Disparities in Testicular Cancer: A Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Biological Etiologies
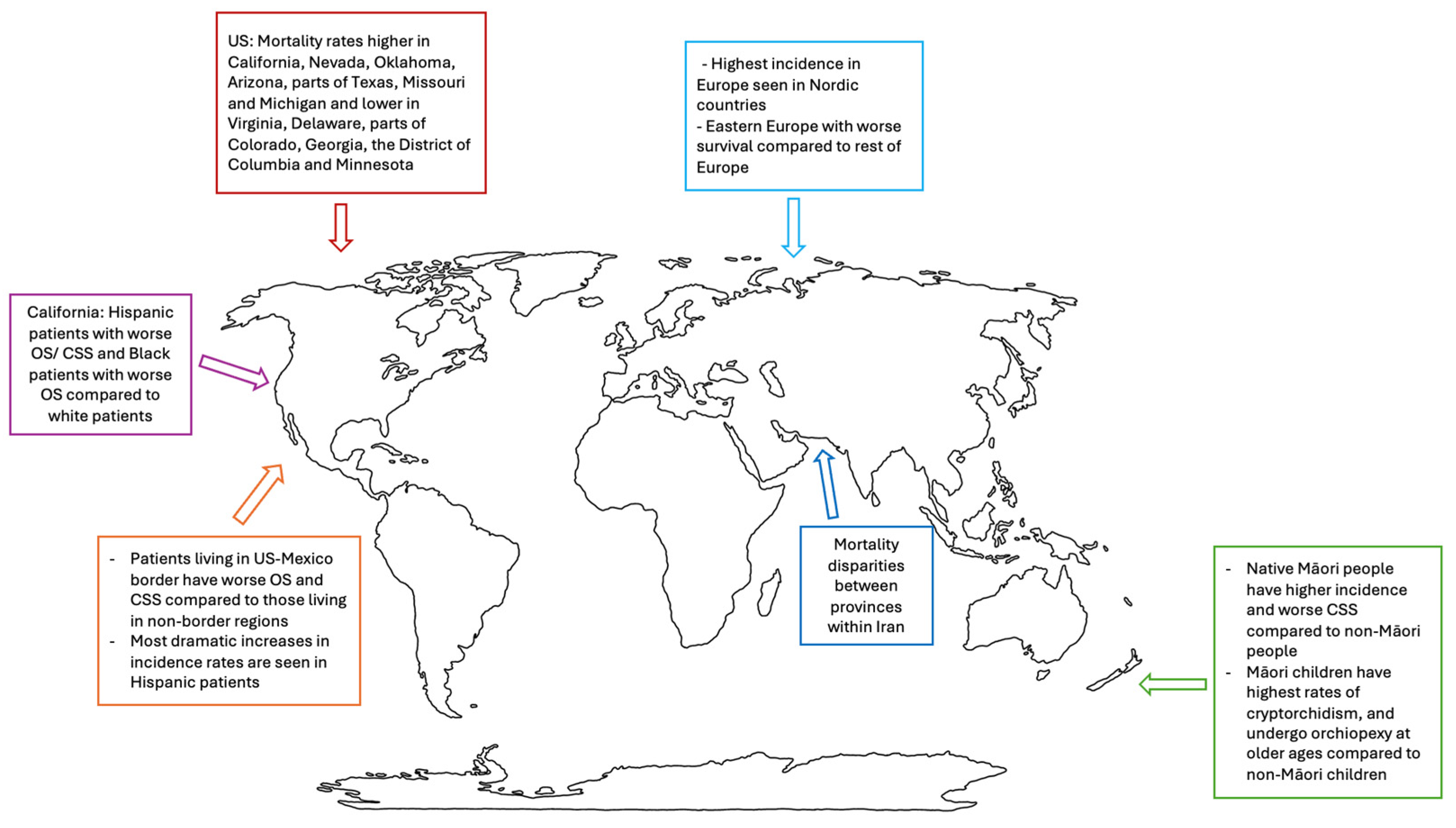
3.2. Worldwide Trends
3.3. New Zealand
3.4. Mexico
3.5. Europe
3.6. United States (US)
3.7. SEER
3.8. NCDB
3.9. CDC-WONDER
3.10. Histology-Specific Outcomes
3.10.1. Non-Seminoma
3.10.2. Seminoma
3.11. Fertility Preservation
3.12. Lesbian, Gay, Bisexual, Transgender and QUEER + (LGBTQ+) Community
3.13. Insurance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Primers 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.J.; Jemal, A.; Wiese, D.; Sung, H.; Kratzer, T.B.; Islami, F.; Dahut, W.L.; Knudsen, K.E. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur. Urol. 2023, 84, 117–126. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Devesa, S.S.; Graubard, B.I.; Castle, P.E. Increasing Incidence of Testicular Germ Cell Tumors Among Black Men in the United States. J. Clin. Oncol. 2005, 23, 5757–5761. [Google Scholar] [CrossRef]
- Ghazarian, A.A.; Kelly, S.P.; Altekruse, S.F.; Rosenberg, P.S.; McGlynn, K.A. Future of testicular germ cell tumor incidence in the United States: Forecast through 2026. Cancer 2017, 123, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- DeRouen, M.C.; McKinley, M.; Shah, S.A.; Borno, H.T.; Aoki, R.; Lichtensztajn, D.Y.; Leppert, J.T.; Brooks, J.D.; Chung, B.I.; Gomez, S.L.; et al. Testicular cancer in Hispanics: Incidence of subtypes over time according to neighborhood sociodemographic factors in California. Cancer Causes Control 2020, 31, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, F.; Haj-Mirzaian, A.; Ebrahimi, H.; Saeedi Moghaddam, S.; Mohajer, B.; Nowroozi, M.R.; Ayati, M.; Farzadfar, F.; Fitzmaurice, C.; Amini, E. Global, regional and national burden of testicular cancer, 1990–2016: Results from the Global Burden of Disease Study 2016. BJU Int. 2019, 124, 386–394. [Google Scholar] [CrossRef]
- Rosen, A.; Jayram, G.; Drazer, M.; Eggener, S.E. Global Trends in Testicular Cancer Incidence and Mortality. Eur. Urol. 2011, 60, 374–379. [Google Scholar] [CrossRef]
- Mourão, T.C.; Curado, M.P.; De Oliveira, R.A.R.; Santana, T.B.M.; Favaretto, R.D.L.; Guimarães, G.C. Epidemiology of Urological Cancers in Brazil: Trends in Mortality Rates Over More Than Two Decades. J. Epidemiol. Glob. Health 2022, 12, 239–247. [Google Scholar] [CrossRef]
- Pishgar, F.; Amini, E.; Gohari, K.; Aminorroaya, A.; Sheidaei, A.; Rostamabadi, Y.; Ebrahimi, H.; Yoosefi, M.; Naderimagham, S.; Rezaei, N.; et al. National and subnational mortality of urological cancers in Iran, 1990–2015. Asia-Pac. J. Clin. Oncol. 2019, 15, e43–e48. [Google Scholar] [CrossRef]
- Banks, K.; Tuazon, E.; Berhane, K.; Koh, C.J.; De Filippo, R.E.; Chang, A.; Kim, S.S.; Daneshmand, S.; Davis-Dao, C.; Lewinger, J.P.; et al. Cryptorchidism and testicular germ cell tumors: Comprehensive meta-analysis reveals that association between these conditions diminished over time and is modified by clinical characteristics. Front. Endocrinol. 2013, 3, 182. [Google Scholar] [CrossRef]
