Simple Summary
Neoadjuvant radiochemotherapy (RCT) and total neoadjuvant therapy (TNT) have significantly reduced local recurrence rates in rectal cancer. However, late local recurrences remain a distinct issue. Most recurrences occur within two years; general follow-up programs typically last for 5 years. We found four patients with late local recurrences > 8 years after surgery. This study highlights that neoadjuvant therapy delays local recurrence.
Abstract
Neoadjuvant radiochemotherapy (RCT) and lately total neoadjuvant therapy (TNT) improved local recurrence rates of rectal cancer significantly compared to total mesorectal excision (TME) alone. Yet the occurrence and impact of late local recurrences after many years appears to be a distinct biological problem. We included n = 188 patients with rectal cancer after RCT and radical resection in this study; n = 38 of which had recurrent disease (sites: local (8.0%), liver (6.4%), lung (3.7%)). We found that 68% of all recurrences developed within the first two years. Four patients, however, experience recurrence >8 years after surgery. Here, we report and characterize four cases of late local recurrence (10% of patients with recurrent disease), suggesting that neoadjuvant therapy in principle delays local recurrence.
Keywords:
rectal cancer; neoadjuvant; radiochemotherapy; outcome; follow-up; surveillance; local recurrence 1. Introduction
Colorectal cancer remains one of the most prevalent types of cancer, after lung and breast cancer. In 2020, over one million colon cancer cases and over 730,000 rectal cancer cases were reported globally [1]. Rectal cancer accounts for 3.9% of all cancer diagnoses for both sexes and is responsible for 3.2% of cancer-related deaths. The widespread adoption of neoadjuvant radiochemotherapy (RCT) has led to a decrease in local recurrence rates and an improvement in disease-free-survival (DFS). These benefits are significantly amplified when chemotherapy is added to the treatment regimen [2].
Local recurrences occur in 6% of cases following RCT, compared to 13% after postoperative chemoradiotherapy within a five-year post-treatment period [3]. The most common sites for distant recurrences are the lungs, followed by liver metastases and local recurrence. Notably, over 90% of all recurrences manifest within the first three years of monitoring [4]. Despite the decline in local and some distant recurrences, no significant improvement has been observed in overall survival (OS) rates post-neoadjuvant therapy [5].
Postoperative surveillance is crucial in colorectal carcinoma management, particularly as secondary hepatic resection offers another curative opportunity for selected patients [6]. Consequently, a five-year post-treatment surveillance period is recommended in Germany for rectal carcinoma patients following curative surgery [7].
While most recurrences in locally advanced rectal cancer appear within three years post-RCT, there are instances of local recurrences occurring well beyond the standard five-year surveillance period [8].
This study aims to assess the rates of these late local recurrences and evaluate the duration of surveillance. We identified a small subgroup of patients who experienced local recurrences long after routine follow-up had concluded. This cohort may benefit from extended surveillance.
2. Methods
2.1. Patient Population
We retrospectively analyzed the follow-up data of all patients (n = 206), who received RCT followed by resection of the rectal primary at our institution from 2000 to 2017. Patients were identified via a query of our prospective, institutional-based clinical research database. We excluded all patients with R1/2-resections (n = 17). Additionally, a patient who required immediate surgery because of stenosis and perforation was excluded. The pretherapeutic imaging and staging consisted of a physical examination, rigid rectoscopy, and computed tomography of the chest/abdomen/pelvis in all patients. Local staging was completed at the beginning of the study using endosonography, and starting in 2005, all patients received pretherapeutic MRI scans of the pelvis. Since the pathologic assessment of the circumferential resection margin (CRM) was implemented into the German Rectal Cancer Guidelines in 2008, and we accrued patients starting in 2000, pathologic CRM data were only available for 46% of patients in our cohort. Only 2.7% of these patients had a positive CRM; therefore, CRM was not included in further analysis. This study was approved by the ethics committee of the University of Tübingen (149/2012BO2) and written informed consent for collecting follow-up data was obtained by all participants. Table 1 shows the clinical characteristics of the patient population.

Table 1.
Patient characteristics.
