Radiotherapy with 15 × 2.633 Gy vs. 20 × 2.0 Gy in Patients with Malignant Spinal Cord Compression and Favorable Survival Prognoses: A Secondary Analysis of the RAMSES-01 Trial
Abstract
Simple Summary
Abstract
1. Introduction
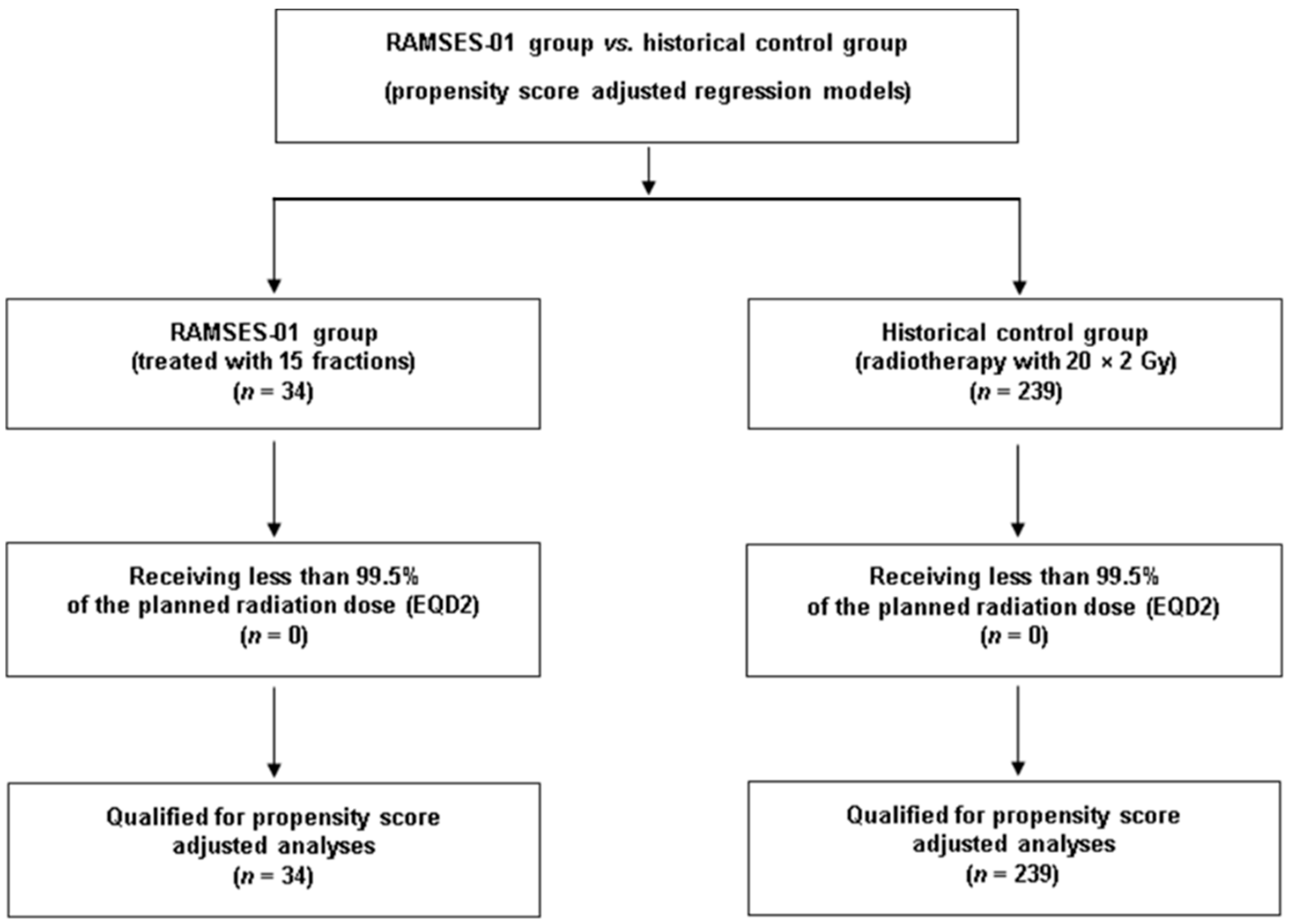
2. Materials and Methods
Statistical Considerations
3. Results
3.1. Outcomes in the RAMSES-01 Group
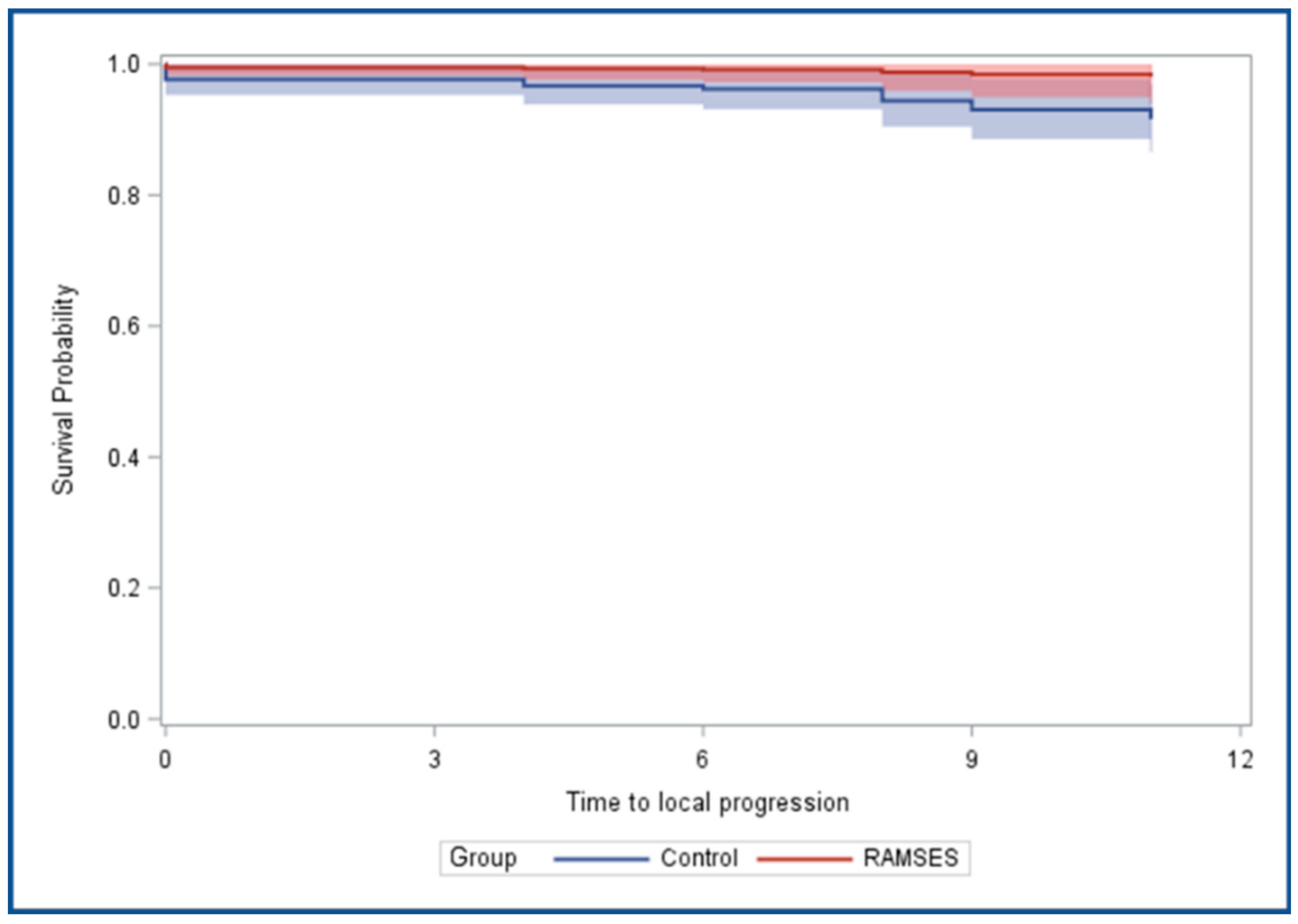
3.2. Comparison of the RAMSES-01 Group and the Historical Control Group
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, D.; Schiff, D. Malignant spinal cord compression. Lancet Oncol. 2005, 6, 15–24. [Google Scholar] [CrossRef]
- Rades, D.; Schild, S.E. Personalization of radiation therapy in the primary treatment of malignant epidural spinal cord compression (MESCC). Semin. Radiat. Oncol. 2023, 33, 148–158. [Google Scholar] [CrossRef]
- Patchell, R.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Rades, D.; Dunst, J.; Schild, S.E. The first score predicting overall survival in patients with metastatic spinal cord compression. Cancer 2008, 112, 157–161. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; McMenemin, R.; Dubois, D.; McKinna, F.; Foran, B.; Madhavan, K.; MacGregor, C.; et al. Effect of single-fraction vs multifraction radiotherapy on ambulatory status among patients with spinal canal compression from metastatic cancer: The SCORAD randomized clinical trial. JAMA 2019, 322, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Thirion, P.G.; Dunne, M.T.; Kelly, P.J.; Flavin, A.; O’Sullivan, J.M.; Hacking, D.; Sasiadek, W.; Small, C.; Pomeroy, M.M.; Martin, J.; et al. Non-inferiority randomised phase 3 trial comparing two radiation schedules (single vs. five fractions) in malignant spinal cord compression. Br. J. Cancer 2020, 122, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Panzner, A.; Rudat, V.; Karstens, J.H.; Schild, S.E. Dose escalation of radiotherapy for metastatic spinal cord compression (MSCC) in patients with relatively favorable survival prognosis. Strahlenther. Onkol. 2011, 187, 729–735. [Google Scholar] [CrossRef]
- Barendsen, G.W. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1981–1997. [Google Scholar] [CrossRef]
- Joiner, M.C.; Van der Kogel, A.J. The linear-quadratic approach to fractionation and calculation of isoeffect relationships. In Basic Clinical Radiobiology; Steel, G.G., Ed.; Oxford University Press: New York, NY, USA, 1997; pp. 106–112. [Google Scholar]
