Leukemic stem cells of acute myeloid leukemia patients carrying NPM1 mutation are candidates for targeted immunotherapy. Leukemia 2014, 28, 1759–1762. [Google Scholar] [CrossRef]
- Kuzelova, K.; Brodska, B.; Fuchs, O.; Dobrovolna, M.; Soukup, P.; Cetkovsky, P. Altered HLA Class I Profile Associated with Type A/D Nucleophosmin Mutation Points to Possible Anti-Nucleophosmin Immune Response in Acute Myeloid Leukemia. PLoS ONE 2015, 10, e0127637. [Google Scholar] [CrossRef]
- van der Lee, D.I.; Reijmers, R.M.; Honders, M.W.; Hagedoorn, R.S.; de Jong, R.C.; Kester, M.G.; van der Steen, D.M.; de Ru, A.H.; Kweekel, C.; Bijen, H.M.; et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Investig. 2019, 129, 774–785. [Google Scholar] [CrossRef]
- Narayan, R.; Olsson, N.; Wagar, L.E.; Medeiros, B.C.; Meyer, E.; Czerwinski, D.; Khodadoust, M.S.; Zhang, L.; Schultz, L.; Davis, M.M.; et al. Acute myeloid leukemia immunopeptidome reveals HLA presentation of mutated nucleophosmin. PLoS ONE 2019, 14, e0219547. [Google Scholar] [CrossRef]
- Morris, V.S.; Ghazi, H.; Fletcher, D.M.; Guinn, B.A. A Direct Comparison, and Prioritisation, of the Immunotherapeutic Targets Expressed by Adult and Paediatric Acute Myeloid Leukaemia Cells: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 9667. [Google Scholar] [CrossRef]
- Adams, S.P.; Sahota, S.S.; Mijovic, A.; Czepulkowski, B.; Padua, R.A.; Mufti, G.J.; Guinn, B.A. Frequent expression of HAGE in presentation chronic myeloid leukaemias. Leukemia 2002, 16, 2238–2242. [Google Scholar] [CrossRef] [PubMed]
- Guinn, B.A.; Bland, E.A.; Lodi, U.; Liggins, A.P.; Tobal, K.; Petters, S.; Wells, J.W.; Banham, A.H.; Mufti, G.J. Humoral detection of leukaemia-associated antigens in presentation acute myeloid leukaemia. Biochem. Biophys. Res. Commun. 2005, 335, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.E.; Bonney, S.A.; Lee, C.; Publicover, A.; Khan, G.; Smits, E.L.; Sigurdardottir, D.; Arno, M.; Li, D.; Mills, K.I.; et al. Application of the pMHC Array to Characterise Tumour Antigen Specific T Cell Populations in Leukaemia Patients at Disease Diagnosis. PLoS ONE 2015, 10, e0140483. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, N.; Buchan, S.; Ingram, W.; Khan, G.; Vittes, G.; Rice, J.; Pulford, K.; Mufti, G.; Stevenson, F.; Guinn, B.A. An analogue peptide from the Cancer/Testis antigen PASD1 induces CD8+ T cell responses against naturally processed peptide. Cancer Immun. 2013, 13, 16. [Google Scholar] [PubMed]
- Almshayakhchi, R.; Nagarajan, D.; Vadakekolathu, J.; Guinn, B.A.; Reeder, S.; Brentville, V.; Metheringham, R.; Pockley, A.G.; Durrant, L.; McArdle, S. A Novel HAGE/WT1-ImmunoBody((R)) Vaccine Combination Enhances Anti-Tumour Responses When Compared to Either Vaccine Alone. Front. Oncol. 2021, 11, 636977. [Google Scholar] [CrossRef]
- Schmitt, M.; Schmitt, A.; Rojewski, M.T.; Chen, J.; Giannopoulos, K.; Fei, F.; Yu, Y.; Gotz, M.; Heyduk, M.; Ritter, G.; et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 2008, 111, 1357–1365. [Google Scholar] [CrossRef]
