Primary Multi-Systemic Metastases in Osteosarcoma: Presentation, Treatment, and Survival of 83 Patients of the Cooperative Osteosarcoma Study Group
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Initial Diagnostics and Intended Treatment
2.3. Follow-Up and Detection of Recurrence
3. Ethics Approval and Patient Consent
4. Data Collection and Definition of Variables
5. Statistics
6. Results
6.1. Patient and Tumour Characteristics
6.2. Treatment Strategy at Initial Disease Presentation
6.3. Prognostic Factors
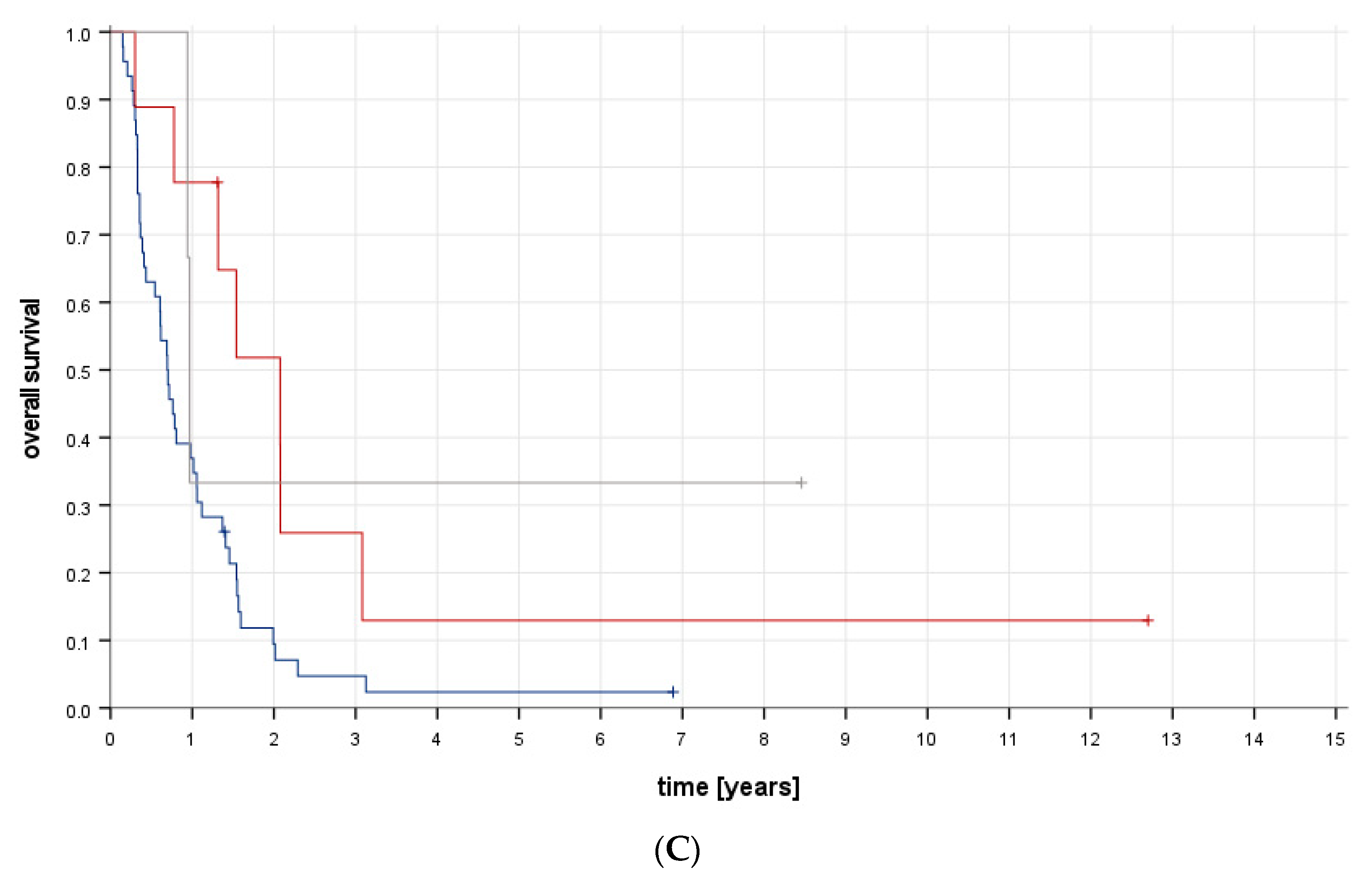
6.4. Survival and Follow-Up
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marko, T.A.; Diessner, B.J.; Spector, L.G. Prevalence of Metastasis at Diagnosis of Osteosarcoma: An International Comparison. Pediatr. Blood Cancer 2016, 63, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Pratt, C.B.; Cain, A.M.; Jones-Wallace, D.J.; Rao, B.N. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: Imaging features. Cancer 1999, 86, 1602–1608. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Longhi, A.; Forni, C.; Zavatta, M.; Versari, M.; Smith, K. High-grade osteosarcoma of the extremity: Differences between localized and metastatic tumors at presentation. J. Pediatr. Hematol. Oncol. 2002, 24, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Cram, P.; Lynch, C.F.; Buckwalter, J.A. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J. Bone Joint Surg. Am. 2013, 95, e89. [Google Scholar] [CrossRef] [PubMed]
- Duchman, K.R.; Gao, Y.; Miller, B.J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015, 39, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Zoubek, A.; Potschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef]
- Meyers, P.A.; Heller, G.; Healey, J.H.; Huvos, A.; Applewhite, A.; Sun, M.; LaQuaglia, M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 1993, 11, 449–453. [Google Scholar] [CrossRef]
- Harris, M.B.; Gieser, P.; Goorin, A.M.; Ayala, A.; Shochat, S.J.; Ferguson, W.S.; Holbrook, T.; Link, M.P. Treatment of metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group Study. J. Clin. Oncol. 1998, 16, 3641–3648. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020, 43, e345–e358. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28352. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21 (Suppl. S7), vii320–vii325. [Google Scholar] [CrossRef] [PubMed]
- Mialou, V.; Philip, T.; Kalifa, C.; Perol, D.; Gentet, J.C.; Marec-Berard, P.; Pacquement, H.; Chastagner, P.; Defaschelles, A.S.; Hartmann, O. Metastatic osteosarcoma at diagnosis: Prognostic factors and long-term outcome--the French pediatric experience. Cancer 2005, 104, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.D.; Warneke, C.L.; Hughes, D.P.; Lally, P.A.; Lally, K.P.; Hayes-Jordan, A.A.; Austin, M.T. Effect of concurrent metastatic disease on survival in children and adolescents undergoing lung resection for metastatic osteosarcoma. J. Pediatr. Surg. 2015, 50, 157–160; discussion 160. [Google Scholar] [CrossRef] [PubMed]