- Rades, D.; Hansen, O.; Jensen, L.H.; Dziggel, L.; Staackmann, C.; Doemer, C.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; Ciervide-Jurio, R.; et al. Radiotherapy for metastatic spinal cord compression with increased radiation doses (RAMSES-01): A prospective multicenter study. BMC Cancer 2019, 19, 1163. [Google Scholar] [CrossRef]
- Rades, D.; Lomidze, D.; Jankarashvili, N.; Lopez Campos, F.; Navarro-Martin, A.; Segedin, B.; Groselj, B.; Staackmann, C.; Kristiansen, C.; Dennis, K.; et al. Radiotherapy for metastatic epidural spinal cord compression with increased doses: Final results of the RAMSES-01 trial. Cancers 2024, 16, 1149. [Google Scholar] [CrossRef]
- National Institutes of Health/National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03; National Institutes of Health/National Cancer Institute: Bethesda, MD, USA, 2010.
- Chow, E.; Hoskin, P.; Mitera, G.; Zeng, L.; Lutz, S.; Roos, D.; Hahn, C.; van der Linden, Y.; Hartsell, W.; Kumar, E.; et al. Update of the international consenus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.C.; Andersen, B.; Breitbart, W.S.; Buchmann, L.O.; Compas, B.; Deshields, T.L.; Dudley, M.M.; Fleishman, S.; Fulcher, C.D.; Greenberg, D.B.; et al. National Comprehensive Cancer Network. Distress management clinical practice guidelines in oncology. J. Natl. Comp. Cancer Netw. 2013, 11, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Galicich, J.H.; Sundaresan, N. Radiation therapy for spinal epidural metastases with complete block. Acta Radiol. Oncol. 1983, 22, 135–143. [Google Scholar] [CrossRef]
- Franklin, J.M.; Eddings, W.; Austin, P.C.; Stuart, E.A.; Schneeweiss, S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat. Med. 2017, 36, 1946–1963. [Google Scholar] [PubMed]
- Giammalva, G.R.; Ferini, G.; Torregrossa, F.; Brunasso, L.; Musso, S.; Benigno, U.E.; Gerardi, R.M.; Bonosi, L.; Costanzo, R.; Paolini, F.; et al. The palliative care in the metastatic spinal tumors. A systematic review on the radiotherapy and surgical perspective. Life 2022, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Stalpers, L.J.A.; Veninga, T.; Schulte, R.; Hoskin, P.J.; Obralic, N.; Bajrovic, A.; Rudat, V.; Schwarz, R.; Hulshof, M.C.; et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression in a series of 1304 patients. J. Clin. Oncol. 2005, 23, 3366–3375. [Google Scholar] [CrossRef]
- Rades, D.; Lange, M.; Veninga, T.; Stalpers, L.J.; Bajrovic, A.; Adamietz, I.A.; Rudat, V.; Schild, S.E. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 524–530. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Cacciola, A.; Parisi, S.; Lillo, S.; Tamburella, C.; Santacaterina, A.; Ferini, G.; Cellini, F.; Draghini, L.; Trippa, F.; et al. An Italian survey on “palliative intent” radiotherapy. Rep. Pract. Oncol. Radiother. 2022, 27, 419–427. [Google Scholar] [CrossRef]