- Klaassen, Z.; Reinstatler, L.; Wilson, S.N.; Ellington, C.; Li, Q.; Terris, M.K.; Moses, K.A. Clinical Disparities for Minorities and Foreign-Born Men With Undescended Versus Descended Testicular Germ Cell Tumors. Clin. Genitourin. Cancer 2016, 14, e251–e255. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.M.; Cheaib, J.G.; Su, Z.T.; Biles, M.J.; Sharma, R.; Zhang, A.; Singla, N.; Bass, E.B.; Pierorazio, P.M. Assessing quality of care in the diagnosis and treatment of early-stage testicular cancer: A critical review and summary. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 400–408. [Google Scholar] [CrossRef]
- Richardson, L.C.; Neri, A.J.; Tai, E.; Glenn, J.D. Testicular cancer: A narrative review of the role of socioeconomic position from risk to survivorship. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, F.P.; Johnson, C.J.; Sherman, R.L.; Stinchcomb, D.G.; Lin, G.; Henry, K.A. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer 2014, 120, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Powe, B.D.; Ross, L.; Wilkerson, D.; Brooks, P.; Cooper, D. Testicular Cancer Among African American College Men: Knowledge, Perceived Risk, and Perceptions of Cancer Fatalism. Am. J. Mens Health 2007, 1, 73–80. [Google Scholar] [CrossRef]
- Kaufman, M. Advanced testicular cancer in a society of racial and socio-economic health disparity. BMJ Case Rep. 2013, 2013, bcr2013009277. [Google Scholar] [CrossRef] [PubMed]
- Uzamere, I.; Wang, Y.; Zheng, T.; Zhu, Y. Genetic determinants for the racial disparities in the risk of prostate and testicular cancers. Commun. Med. 2022, 2, 138. [Google Scholar] [CrossRef]
- Sarfati, D.; Shaw, C.; Blakely, T.; Atkinson, J.; Stanley, J. Ethnic and socioeconomic trends in testicular cancer incidence in New Zealand. Int. J. Cancer 2011, 128, 1683–1691. [Google Scholar] [CrossRef]
- Ballantine, K.R.; Watson, H.; Macfarlane, S.; Winstanley, M.; Corbett, R.P.; Spearing, R.; Stevanovic, V.; Yi, M.; Sullivan, M.J. Small Numbers, Big Challenges: Adolescent and Young Adult Cancer Incidence and Survival in New Zealand. J. Adolesc. Young Adult Oncol. 2017, 6, 277–285. [Google Scholar] [CrossRef]
- Gurney, J.K.; Sarfati, D.; Stanley, J. Obscure etiology, unusual disparity: The epidemiology of testicular cancer in New Zealand. Cancer Causes Control 2015, 26, 561–569. [Google Scholar] [CrossRef]
- Dass, P.H.; Jameson, M.B. Testicular cancer: A 13-year retrospective review of ethnic disparities in the Waikato region, New Zealand. Intern. Med. J. 2020, 50, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.K. The puzzling incidence of testicular cancer in New Zealand: What can we learn? Andrology 2019, 7, 394–401. [Google Scholar] [CrossRef]