2.2. Neoadjuvant Radiochemotherapy
All patients received external-beam radiation therapy as either 3D-conformable or intensity-modulated radiotherapy (IMRT). The most common regimen consisted of 28 fractions with 1.8 Gy, for a total dose of 50.4 Gy. All patients received preoperative 5-fluorouracil (5-FU)-based chemotherapy. The most common protocol was continuous infusion therapy with 5-FU (225 mg/m2/d) for a 6-week continuous cycle. Since 2014, all patients have been recommended adjuvant treatment following neoadjuvant therapy; before 2014, this decision was made on an individual basis. Therefore, 103 patients (50%) in this patient population received adjuvant therapy.
2.3. Surgery
All patients underwent radical resection of the rectal primary. For rectal tumors greater than 10 cm from the anal verge, a partial mesorectal excision with transection of the mesorectum 5 cm distal to the distal edge of the rectal cancer was performed. For rectal tumors less than 10 cm from the anal verge, a total mesorectal excision (TME) was performed. In most cases, this was achieved with a low anterior resection (147 cases) with a protective loop ileostomy in 106 cases. Because of low-lying tumors or sphincter involvement, 36 patients (19.5%) were treated with an abdominoperineal resection (APR). Surgery was performed 6–8 weeks (median 6.14) after the completion of RCT.
2.4. Local Recurrence
Local recurrence was defined as a recurrent tumor within the pelvis below the promontory. A histological confirmation could be obtained in 93.3% of patients (14 cases). In cases without histological confirmation, a size progression of a pelvic mass was defined as local recurrence.
2.5. Statistical Analysis
We used our electronic hospital information system iMedOne (Deutsche Telekom Clinical Solutions GmbH, 50676 Köln, Germany) and GapIT (i-Solutions Health/Mesalvo Mannheim GmbH, 68167 Mannheim, Germany) as the data source. The information was collected and processed anonymously in a secured electronic database. Statistical analysis was performed with Bias for Windows Version 11.06 (Hanns Ackermann, University of Frankfurt, 60590 Frankfurt, Germany) and SPSS Statistics Version 24 (IBM Deutschland GmbH, 71139 Ehningen, Germany). The OS as well as DFS was assessed using Kaplan–Meier survival analysis. Group comparisons in the survival analysis were conducted using the Log-Rank test. Differences between individual subgroups were calculated using the Mann–Whitney test. A p-value below 0.05 was considered statistically significant.
3. Results
During a median follow-up of 88.5 months, 38 patients (20.2%) experienced recurrences. Local recurrences occurred in 15 patients, equating to a rate of 8.0%. Liver and lung metastases were observed in 12 (6.4%) and 7 (3.7%) patients, respectively. Three patients (1.6%) had multi-site metastases. One patient had a metastatic cervical lymph node as the sole site of recurrence. Figure 1 illustrates the cumulative appearance of recurrences. Table 2 and Table 3 provide an overview of the pathological findings and recurrences. Results of the submitted doctoral thesis have been incorporated [9].
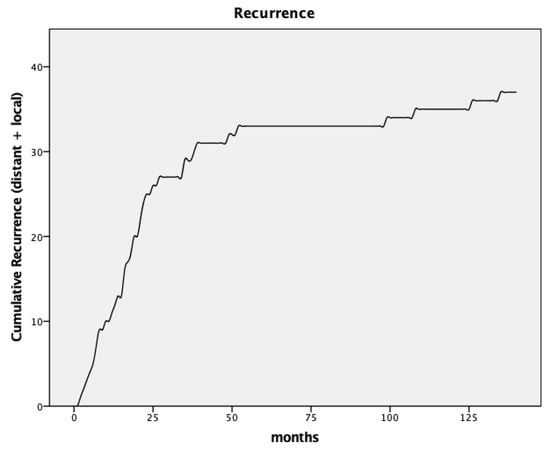
Figure 1.
Curve for cumulative recurrence.

Table 2.
Pathological stage.

Table 3.
Recurrence.
The median duration between surgery and local recurrence was 23 months (range 6–135 months, SD 46.0). No significant difference in initial T-stage was found between patients with and without local recurrence (Mann–Whitney U-Test, p = 0.46). The distribution of initial T-stage was identical in the subgroup of patients with local recurrence (69% with cT3, 31% with cT4). This is comparable to the T-stage of the initial staging with 78% of patients with cT3 and 21% cT4 tumors. However, the nodal stage significantly influenced recurrence rates. Patients with positive lymph nodes had a higher rate of recurrence (38.6%) compared to node-negative patients (14.6%, p = 0.001). Concerning local recurrence, there was also a higher rate in patients with positive lymph nodes (13.6% local recurrences in node-positive vs. 6.3% in node-negative patients), which was not statistically significant (p = 0.16).