- Steenland, E.; Leer, J.W.; van Houwelingen, H.; Post, W.J.; van den Hout, W.B.; Kievit, J.; de Haes, H.; Martijn, H.; Oei, B.; Vonk, E.; et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999, 52, 101–109. [Google Scholar] [CrossRef]
- Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain (randomised comparison with a multifraction schedule over 12 months of patient follow-up). Radiother. Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Hartsell, W.E.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M., 3rd; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar] [CrossRef]
- Roos, D.E.; Turner, S.L.; O’Brien, P.C.; Smith, J.G.; Spry, N.A.; Burmeister, B.H.; Hoskin, P.J.; Ball, D.L.; Trans-Tasman Radiation Oncology Group. TROG 96.05. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother. Oncol. 2005, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bendfeldt, G.A.; Chanbour, H.; Chen, J.W.; Gangavarapu, L.S.; LaBarge, M.E.; Ahmed, M.; Jonzzon, S.; Roth, S.G.; Chotai, S.; Luo, L.Y.; et al. Does Low-Grade Versus High-Grade Bilsky Score Influence Local Recurrence and Overall Survival in Metastatic Spine Tumor Surgery? Neurosurgery 2023, 93, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Robin, A.M.; O’Toole, J.E.; Laufer, I. Minimally invasive surgery strategies: Changing the time of spine tumors. Neurosurg. Clin. N. Am. 2020, 31, 201–209. [Google Scholar]
- Laufer, I.; Iorgulescu, J.B.; Chapman, T.; Lis, E.; Shi, W.; Zhang, Z.; Cox, B.W.; Yamada, Y.; Bilsky, M.H. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J. Neurosurg. Spine 2013, 18, 207–214. [Google Scholar] [CrossRef]
- Turel, M.K.; Kerolus, M.G.; O’Toole, J.E. Minimally invasive “separation surgery” plus adjuvant stereotactic radiotherapy in the management of spinal epidural metastases. J. Craniovertebr. Junction Spine 2017, 8, 119–126. [Google Scholar] [CrossRef]
- Ito, K.; Sugita, S.; Nakajima, Y.; Furuya, T.; Hiroaki, O.; Hayakawa, S.; Hozumi, T.; Saito, M.; Karasawa, K. Phase 2 clinical trial of separation surgery followed by stereotactic body radiation therapy for metastatic epidural spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 106–113. [Google Scholar] [CrossRef]
- Moussazadeh, N.; Laufer, I.; Yamada, Y.; Bilsky, M.H. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Control 2014, 21, 168–174. [Google Scholar] [CrossRef]