- Qazilbash, M.H.; Wieder, E.; Thall, P.F.; Wang, X.; Rios, R.; Lu, S.; Kanodia, S.; Ruisaard, K.E.; Giralt, S.A.; Estey, E.H.; et al. PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia 2017, 31, 697–704. [Google Scholar] [CrossRef]
- Brayer, J.; Lancet, J.E.; Powers, J.; List, A.; Balducci, L.; Komrokji, R.; Pinilla-Ibarz, J. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am. J. Hematol. 2015, 90, 602–607. [Google Scholar] [CrossRef]
- Greiner, J.; Goetz, M.; Schuler, P.J.; Bulach, C.; Hofmann, S.; Schrezenmeier, H.; Dhner, H.; Schneider, V.; Guinn, B.A. Enhanced stimulation of antigen-specific immune responses against nucleophosmin 1 mutated acute myeloid leukaemia by an anti-programmed death 1 antibody. Br. J. Haematol. 2022, 198, 866–874. [Google Scholar] [CrossRef]
- Ehninger, A.; Kramer, M.; Röllig, C.; Thiede, C.; Bornhäuser, M.; Von Bonin, M.; Wermke, M.; Feldmann, A.; Bachmann, M.; Ehninger, G. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014, 4, e218. [Google Scholar] [CrossRef]
- Caron, P.C.; Dumont, L.; Scheinberg, D.A. Supersaturating infusional humanized anti-CD33 monoclonal antibody HuM195 in myelogenous leukemia. Clin. Cancer Res. 1998, 4, 1421–1428. [Google Scholar] [PubMed]
- Raza, A.; Jurcic, J.G.; Roboz, G.J.; Maris, M.; Stephenson, J.J.; Wood, B.L.; Feldman, E.J.; Galili, N.; Grove, L.E.; Drachman, J.G.; et al. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: A phase 1 trial. Leuk. Lymphoma 2009, 50, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.; Kalaycio, M.; Weiner, G.; Frankel, S.; Schulman, P.; Schwartzberg, L.; Jurcic, J.; Velez-Garcia, E.; Seiter, K.; Scheinberg, D.; et al. Treatment of relapsed or refractory acute myeloid leukemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia 2003, 17, 314–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laszlo, G.S.; Estey, E.H.; Walter, R.B. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014, 28, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.I. How close are we to CAR T-cell therapy for AML? Best Pract. Res. Clin. Haematol. 2019, 32, 101104. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terre, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Dohner, H.; Weber, D.; Krzykalla, J.; Fiedler, W.; Kuhn, M.W.M.; Schroeder, T.; Mayer, K.; Lubbert, M.; Wattad, M.; Gotze, K.; et al. Intensive chemotherapy with or without gemtuzumab ozogamicin in patients with NPM1-mutated acute myeloid leukaemia (AMLSG 09-09): A randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2023, 10, e495–e509. [Google Scholar] [CrossRef]
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin. Biol. Ther. 2014, 14, 1185–1195. [Google Scholar] [CrossRef]