Most local recurrences (nine patients) occurred within the first 3 years post-surgery. One patient was diagnosed with recurrent disease 39 months after surgery and another one 49 months after surgery. Four additional patients developed late local recurrences after 8–11 years, accounting for 26.7% of all local recurrences. Patients with local recurrences are depicted in Table 4.

Table 4.
Local recurrence.
The time to recurrence varied by site, with distant metastases occurring at a median of 17 months and local recurrences occurring at 23 months post-surgery (p = 0.022).
The 5-year OS and DFS rates were 79.2% and 72.6%, respectively. Ten years post-surgery, 58.5% of patients remained alive. The 5-year OS was significantly worse in patients with node-positive tumor stages (ypN+). The 5-year OS in node-negative patients was 84.1% compared to 63.0% for patients with node-positive tumors. After 10 years of follow-up, 64.0% of node-negative patients remained alive compared to only 40.9% of node-positive patients (p = 0.001).
The DFS was significantly different in both groups as well, with a 5-year DFS of 79.7% in node-negative patients and 49.5% in node-positive patients (p < 0.001). Both OS and DFS in relation to nodal stage are depicted in Figure 2 and Figure 3.
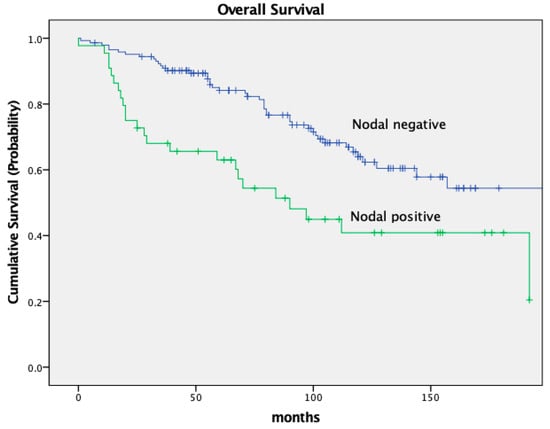
Figure 2.
Kaplan–Meier curves for OS, nodal stage.
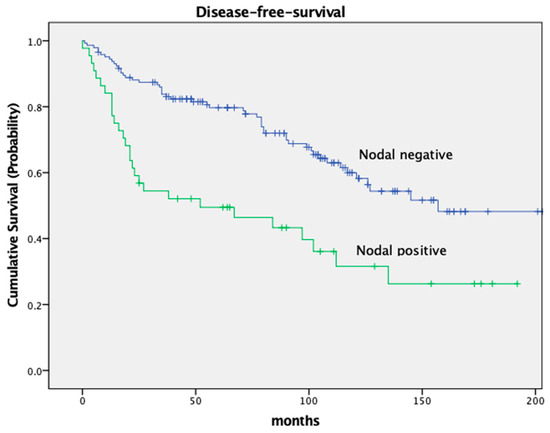
Figure 3.
Kaplan–Meier curves for disease-free-survival, nodal stage.
The OS was worse in patients who developed recurrence (local and distant) in comparison to patients with no recurrence with a 5-year OS of 89.6% and 41.3%, respectively. The 10-year OS was 66.0% without recurrence and 26.7% with recurrence (p < 0.001). See Figure 4.
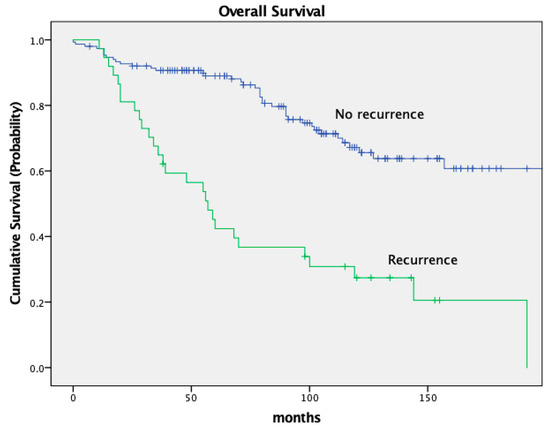
Figure 4.