- Guckenberger, M.; Mantel, F.; Gerszten, P.C.; Flickinger, J.C.; Sahgal, A.; Létourneau, D.; Grills, I.S.; Jawad, M.; Fahim, D.K.; Shin, J.H.; et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiat. Oncol. 2014, 9, 226. [Google Scholar] [CrossRef]
- Wong, H.C.Y.; Lee, S.F.; Chan, A.W.; Caini, S.; Hoskin, P.; Simone, C.B., 2nd; Johnstone, P.; van der Linden, Y.; van der Velden, J.M.; Martin, E.; et al. Stereotactic body radiation therapy versus conventional external beam radiotherapy for spinal metastases: A systematic review and meta-analysis of randomized controlled trials. Radiother. Oncol. 2023, 189, 109914. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic body radiation therapy for spinal metastases: Benefits and limitations. Semin. Radiat. Oncol. 2023, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Palmisciano, P.; Scalia, G.; Haider, A.S.; Bin-Alamer, O.; Sagoo, N.S.; Bozkurt, I.; Deora, H.; Priola, S.M.; Aoun, S.G.; et al. The role of radiation therapy in the treatment of spine metastases from hepatocellular carcinoma: A systematic review and meta-analysis. Neurosurg. Focus 2022, 53, E12. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Shiu, A.S.; Yang, J.; Wang, X.S.; Allen, P.; Brown, B.W.; Grossman, P.; Frija, E.K.; McAleer, M.F.; Azeem, S.; et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 2012, 118, 5069–5077. [Google Scholar] [CrossRef]
- Versteeg, A.L.; van der Velden, J.M.; Hes, J.; Eppinga, W.; Kasperts, N.; Verkooijen, H.M.; Oner, F.C.; Seravalli, E.; Verlaan, J.J. Stereotactic radiotherapy followed by surgical stabilization within 24 h for unstable spinal metastases; A stage I/II a study according to the IDEAL Framework. Front. Oncol. 2018, 8, 626. [Google Scholar] [CrossRef]


| Characteristic | RAMSES Group N Patients (%) | Control Group N Patients (%) |
|---|---|---|
| Age | ||
| ≤64 years | 15 (44.1) | 136 (56.9) |
| ≥65 years (elderly) | 19 (55.9) | 103 (43.1) |
| Gender | ||
| Female | 10 (29.4) | 120 (50.2) |
| Male | 24 (70.6) | 119 (49.8) |
| Interval diagnosis of malignancy to MSCC | ||
| ≤15 months | 14 (41.2) | 82 (34.3) |
| >15 months | 20 (58.8) | 158 (65.7) |
| Additional bone metastases | ||
| No | 8 (23.5) | 132 (55.2) |
| Yes | 26 (76.5) | 107 (44.8) |
| Hematogenous metastases outside bone | ||
| No | 29 (85.3) | 227 (95.0) |
| Yes | 5 (14.7) | 12 (5.0) |
| Type of malignancy | ||
| Breast cancer | 6 (17.6) | 80 (33.5) |
| Prostate cancer | 14 (41.2) | 42 (17.6) |
| Myeloma/lymphoma | 7 (20.6) | 70 (29.3) |
| Lung cancer | 4 (11.8) | 11 (4.6) |
| Less radiosensitive tumors | 2 (5.9) | 8 (3.3) |
| Other malignancies | 1 (2.9) | 28 (11.7) |
| Dynamic (time developing) of motor deficits | ||
| 8–14 days | 7 (20.6) | 59 (24.7) |