- O’Hear, C.; Inaba, H.; Pounds, S.; Shi, L.; Dahl, G.; Bowman, W.P.; Taub, J.W.; Pui, C.H.; Ribeiro, R.C.; Coustan-Smith, E. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 2013, 119, 4036–4043. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Perriello, V.M.; Gionfriddo, I.; Rossi, R.; Milano, F.; Mezzasoma, F.; Marra, A.; Spinelli, O.; Rambaldi, A.; Annibali, O.; Avvisati, G.; et al. CD123 Is Consistently Expressed on NPM1-Mutated AML Cells. Cancers 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- He, S.Z.; Busfield, S.; Ritchie, D.S.; Hertzberg, M.S.; Durrant, S.; Lewis, I.D.; Marlton, P.; McLachlan, A.J.; Kerridge, I.; Bradstock, K.F. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk. Lymphoma 2015, 56, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Montesinos, P.; Aribi, A.; Marconi, G.; Altman, J.K.; Wang, E.S.; Roboz, G.J.; Burke, P.W.; Gaidano, G.; Walter, R.B.; et al. Broad Activity for the Pivekimab Sunirine (PVEK, IMGN632), Azacitidine, and Venetoclax Triplet in High-Risk Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML). Blood 2022, 140, 145–149. [Google Scholar] [CrossRef]
- Kaur, M.; Drake, A.C.; Hu, G.; Rudnick, S.; Chen, Q.; Phennicie, R.; Attar, R.; Nemeth, J.; Gaudet, F.; Chen, J. Induction and therapeutic targeting of human NPM1c+ myeloid leukemia in the presence of autologous immune system in mice. J. Immunol. 2019, 202, 1885–1894. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhang, F.; Liu, P. A perspective of immunotherapy for acute myeloid leukemia: Current advances and challenges. Front. Pharmacol. 2023, 14, 1151032. [Google Scholar] [CrossRef]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, Y.; Luo, J.; Huang, Z.; Yu, J.; Meng, X. Looking for the Optimal PD-1/PD-L1 Inhibitor in Cancer Treatment: A Comparison in Basic Structure, Function, and Clinical Practice. Front. Immunol. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Greiner, J.; Hofmann, S.; Schmitt, M.; Gotz, M.; Wiesneth, M.; Schrezenmeier, H.; Bunjes, D.; Dohner, H.; Bullinger, L. Acute myeloid leukemia with mutated nucleophosmin 1: An immunogenic acute myeloid leukemia subtype and potential candidate for immune checkpoint inhibition. Haematologica 2017, 102, e499–e501. [Google Scholar] [CrossRef]
- Guinn, B.A.; Schuler, P.J.; Schrezenmeier, H.; Hofmann, S.; Weiss, J.; Bulach, C.; Gotz, M.; Greiner, J. A Combination of the Immunotherapeutic Drug Anti-Programmed Death 1 with Lenalidomide Enhances Specific T Cell Immune Responses against Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 9285. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Uy, G.L.; Trinkaus, K.; Nelson, A.D.; Demland, J.; Abboud, C.N.; Cashen, A.F.; Stockerl-Goldstein, K.E.; Westervelt, P.; DiPersio, J.F.; et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood 2011, 117, 1828–1833. [Google Scholar] [CrossRef]
- Huang, Z.W.; Zhang, X.N.; Zhang, L.; Liu, L.L.; Zhang, J.W.; Sun, Y.X.; Xu, J.Q.; Liu, Q.; Long, Z.J. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct. Target. Ther. 2023, 8, 391. [Google Scholar] [CrossRef]
- Greiner, J.; Schuler, P.J.; Schrezenmeier, H.; Weiss, J.; Bulach, C.; Goetz, M.; Guinn, B. Combinations of Different Immunotherapeutics Enhance Specific T Cell Immune Responses Against Leukemic Cells, as well as Leukemic Progenitor and Stem Cells in Acute Myeloid Leukemia In Preparation.