- Meredith, I.; Sarfati, D.; Ikeda, T.; Blakely, T. Cancer in Pacific people in New Zealand. Cancer Causes Control 2012, 23, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.; Sarfati, D.; Stanley, J.; Studd, R. Do Ethnic Patterns in Cryptorchidism Reflect Those Found in Testicular Cancer? J. Urol. 2013, 190, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Rodríguez, J.A.; Montalvo-Casimiro, M.; Álvarez-López, D.I.; Reynoso-Noverón, N.; Cuevas-Estrada, B.; Mendoza-Pérez, J.; Jiménez-Ríos, M.A.; Wegman-Ostrosky, T.; Salcedo-Tello, P.; Scavuzzo, A.; et al. Understanding Sociodemographic Factors among Hispanics Through a Population-Based Study on Testicular Cancer in Mexico. J. Racial Ethn. Health Disparities 2023. [Google Scholar] [CrossRef]
- Taylor, Z.D.; Chew, L.; Tumey, T.; Gard, C.C.; Woods, M.E. Differences in incidence, staging, and survival of urologic cancers in patients under 65 living in the US-Mexico border region. Curr. Urol. 2023, 17, 118–124. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Dwyer-Lindgren, L.; Fitzmaurice, C.; Stubbs, R.W.; Bertozzi-Villa, A.; Morozoff, C.; Charara, R.; Allen, C.; Naghavi, M.; Murray, C.J.L. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980–2014. JAMA 2017, 317, 388. [Google Scholar] [CrossRef]
- Trama, A.; Stark, D.; Bozovic-Spasojevic, I.; Gaspar, N.; Peccatori, F.; Toss, A.; Bernasconi, A.; Quarello, P.; Scheinemann, K.; Jezdic, S.; et al. Cancer burden in adolescents and young adults in Europe. ESMO Open 2023, 8, 100744. [Google Scholar] [CrossRef]
- Gatta, G.; Trama, A.; Capocaccia, R.; Hackl, M.; Eycken, E.V.; Henau, K.; Dimitrova, N.; Sekerija, M.; Dušek, L.; Mägi, M.; et al. Epidemiology of rare cancers and inequalities in oncologic outcomes. Eur. J. Surg. Oncol. 2019, 45, 3–11. [Google Scholar] [CrossRef]
- Schaffar, R.; Pant, S.; Bouchardy, C.; Schubert, H.; Rapiti, E. Testicular cancer in Geneva, Switzerland, 1970–2012: Incidence trends, survival and risk of second cancer. BMC Urol. 2019, 19, 64. [Google Scholar] [CrossRef]
- Nur, U.; Rachet, B.; Parmar, M.K.B.; Sydes, M.R.; Cooper, N.; Stenning, S.; Read, G.; Oliver, T.; Mason, M.; Coleman, M.P. Socio-economic inequalities in testicular cancer survival within two clinical studies. Cancer Epidemiol. 2012, 36, 217–221. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Wang, Y.; Ma, S. Racial differences in testicular cancer in the United States: Descriptive epidemiology. BMC Cancer 2020, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, J.; Wu, W.; Fang, D.; Wang, K.; Yang, L.; Jiang, X.; Wang, Q.; Li, L. Racial disparities in young-onset patients with colorectal, breast and testicular cancer. J. Cancer 2019, 10, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, Y.B.; Tang, B.W.; Brown, M.; Wang, B.H.; Du, C.L.; Du, J.S.; Wang, X.M.; Cai, L.J.; Wu, G.Y.; et al. Assessment of Prognostic Factors of Racial Disparities in Testicular Germ Cell Tumor Survival in the United States (1992–2015). Biomed. Environ. Sci. BES 2021, 34, 152–162. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Ellison, L.M. Testicular cancer patterns in Asian-American males: An opportunity for public health education to impact outcomes. Urology 2005, 66, 606–609. [Google Scholar] [CrossRef]