| >14 days | 27 (79.4) | 180 (75.3) |
| Pre-treatment walking ability | ||
| Not able to walk | 4 (11.8) | 22 (9.2) |
| Able to walk | 30 (88.2) | 217 (90.8) |
| Number of vertebrae associated with MSCC | ||
| 1 or 2 | 20 (58.8) | 122 (51.0) |
| 3 or more | 14 (41.2) | 117 (49.0) |
| ECOG performance score | ||
| 0–2 | 22 (64.7) | 197 (82.4) |
| 3–4 | 12 (35.3) | 42 (17.6) |
| Endpoint | Hazard Ratio Estimate | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Local progression-free survival | |||
| Propensity score adjusted | 0.211 | 0.014–3.254 | 0.265 |
| Overall survival | |||
| Propensity score adjusted | 1.195 | 0.477–2.994 | 0.704 |
| Endpoint | Odds Ratio Estimate | 95% Confidence Interval | p-Value |
| Improvement of motor function | |||
| Propensity score adjusted | 2.236 | 0.929–5.382 | 0.073 |
| Post-treatment walking ability | |||
| Propensity score adjusted | 0.800 | 0.114–5.591 | 0.822 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rades, D.; Lomidze, D.; Jankarashvili, N.; Lopez Campos, F.; Navarro-Martin, A.; Segedin, B.; Groselj, B.; Staackmann, C.; Yu, N.Y.; Cacicedo, J. Radiotherapy with 15 × 2.633 Gy vs. 20 × 2.0 Gy in Patients with Malignant Spinal Cord Compression and Favorable Survival Prognoses: A Secondary Analysis of the RAMSES-01 Trial. Cancers 2024, 16, 3436. https://doi.org/10.3390/cancers16203436
Rades D, Lomidze D, Jankarashvili N, Lopez Campos F, Navarro-Martin A, Segedin B, Groselj B, Staackmann C, Yu NY, Cacicedo J. Radiotherapy with 15 × 2.633 Gy vs. 20 × 2.0 Gy in Patients with Malignant Spinal Cord Compression and Favorable Survival Prognoses: A Secondary Analysis of the RAMSES-01 Trial. Cancers. 2024; 16(20):3436. https://doi.org/10.3390/cancers16203436
Chicago/Turabian StyleRades, Dirk, Darejan Lomidze, Natalia Jankarashvili, Fernando Lopez Campos, Arturo Navarro-Martin, Barbara Segedin, Blaz Groselj, Christian Staackmann, Nathan Y. Yu, and Jon Cacicedo. 2024. "Radiotherapy with 15 × 2.633 Gy vs. 20 × 2.0 Gy in Patients with Malignant Spinal Cord Compression and Favorable Survival Prognoses: A Secondary Analysis of the RAMSES-01 Trial" Cancers 16, no. 20: 3436. https://doi.org/10.3390/cancers16203436
APA StyleRades, D., Lomidze, D., Jankarashvili, N., Lopez Campos, F., Navarro-Martin, A., Segedin, B., Groselj, B., Staackmann, C., Yu, N. Y., & Cacicedo, J. (2024). Radiotherapy with 15 × 2.633 Gy vs. 20 × 2.0 Gy in Patients with Malignant Spinal Cord Compression and Favorable Survival Prognoses: A Secondary Analysis of the RAMSES-01 Trial. Cancers, 16(20), 3436. https://doi.org/10.3390/cancers16203436