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wong, M.P.M.; Ng, R.K. Aberrant DNA Methylation in Acute Myeloid Leukemia and Its Clinical Implications. Int. J. Mol. Sci. 2019, 20, 4576. [Google Scholar] [CrossRef]
- Chin, L.; Wong, C.Y.G.; Gill, H. Targeting and Monitoring Acute Myeloid Leukaemia with Nucleophosmin-1 (NPM1) Mutation. Int. J. Mol. Sci. 2023, 24, 3161. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, D.Y.; et al. A Phase Ib/II Study of Ivosidenib with Venetoclax +/- Azacitidine in IDH1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Wong, K.K.; Hassan, R.; Yaacob, N.S. Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Front. Oncol. 2021, 11, 624742. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ravandi, F.; Wei, A.H.; Dombret, H.; Thol, F.; Voso, M.T.; Schuh, A.C.; Porkka, K.; La Torre, I.; Skikne, B.; et al. Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status. Blood 2022, 139, 2145–2155. [Google Scholar] [CrossRef]
- Wu, H.-C.; Rérolle, D.; Berthier, C.; Hleihel, R.; Sakamoto, T.; Quentin, S.; Benhenda, S.; Morganti, C.; Wu, C.; Conte, L. Actinomycin D targets NPM1c-primed mitochondria to restore PML-driven senescence in AML therapy. Cancer Discov. 2021, 11, 3198–3213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, F.; Yang, L.; Xian, J.; Zou, Q.; Jin, H.; Wang, L.; Zhang, L. Nucleophosmin mutations induce chemosensitivity in THP-1 leukemia cells by suppressing NF-κB activity and regulating Bax/Bcl-2 expression. J. Cancer 2016, 7, 2270. [Google Scholar] [CrossRef][Green Version]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Reville, P.K.; Kantarjian, H.; Jabbour, E.; Borthakur, G.; Daver, N.; Issa, G.; Furudate, K.; Tanaka, T.; Pierce, S. Contemporary outcomes in IDH-mutated acute myeloid leukemia: The impact of co-occurring NPM1 mutations and venetoclax-based treatment. Am. J. Hematol. 2022, 97, 1443–1452. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Ravandi, F.; Liu-Dumlao, T.; Brandt, M.; Faderl, S.; Pierce, S.; Borthakur, G.; Garcia-Manero, G.; Cortes, J.; Kantarjian, H. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2012, 120, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with> 30% blasts. Blood J. Am. Soc. Hematol. 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Veselý, J. Mode of action and effects of 5-azacytidine and of its derivatives in eukaryotic cells. Pharmacol. Ther. 1985, 28, 227–235. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.-Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood J. Am. Soc. Hematol. 2020, 135, 2137–2145. [Google Scholar] [CrossRef]
- Dick, J.E.; Bhatia, M.; Gan, O.; Kapp, U.; Wang, J.C. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells 1997, 15 (Suppl. S1), 199–203. [Google Scholar] [CrossRef]
- Becker, H.; Marcucci, G.; Maharry, K.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Whitman, S.P.; Wu, Y.Z.; Schwind, S.; Paschka, P.; et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 596–604. [Google Scholar] [CrossRef]
- Ranieri, R.; Pianigiani, G.; Sciabolacci, S.; Perriello, V.M.; Marra, A.; Cardinali, V.; Pierangeli, S.; Milano, F.; Gionfriddo, I.; Brunetti, L.; et al. Current status and future perspectives in targeted therapy of NPM1-mutated AML. Leukemia 2022, 36, 2351–2367. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Brodska, B.; Otevrelova, P.; Salek, C.; Fuchs, O.; Gasova, Z.; Kuzelova, K. High PD-L1 Expression Predicts for Worse Outcome of Leukemia Patients with Concomitant NPM1 and FLT3 Mutations. Int. J. Mol. Sci. 2019, 20, 2823. [Google Scholar] [CrossRef] [PubMed]
- Orskov, A.D.; Treppendahl, M.B.; Skovbo, A.; Holm, M.S.; Friis, L.S.; Hokland, M.; Gronbaek, K. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 2015, 6, 9612–9626. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Weinzierl, A.O.; Flad, T.; Guinn, B.A.; Mueller, L.; Mufti, G.J.; Stevanovic, S.; Pawelec, G. A novel MHC-associated proteinase 3 peptide isolated from primary chronic myeloid leukaemia cells further supports the significance of this antigen for the immunotherapy of myeloid leukaemias. Leukemia 2006, 20, 1067–1072. [Google Scholar] [CrossRef]