Kaplan–Meier curves for recurrence.
OS and DFS also varied based on the tumor’s location within the rectum. Low-lying tumors (<6 cm from the anal verge) had worse OS and DFS compared to tumors located in the middle or upper third of the rectum. Patients with a tumor in the lower third had an OS of 72.1% and a DFS of 67.2%; patients with a tumor in the middle third of the rectum had an OS of 83.8% and a DFS of 77.3% (OS p = 0.09, DFS p = 0.011). For details, see Figure 5 and Figure 6 and Table 5.
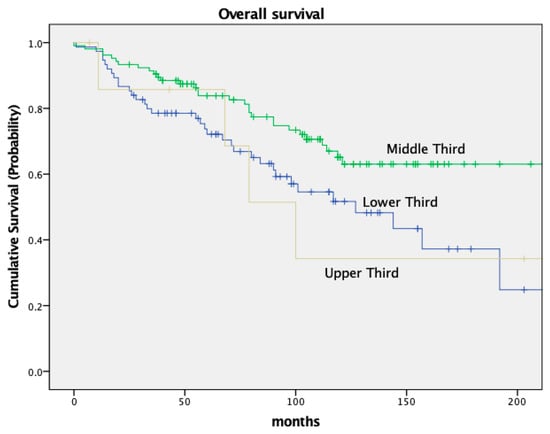
Figure 5.
Kaplan–Meier curves for OS, location of tumor.
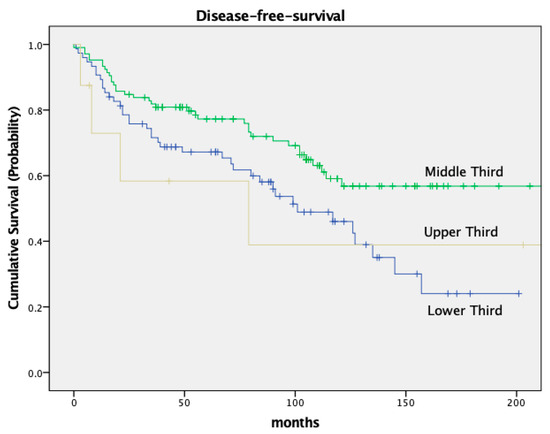
Figure 6.
Kaplan–Meier curves for disease-free-survival, location of tumor.

Table 5.
Survival.
Out of four patients with late local recurrence, only one could be treated curatively with an APR and is still alive after 134 months follow-up. This 73-year-old female patient was initially treated with an AR including TME in September 2008. The preoperative restaging showed an ycT3N2-tumor 5 cm above the anal verge. The histological examination of the specimen revealed an ypT2 with a negative nodal status (0/23 lymph nodes). Due to severe side effects caused by the neoadjuvant RCT, the patient refused adjuvant chemotherapy. Regular follow-up has been completed without evidence of recurrence. However, in January 2017, 99 months after surgery, a routinely performed colonoscopy showed a local recurrence in the anastomotic area. After distant metastases had been excluded via CT scan, the patient underwent an APR. Histologically detected infiltration of the dorsal wall of the vagina made a re-resection necessary. Finally, a R0-resection could be achieved. Later on, a presacral mass was detected in a CT scan in May 2018, 16 months after the APR. A PET-scan could not clearly confirm malignancy, so the interdisciplinary decision was to follow up with the patient. During the following 19 months, the presacral mass was stable.
Another female patient received brachytherapy after a late recurrence was detected and biopsy proven. Initially, the 68-year-old woman was staged T3N0 and underwent AR in August 2004. Histology confirmed a node-negative ypT2 tumor. The patient did not receive adjuvant therapy, and regular follow-up did not reveal any evidence of recurrence. Due to vaginal bleeding, further diagnostic investigation was performed in 2013. A biopsy confirmed a local recurrence at the introitus of the vagina 108 months after AR. A palliative brachytherapy was performed. The patient passed away less than one year later, in August 2014.