- Townsend, J.S.; Richardson, L.C.; German, R.R. Incidence of Testicular Cancer in the United States, 1999–2004. Am. J. Mens Health 2010, 4, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lou Biggs, M.; Schwartz, S.M. Differences in Testis Cancer Survival by Race and Ethnicity: A Population-Based Study, 1973–1999 (United States). Cancer Causes Control 2004, 15, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gleason, A.M. Racial Disparities in Testicular Cancer: Impact on Health Promotion. J. Transcult. Nurs. 2006, 17, 58–64. [Google Scholar] [CrossRef]
- Sun, M.; Abdollah, F.; Liberman, D.; Abdo, A.; Thuret, R.; Tian, Z.; Shariat, S.F.; Montorsi, F.; Perrotte, P.; Karakiewicz, P.I. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: A survival analysis. Cancer 2011, 117, 4277–4285. [Google Scholar] [CrossRef]
- Woldu, S.L.; Aydin, A.M.; Rao, A.V.; Hutchinson, R.C.; Singla, N.; Clinton, T.N.; Krabbe, L.-M.; Passoni, N.M.; Raj, G.V.; Miller, D.S.; et al. Differences at Presentation and Treatment of Testicular Cancer in Hispanic Men: Institutional and National Hospital-based Analyses. Urology 2018, 112, 103–111. [Google Scholar] [CrossRef]
- Shah, Y.B.; Goldberg, H.; Hu, B.; Daneshmand, S.; Chandrasekar, T. Metastatic Testicular Cancer Patterns and Predictors: A Contemporary Population-based SEER Analysis. Urology 2023, 180, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Frazier, A.L.; Poynter, J.N. Survival differences by race/ethnicity among children and adolescents diagnosed with germ cell tumors. Int. J. Cancer 2020, 146, 2433–2441. [Google Scholar] [CrossRef]
- Shaikh, F.; Murray, M.J.; Amatruda, J.F.; Coleman, N.; Nicholson, J.C.; Hale, J.P.; Pashankar, F.; Stoneham, S.J.; Poynter, J.N.; Olson, T.A.; et al. Paediatric extracranial germ-cell tumours. Lancet Oncol. 2016, 17, e149–e162. [Google Scholar] [CrossRef] [PubMed]
- DeRouen, M.C.; Mujahid, M.; Srinivas, S.; Keegan, T.H.M. Disparities in Adolescent and Young Adult Survival After Testicular Cancer Vary by Histologic Subtype: A Population-Based Study in California 1988–2010. J. Adolesc. Young Adult Oncol. 2016, 5, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Robbins, A.S.; Fedewa, S.A.; Ward, E.M. Disparities in stage at diagnosis among adults with testicular germ cell tumors in the National Cancer Data Base. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 23.e15–23.e21. [Google Scholar] [CrossRef]
- Macleod, L.C.; Cannon, S.S.; Ko, O.; Schade, G.R.; Wright, J.L.; Lin, D.W.; Holt, S.K.; Gore, J.L.; Dash, A. Disparities in Access and Regionalization of Care in Testicular Cancer. Clin. Genitourin. Cancer 2018, 16, e785–e793. [Google Scholar] [CrossRef]
- Webster, B.R.; Riley, J.M.; Davis, M.S. Initial Presentation and Outcomes of Testicular Cancer among Different Racial and Ethnic Groups at a Tertiary Care Facility in New Mexico. Urol. Pract. 2021, 8, 71–77. [Google Scholar] [CrossRef]
- Agarwal, G.; Sharma, P.; Valderrama, O.; Lin, H.-Y.; Yue, B.; Nguyen, S.; Fishman, M.; Luchey, A.; Pow-Sang, J.M.; Spiess, P.E.; et al. Sociodemographic and Provider Based Disparities in the Management of Stage I Testicular Cancer. Urol. Pract. 2017, 4, 36–42. [Google Scholar] [CrossRef]