- Tettamanti, S.; Pievani, A.; Biondi, A.; Dotti, G.; Serafini, M. Catch me if you can: How AML and its niche escape immunotherapy. Leukemia 2022, 36, 13–22. [Google Scholar] [CrossRef]
- Nelde, A.; Schuster, H.; Heitmann, J.S.; Bauer, J.; Maringer, Y.; Zwick, M.; Volkmer, J.P.; Chen, J.Y.; Stanger, A.M.P.; Lehmann, A.; et al. Immune Surveillance of Acute Myeloid Leukemia Is Mediated by HLA-Presented Antigens on Leukemia Progenitor Cells. Blood Cancer Discov. 2023, 4, 468–489. [Google Scholar] [CrossRef]
| Associated Characteristics | AML NPM1mut | AML NPM1WT |
|---|---|---|
| Key information | Gatekeeper mutation, association with a specific subgroup of AML patients, has its own WHO subgroup | |
| Marker stability | NPM1mut is a stable marker Observed again at relapse | |
| Age/sex | Associated with older AML patients > 35 years of age, de novo AML, and an increased frequency in females | More common in patients < 35 years of age. |
| Response to treatment | Good response to induction therapy | |
| Prognosis | Better prognosis in older but not younger AML patients; MRD status affects prognosis; NPM1mut/FLT3-ITD− patients have a better prognosis than NPM1mut FLT3-ITD+ patients | |
| Clinical features | Presents with high blast percentages, elevated white cell and platelet counts, and a high frequency of NK cells | |
| Karyotype | Normal karyotype | t(8;21), inv(16), t(15;17) |
| FAB subtype | FAB M1-M6; more often M4 and M5 | FAB M0 |
| Diseased cell phenotype | Restricted to myeloid cells Diseased cells are CD33+ and for > 90% of AML patients are CD34− LSCs co-express CD96, IL12RB1 | LSCs are CD34+CD38− |
| Mutations | FLT3-ITD (2 × more common); co-mutations include with DNMT3A > FLT3-ITD > tet methylcytosine dioxygenase 2 (TET2). | Biallelic CEPBA mutations occur |
| Associated gene expression | Upregulated HOX genes (A4, A5, A6, A7, A9, A10, B2, B3, B5, B6) and upregulated HOX-related genes (PBX3 and MEIS1). |
| Risk | Molecular and Cytogenetic Indicators in AML NPM1mut Patients |
|---|---|
| Poor | Partial tandem duplication of the mixed lineage leukemia (MLL) gene [25,26] and increased expression of the transcription factor ecotropic virus integration site 1 (EVI1) [27]; DNMT3A, MN1, BAALC, EGR-1, AF1q [28]; adverse cytogenetic abnormalities [29] |
| Intermediate | Isocitrate dehydrogenase 1 (nicotinamide adenine dinucleotide phosphate (NADP+)), soluble (IDH1), isocitrate dehydrogenase 2 (NADP+), mitochondrial (IDH2), and TET2 [30]; t(9;11)(p21.3;q23.3); MLLT3-KMT2A [31]; FLT3-ITD [12]; dependent on MRD status [12] |
| Favorable | NPM1mut without any other genetic abnormality [12] or with a secondary type of mutation [32]; mutations in the transcription factor CEBPA indicates response to therapy [33,34]; concurrence with t(8;21), inv(16)/t(16;16), t(8;21) [12]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, J.; Mohamed, E.; Fletcher, D.M.; Schuler, P.J.; Schrezenmeier, H.; Götz, M.; Guinn, B.-a. Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia. Cancers 2024, 16, 3443. https://doi.org/10.3390/cancers16203443
Greiner J, Mohamed E, Fletcher DM, Schuler PJ, Schrezenmeier H, Götz M, Guinn B-a. Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia. Cancers. 2024; 16(20):3443. https://doi.org/10.3390/cancers16203443
Chicago/Turabian StyleGreiner, Jochen, Eithar Mohamed, Daniel M. Fletcher, Patrick J. Schuler, Hubert Schrezenmeier, Marlies Götz, and Barbara-ann Guinn. 2024. "Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia" Cancers 16, no. 20: 3443. https://doi.org/10.3390/cancers16203443
APA StyleGreiner, J., Mohamed, E., Fletcher, D. M., Schuler, P. J., Schrezenmeier, H., Götz, M., & Guinn, B.-a. (2024). Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia. Cancers, 16(20), 3443. https://doi.org/10.3390/cancers16203443