A lateral local recurrence with infiltration of the ureter and bladder was found in a male patient after AR in July 2001. The initial histological finding revealed an ypT2ypN1-tumor. Adjuvant therapy was recommended but was refused by the patient. The five years of regular follow-up were unobtrusive. Eventually, the patient underwent further diagnostic investigation in 2012 because of elevated tumor markers (CEA levels). A CT scan confirmed recurrence in the pelvic wall 135 months after surgery (see Figure 7). The tumor was histologically confirmed through transanal biopsy. The therapy consisted of an extended surgery with resection of the urethra and bladder after local radiation and chemotherapy, but an R0-resection could not be achieved. Furthermore, the patient developed lung metastases and received radiotherapy. The patient died 5 years after diagnosis of local recurrence in 2017.
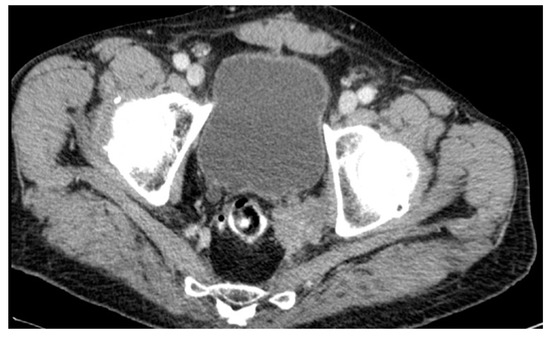
Figure 7.
CT scan #1.
Lastly, one patient developed a presacral mass and received local radiation in combination with chemotherapy, unfortunately without any response. The 68-year-old male initially had a bulky, low-lying T4-tumor with stenosis. He received an end-colostomy prior to RCT and a radical APR afterwards in September 2001. Pathology revealed a node-negative ypT3. The patient refused adjuvant treatment. A CT scan in 2012, which was undertaken because of perineal pain, showed a presacral mass (see Figure 8). CT-guided biopsy confirmed a local recurrence 126 months after APR. The palliative radiation was well tolerated by the patient and was followed by chemotherapy. The patient died in September 2013, 18 months after diagnosis of recurrence.
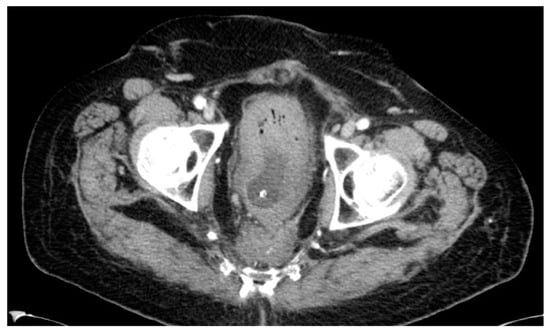
Figure 8.
CT scan #2.
4. Discussion
In this comprehensive study, we examined recurrence patterns in a cohort of n = 188 patients diagnosed with locally advanced rectal cancer. All patients underwent neoadjuvant radiochemotherapy, followed by radical resection. The median follow-up period for this cohort was 88 months, providing a robust dataset for long-term outcomes. We identified a subset of patients who developed local recurrence beyond a commonly used follow-up timeframe of five years. This group of patients with “late local recurrences” accounted for approximately one-quarter of all local recurrences (26.7%). Our finding underscores the fact that a select group of patients locally recurs beyond the standard five-year window. This group of patients is biologically interesting and warrants further investigation.
Our survival data are in solid agreement with previously published results from randomized controlled trials. For instance, a significant survival difference based on nodal stage has been well documented and is confirmed by our present study. Pooled data of five major European rectal cancer studies revealed a 5-year OS of 77.9% in pathologically node-negative patients and 53.1% in node-positive patients. These data align with our 5-year OS rates of 84.1% for node-negative patients and 63.0% for node-positive patients. Similarly, the DFS of 79.7% in node-negative patients and 49.5% in node-positive patients in the current trial are consistent with the above data, which reported DFS rates of 65.7% and 40.2%, respectively [10]. We believe that this consistency adds credibility to our findings and suggests that they may well be generalized to a broader population and a true cancer phenomenon. Adding to the above, we also studied the impact of tumor location within the rectum on survival. Our data revealed that patients with tumors located in the middle third of the rectum had superior survival rates (83.8% OS and 77.3% DFS) compared to those with distal tumors (72.1% OS and 67.2% DFS). These findings further corroborate previous data, reporting varying 5-year OS and DFS rates based on tumor location [11].