- Clemons, J.; Zahnd, W.E.; Nutt, M.; Sadowski, D.; Dynda, D.; Alanee, S. Impact of Urologist Density and County Rurality on the Practice of Retroperitoneal Lymph Node Dissection and Cancer-Specific Death in Patients with Nonseminomatous Germ Cell Tumors. J. Adolesc. Young Adult Oncol. 2017, 6, 83–90. [Google Scholar] [CrossRef]
- Rovito, M.J.; Taylor, S.; Lockwood, R.; Adams, W.B.; Craycraft, M. Testicular Cancer Incidence and Mortality Within Rural and Urban Regions. J. Adolesc. Young Adult Oncol. 2020, 9, 202–207. [Google Scholar] [CrossRef]
- Jim, M.A.; Arias, E.; Seneca, D.S.; Hoopes, M.J.; Jim, C.C.; Johnson, N.J.; Wiggins, C.L. Racial Misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am. J. Public Health 2014, 104, S295–S302. [Google Scholar] [CrossRef]
- Wolff, D.T.; Monaghan, T.F.; Gordon, D.J.; Michelson, K.P.; Jones, T.; Khargi, R.; Smith, M.T.; Maffucci, F.; Kwun, H.; Suss, N.R.; et al. Racial Differences in Incident Genitourinary Cancer Cases Captured in the National Cancer Database. Medicina 2021, 57, 671. [Google Scholar] [CrossRef]
- Melkonian, S.C.; Said, N.; Weir, H.K.; Jim, M.A.; Siegel, D.A. Incidence of selected cancers in Non-Hispanic American Indian and Alaska Native adolescent and young adult populations, 1999–2019. Ann. Epidemiol. 2023, 83, 78–86.e2. [Google Scholar] [CrossRef] [PubMed]
- Ghazwani, Y.; Alghafees, M.; Suheb, M.K.; Shafqat, A.; Sabbah, B.N.; Arabi, T.Z.; Razak, A.; Sabbah, A.N.; Alaswad, M.; AlKattan, W.; et al. Trends in genitourinary cancer mortality in the United States: Analysis of the CDC-WONDER database 1999–2020. Front. Public Health 2024, 12, 1354663. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.O.; Ghosh, A.; Goldberg, S.I.; Chino, F.; Efstathiou, J.A.; Kamran, S.C. Disparities in testicular cancer incidence, mortality, and place of death trends from 1999 to 2020: A comprehensive cohort study. Cancer Rep. 2023, 6, e1880. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Lupo, P.J.; Roth, M.E.; Winick, N.J.; Pruitt, S.L. Disparities in Cancer Survival Among Adolescents and Young Adults: A Population-Based Study of 88 000 Patients. JNCI J. Natl. Cancer Inst. 2021, 113, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Morra, S.; Cano Garcia, C.; Piccinelli, M.L.; Tappero, S.; Barletta, F.; Incesu, R.; Scheipner, L.; Baudo, A.; Tian, Z.; De Angelis, M.; et al. Survival of stage III non-seminoma testis cancer patients versus simulated controls, according to race/ethnicity. Int. J. Urol. 2024, 31, 1137–1143. [Google Scholar] [CrossRef]
- Incesu, R.-B.; Barletta, F.; Tappero, S.; Piccinelli, M.L.; Garcia, C.C.; Morra, S.; Scheipner, L.; Tian, Z.; Saad, F.; Shariat, S.F.; et al. Survival differences in non-seminoma testis cancer patients according to race/ethnicity. Cancer Epidemiol. 2024, 89, 102538. [Google Scholar] [CrossRef]
- Bhambhvani, H.P.; Greenberg, D.R.; Kasman, A.M.; DeRouen, M.C.; Cheng, I.; Eisenberg, M.L.; Shah, S.A. Racial and socioeconomic disparities in retroperitoneal lymph node dissection and survival in nonseminomatous germ cell tumor: A population-based study. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 197.e1–197.e8. [Google Scholar] [CrossRef]