Lower survival rates in patients with distal rectal cancer can generally be attributed to multiple factors. One significant factor is the surgical complexity associated with these tumors, often necessitating APR, which has been shown to increase the risk of a less favorable oncologic outcome compared to AR [10,12,13].
Our analysis showed a sphincter preservation rate of 81%. This is concordant to randomized trials like the German and Polish Rectal Cancer trials, in which overall sphincter preservation rates of 52% and 69% have been reported [2,3].
Additionally, distal tumors are at a higher risk for lateral lymph node involvement, a factor that has been extensively studied, particularly in Japan [14,15,16]. Although lateral lymph node dissection is a standard procedure in Japan for advanced distal rectal cancer, its ability to influence progression-free survival remains a subject of ongoing research [17]. Nonetheless, some investigators strongly believe that it reduces the rate of local recurrence and should be used for all patients with advanced local disease.
Taking into account the extended observation period of the current study (commencing in the year 2000), we consider it challenging to definitively state if lateral lymph node involvement was responsible for the local recurrences observed, particularly the late ones. Not all our patients with local recurrence underwent initial MRI staging of the pelvis, making it difficult to retrospectively study this aspect. However, it is certainly a possibility, and it is thus of high importance that future studies incorporate more advanced imaging techniques for better preoperative staging, as this is good clinical practice in many regions today.
Albeit the exact mechanism behind late local recurrence remains elusive, several risk factors for late recurrence have been reported, including tumor location, tumor stage at diagnosis, preoperative CEA levels, and lymph node status [18,19]. The most significant prognostic factors for DFS and OS are lymph node status and circumferential resection margin [19,20,21,22]. Additionally, the distribution of positive lymph-nodes has prognostic implications. In this study, shorter survival (OS and DFS) [23] and a high incidence of metastatic disease at the time of surgery [24], were associated with more centrally located positive lymph-nodes. The most important predictors for local recurrence are T-status, nodal status, and residual tumor status (R0 versus R1/R2). Surgery type (TME vs. Conventional) and administration of radiotherapy are also influencers of local recurrence. However, these factors have no effect on DFS or OS [23]. Our cohort only showed a significant difference in nodal status concerning local recurrences. Late local recurrences exhibited no substantial difference in this regard, as three out of four were node-negative. Overall, we assume that all known risk factors favor a relatively early onset of recurrences, and the factors for late onset are not known. Due to the low absolute number, statistically, no factor can be excluded in our cohort.
Taken together, the overall recurrence patterns in our cohort were consistent with the existing literature. Most recurrences manifested within the first three years, primarily in the liver and locally. Hence, the distribution and timing of recurrent disease was similar to that in other studies, with liver metastases appearing first, followed by lung metastases and local recurrences [8]. Interestingly, we observed four cases of recurrent disease after nine years of follow-up (approximately 10% of all recurrences), all of which were local. Although statistical considerations on an epidemiological scale must be taken into further account, this might suggest that an extended follow-up period may be beneficial for early detection of late local recurrences for some patients, increasing the possibility of curative re-resection.
Recent advancements in TNT have shown promising results, particularly in controlling distant metastases [24,25,26]. However, they also make the overall management of rectal cancer even more complicated, since stringent, long-term follow-up becomes crucial, particularly in those patients taking part in watchful waiting trials. Some data from the 5-year follow-up of the RAPIDO trial even suggest that local recurrence rates were higher following TNT as compared to long-course chemoradiotherapy (7.2% in comparison to 3.9% (p = 0.049) in patients with R0 resections) [27]. Therefore, the selection of patients who can benefit from TNT must remain an area of ongoing research that has to take into account the problems of late local recurrence discussed in the present work.
In conclusion, our study suggests that neoadjuvant therapy may delay local recurrence in patients with locally advanced rectal cancer in principle. This raises questions about the long-term efficacy of neoadjuvant RCT in reducing local recurrence rates, particularly if lateral nodes are involved. Longer-term follow-up of some patients might be warranted, since good results can be achieved even in the case of local recurrence that makes secondary pelvic exenteration necessary [28,29]. And the very problem of late local recurrence discussed in the present work has become even more pressing with the further introduction of TNT- and watchful-waiting strategies that sometimes trouble the surgeon.