- Gray, P.J.; Lin, C.C.; Sineshaw, H.; Paly, J.J.; Jemal, A.; Efstathiou, J.A. Management trends in stage I testicular seminoma: Impact of race, insurance status, and treatment facility. Cancer 2015, 121, 681–687. [Google Scholar] [CrossRef]
- Patel, A.; Howard, J.M.; Chertack, N.; Badia, R.R.; Margulis, V.; Woldu, S.L.; Courtney, K.; Bowman, I.A.; Arafat, W.; Meng, X.; et al. Disparities in Pre-Orchiectomy Sperm Cryopreservation Among Testicular Cancer Patients at a Public Safety Net Hospital and a Private Tertiary Care Center. Urology 2022, 163, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.L.; Picton, H.M.; Friend, A.J.; Hayden, C.M.; Brougham, M.; Cox, R.; Grandage, V.; Kwok-Williams, M.; Lane, S.; Mitchell, R.T.; et al. Inconsistencies in fertility preservation for young people with cancer in the UK. Arch. Dis. Child. 2022, 107, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Block, R.G.; Clayman, M.L.; Kelvin, J.; Arvey, S.R.; Lee, J.-H.; Reinecke, J.; Sehovic, I.; Jacobsen, P.B.; Reed, D.; et al. If You Did Not Document It, It Did Not Happen: Rates of Documentation of Discussion of Infertility Risk in Adolescent and Young Adult Oncology Patients’ Medical Records. J. Oncol. Pract. 2015, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Goodman, M.; Adams, N.; Corneil, T.; Hashemi, L.; Kreukels, B.; Motmans, J.; Snyder, R.; Coleman, E. Epidemiological considerations in transgender health: A systematic review with focus on higher quality data. Int. J. Transgender Health 2020, 21, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.B.; Komatsoulis, G.A.; Meersman, S.C.; Garrett-Mayer, E.; Bruinooge, S.S.; Miller, R.S.; Potter, D.; Koronkowski, B.; Stepanski, E.; Dizon, D.S. Identification of Transgender People With Cancer in Electronic Health Records: Recommendations Based on CancerLinQ Observations. JCO Oncol. Pract. 2021, 17, e336–e342. [Google Scholar] [CrossRef]
- De Vries, E.; Kathard, H.; Müller, A. Debate: Why should gender-affirming health care be included in health science curricula? BMC Med. Educ. 2020, 20, 51. [Google Scholar] [CrossRef]
- De Nie, I.; Wiepjes, C.M.; De Blok, C.J.M.; Van Moorselaar, R.J.A.; Pigot, G.L.S.; Van Der Sluis, T.M.; Barbé, E.; Van Der Voorn, P.; Van Mello, N.M.; Huirne, J.; et al. Incidence of testicular cancer in trans women using gender-affirming hormonal treatment: A nationwide cohort study. BJU Int. 2022, 129, 491–497. [Google Scholar] [CrossRef]
- Tinajero, J.; Rashid, T. Urologic oncology considerations in transgender and gender diverse patients. Curr. Opin. Urol. 2024, 34, 314–322. [Google Scholar] [CrossRef]
- Vaccaro, C.J.; McGrath, M.G.; McFadden, E. Breaking Healthcare Barriers for Transgender Individuals with Rare Tumor Presentation. Cureus 2023, 15, e3379. [Google Scholar] [CrossRef]
- Chipollini, J.; Tang, D.H.; Zhou, J.; Reich, R.R.; Leone, A.R.; Gilbert, S.M.; Sexton, W.J. Trends in Insurance Status during Initial Presentation of Testicular Carcinoma: Examining Health Outcomes and Implications of Health Reform for Young Adults in the United States. Urol. Pract. 2019, 6, 18–23. [Google Scholar] [CrossRef]