Author Contributions
Conceptualization: A.S., K.-P.T., M.-H.D. and T.L.; Methodology, A.S., K.-P.T., M.-H.D. and T.L.; Software, A.S. and J.K.; Validation, A.S. and H.J.S.; Formal analysis, A.S., D.M., J.K. and A.M.; Investigation, A.S., M.M., P.R., D.M., M.-H.D. and T.L.; Resources, A.S., M.M., P.R., W.S. and T.L.; Data curation, A.S. and T.L.; Writing—original draft, A.S. and T.L.; Writing—review and editing, A.S., M.M., P.R., K.-P.T., W.S., D.M., J.K., A.M., H.J.S., M.-H.D. and T.L.; Visualization, A.S.; Supervision, P.R., K.-P.T., W.S., H.J.S., M.-H.D. and T.L.; Project administration, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Tübingen (149/2012BO2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bębenek, M.; Pudełko, M.; Kryj, M.; Olędzki, J.; Szmeja, J.; Słuszniak, J.; et al. Sphincter Preservation Following Preoperative Radiotherapy for Rectal Cancer: Report of a Randomised Trial Comparing Short-Term Radiotherapy vs. Conventionally Fractionated Radiochemotherapy. Radiother. Oncol. 2004, 72, 15–24. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 21, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Liska, D.; Tang, P.; Shia, J.; Saltz, L.; Goodman, K.; Downey, R.J.; Nash, G.M.; Temple, L.K.; Paty, P.B.; et al. Pulmonary Recurrence Predominates after Combined Modality Therapy for Rectal Cancer: An Original Retrospective Study. Ann. Surg. 2012, 256, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, W.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.M.K.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; Van de Velde, C.J.H. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, B.J.; Kronborg, O.; Fenger, C.; Jørgensen, O.D. A Prospective Randomized Study of Follow-up after Radical Surgery for Colorectal Cancer. Br. J. Surg. 1997, 84, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft—Deutsche Krebshilfe—AWMF). S3-Leitlinie Kolorektales Karzinom, Langversion 2.1; AWMF Registernummer 021/007OL; Deutsche Krebshilfe: Bonn, Germany, 2019; pp. 1–328. [Google Scholar]
- Chang, G.J.; Ikoma, N.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Eng, C.; Das, P.; Kopetz, S.; Messick, C.; Skibber, J.M. Impact of Recurrence and Salvage Surgery on Survival after Multidisciplinary Treatment of Rectal Cancer. J. Clin. Oncol. 2017, 35, 2631. [Google Scholar] [CrossRef]
- Salega, A. Outcome nach neoadjuvanter Therapie beim lokal fortgeschrittenen Rektumkarzinom. Ph.D. Thesis, Eberhard Karl University of Tubingen, Tübingen, Germany, 2022. [Google Scholar] [CrossRef]
- Marr, R.; Birbeck, K.; Garvican, J.; Macklin, C.P.; Tiffin, N.J.; Parsons, W.J.; Dixon, M.F.; Mapstone, N.P.; Sebag-Montefiore, D.; Scott, N.; et al. The Modern Abdominoperineal Excision: The next Challenge after Total Mesorectal Excision. Ann. Surg. 2005, 242, 74–82. [Google Scholar] [CrossRef]
- Valentini, V.; Van Stiphout, R.G.P.M.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Nomograms for Predicting Local Recurrence, Distant Metastases, and Overall Survival for Patients with Locally Advanced Rectal Cancer on the Basis of European Randomized Clinical Trials. J. Clin. Oncol. 2011, 29, 3163–3172. [Google Scholar] [CrossRef]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the Plane of Surgery Achieved on Local Recurrence in Patients with Operable Rectal Cancer: A Prospective Study Using Data from the MRC CR07 and NCIC-CTG CO16 Randomised Clinical Trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; van de Velde, C.J.H.; Marijnen, C.A.M.; van Krieken, J.H.J.M.; Quirke, P. Low Rectal Cancer: A Call for a Change of Approach in Abdominoperineal Resection. J. Clin. Oncol. 2005, 23, 9257–9264. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Kobayashi, H.; Kato, T.; Mori, T.; Mochizuki, H.; Kameoka, S.; Shirouzu, K.; Muto, T. Indication and Benefit of Pelvic Sidewall Dissection for Rectal Cancer. Dis. Colon Rectum 2006, 49, 1663–1672. [Google Scholar] [CrossRef]
- Christou, N.; Meyer, J.; Toso, C.; Ris, F.; Buchs, N.C. Lateral Lymph Node Dissection for Low Rectal Cancer: Is It Necessary? World J. Gastroenterol. 2019, 25, 4294–4299. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Oya, M.; Azekura, K.; Yamaguchi, T.; Muto, T. Incidence and Prognostic Significance of Lateral Lymph Node Metastasis in Patients with Advanced Low Rectal Cancer. J. Br. Surg. 2005, 92, 756–763. [Google Scholar] [CrossRef]
- Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Ito, M.; Kinugasa, Y.; Komori, K.; Ohue, M.; Ota, M.; Akazai, Y.; Shiozawa, M.; et al. Mesorectal Excision with or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212). Ann. Surg. 2017, 266, 201–207. [Google Scholar] [CrossRef]
- Seo, S.I.; Lim, S.-B.; Yoon, Y.S.; Kim, C.W.; Yu, C.S.; Kim, T.W.; Kim, J.H.; Kim, J.C. Comparison of Recurrence Patterns between ≤5 Years and >5 Years after Curative Operations in Colorectal Cancer Patients. J. Surg. Oncol. 2013, 108, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Liu, M.C.; Jian, J.J.M.; Horng, C.F.; Cheng, T.I.; Chen, C.M.; Fang, W.T.; Chung, Y.L. Is Final TNM Staging a Predictor for Survival in Locally Advanced Rectal Cancer after Preoperative Chemoradiation Therapy? Ann. Surg. Oncol. 2007, 14, 2766–2772. [Google Scholar] [CrossRef]
- Quah, H.-M.; Chou, J.F.; Gonen, M.; Shia, J.; Schrag, D.; Saltz, L.B.; Goodman, K.A.; Minsky, B.D.; Wong, W.D.; Weiser, M.R. Pathologic Stage Is Most Prognostic of Disease-Free Survival in Locally Advanced Rectal Cancer Patients after Preoperative Chemoradiation. Cancer 2008, 113, 57–64. [Google Scholar] [CrossRef]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic Resonance Imaging-Detected Tumor Response for Locally Advanced Rectal Cancer Predicts Survival Outcomes: MERCURY Experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef]
- Wibe, A.; Rendedal, P.R.; Svensson, E.; Norstein, J.; Eide, T.J.; Myrvold, H.E.; Søreide, O. Prognostic Significance of the Circumferential Resection Margin Following Total Mesorectal Excision for Rectal Cancer. Br. J. Surg. 2002, 89, 327–334. [Google Scholar] [CrossRef]
- Van Gijn, W.; Van Stiphout, R.G.P.M.; Van de Velde, C.J.H.; Valentini, V.; Lammering, G.; Gambacorta, M.A.; Påhlman, L.; Bujko, K.; Lambin, P. Nomograms to Predict Survival and the Risk for Developing Local or Distant Recurrence in Patients with Rectal Cancer Treated with Optional Short-Term Radiotherapy. Ann. Oncol. 2015, 26, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-Course Radiotherapy Followed by Chemotherapy before Total Mesorectal Excision (TME) versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial; The Lancet Oncology: Cambridge, MA, USA, 2021. [Google Scholar]
- Couwenberg, A.M.; Varvoglis, D.N.; Grieb, B.C.; Marijnen, C.A.M.; Ciombor, K.K.; Guillem, J.G. New Opportunities for Minimizing Toxicity in Rectal Cancer Management. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389558. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.P.H.; et al. Locoregional Failure During and After Short-Course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-Course Chemoradiotherapy and Surgery. Ann. Surg. 2023, 278, e766–e772. [Google Scholar] [CrossRef]
- Solomon, M.J.; Brown, K.G.M.; Koh, C.E.; Lee, P.; Austin, K.K.S.; Masya, L. Lateral Pelvic Compartment Excision during Pelvic Exenteration. Br. J. Surg. 2015, 102, 1710–1717. [Google Scholar] [CrossRef]
- Bhangu, A.; Mohammed Ali, S.; Brown, G.; Nicholls, R.J.; Tekkis, P. Indications and Outcome of Pelvic Exenteration for Locally Advanced Primary and Recurrent Rectal Cancer. Ann. Surg. 2014, 259, 315–322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).