- Markt, S.C.; Lago-Hernandez, C.A.; Miller, R.E.; Mahal, B.A.; Bernard, B.; Albiges, L.; Frazier, L.A.; Beard, C.J.; Wright, A.A.; Sweeney, C.J. Insurance status and disparities in disease presentation, treatment, and outcomes for men with germ cell tumors. Cancer 2016, 122, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Koroukian, S.M.; Bakaki, P.M.; Raghavan, D. Survival disparities by Medicaid status: An analysis of 8 cancers. Cancer 2012, 118, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Withington, J.; Cole, A.P.; Meyer, C.P.; Seisen, T.; Schmid, M.; Lipsitz, S.R.; Sweeney, C.J.; Dasgupta, P.; Trinh, Q. Comparison of testis cancer-specific survival: An analysis of national cancer registry data from the USA, UK and Germany. BJU Int. 2019, 123, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Albany, C. Current medical management of patients with poor-risk metastatic germ-cell tumors. Curr. Opin. Urol. 2018, 28, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.B.; Nguyen, C.T.; Saffore, L.; Modlin, C.; Modlin, C.S. Racial Disparities in Urologic Health Care. J. Natl. Med. Assoc. 2010, 102, 108–118. [Google Scholar] [CrossRef]
- Adams, W.B.; Rovito, M.J.; Craycraft, M. The Connection Between Testicular Cancer, Minority Males, and Planned Parenthood. Am. J. Mens Health 2018, 12, 1774–1783. [Google Scholar] [CrossRef]
- Simone, C.B.; Hampshire, M.K.; Vachani, C.; Metz, J.M. The Utilization of Oncology Web-based Resources in Spanish-speaking Internet Users. Am. J. Clin. Oncol. 2012, 35, 520–526. [Google Scholar] [CrossRef]
- Saab, M.M.; Shetty, V.N.; McCarthy, M.; Davoren, M.P.; Flynn, A.; Kirby, A.; Robertson, S.; Shorter, G.W.; Murphy, D.; Rovito, M.J.; et al. Promoting ‘testicular awareness’: Co-design of an inclusive campaign using the World Café Methodology. Health Expect. 2024, 27, e13898. [Google Scholar] [CrossRef]
- Saab, M.M.; Landers, M.; Hegarty, J. Exploring men’s preferred strategies for learning about testicular disorders inclusive of testicular cancer: A qualitative descriptive study. Eur. J. Oncol. Nurs. 2017, 26, 27–35. [Google Scholar] [CrossRef]

| Incidence/Development of Testicular Cancer | Mortality |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, D.; Daneshmand, S. Disparities in Testicular Cancer: A Review of the Literature. Cancers 2024, 16, 3433. https://doi.org/10.3390/cancers16203433
Escobar D, Daneshmand S. Disparities in Testicular Cancer: A Review of the Literature. Cancers. 2024; 16(20):3433. https://doi.org/10.3390/cancers16203433
Chicago/Turabian StyleEscobar, Domenique, and Siamak Daneshmand. 2024. "Disparities in Testicular Cancer: A Review of the Literature" Cancers 16, no. 20: 3433. https://doi.org/10.3390/cancers16203433
APA StyleEscobar, D., & Daneshmand, S. (2024). Disparities in Testicular Cancer: A Review of the Literature. Cancers, 16(20), 3433. https://doi.org/10.3390/cancers16203433