- Salzer-Kuntschik, M.; Brand, G.; Delling, G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe 1983, 4, 135–141. [Google Scholar] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Bacci, G.; Fabbri, N.; Balladelli, A.; Forni, C.; Palmerini, E.; Picci, P. Treatment and prognosis for synchronous multifocal osteosarcoma in 42 patients. J. Bone Joint Surg. Br. 2006, 88, 1071–1075. [Google Scholar] [CrossRef]
- Bacci, G.; Dallari, D.; Battistini, A.; Orlandi, M.; Ferrari, S.; Avella, M.; Picci, P.; Casadei, R.; Ruggieri, P. The prognostic value of serum alkaline phosphatase in osteosarcoma of the limbs. Chir. Organi Mov. 1992, 77, 171–180. [Google Scholar]
- Basoli, S.; Cosentino, M.; Traversari, M.; Manfrini, M.; Tsukamoto, S.; Mavrogenis, A.F.; Bordini, B.; Donati, D.M.; Errani, C. The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities. Curr. Oncol. 2023, 30, 7043–7054. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.C.; Bertie, J.; Rodseth, R.; Sartorius, B.; Ferreira, N. Pre-treatment serum lactate dehydrogenase and alkaline phosphatase as predictors of metastases in extremity osteosarcoma. J. Bone Oncol. 2015, 4, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Saoud, C.; Ali, S.Z. Metastatic sarcomas to pleural effusion: A 10-year large tertiary care center experience with emphasis on clinical features and cytomorphologic characteristics. J. Am. Soc. Cytopathol. 2023, 12, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Buddingh, E.P.; Anninga, J.K.; Versteegh, M.I.; Taminiau, A.H.; Egeler, R.M.; van Rijswijk, C.S.; Hogendoorn, P.C.; Lankester, A.C.; Gelderblom, H. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr. Blood Cancer 2010, 54, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Miyahara, R.; Bando, T.; Okubo, K.; Watanabe, K.; Nakayama, T.; Toguchida, J.; Date, H. Prognostic factors of pulmonary metastasectomy for osteosarcomas of the extremities. Eur. J. Cardiothorac. Surg. 2008, 34, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Kanazawa, Y.; Abdel-Wanis, M.E.; Asada, N.; Abe, S.; Isu, K.; Sugita, T.; Tomita, K. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: The Japanese Musculoskeletal Oncology Group study. J. Clin. Oncol. 2002, 20, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Akiyama, T.; Fukushima, T.; Iwata, S.; Takeshita, K.; Kawai, A.; Tanaka, S.; Kobayashi, H. Surgical resection of the primary lesion for osteosarcoma patients with metastasis at initial diagnosis. Jpn. J. Clin. Oncol. 2021, 51, 416–423. [Google Scholar] [CrossRef]
- Kayton, M.L.; Huvos, A.G.; Casher, J.; Abramson, S.J.; Rosen, N.S.; Wexler, L.H.; Meyers, P.; LaQuaglia, M.P. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J. Pediatr. Surg. 2006, 41, 200–206; discussion 200–206. [Google Scholar] [CrossRef]
- Ciccarese, F.; Bazzocchi, A.; Ciminari, R.; Righi, A.; Rocca, M.; Rimondi, E.; Picci, P.; Bacchi Reggiani, M.L.; Albisinni, U.; Zompatori, M.; et al. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur. J. Radiol. 2015, 84, 2679–2685. [Google Scholar] [CrossRef]
- Gao, E.; Li, Y.; Zhao, W.; Zhao, T.; Guo, X.; He, W.; Wu, W.; Zhao, Y.; Yang, Y. Necessity of thoracotomy in pulmonary metastasis of osteosarcoma. J. Thorac. Dis. 2019, 11, 3578–3583. [Google Scholar] [CrossRef]
- Su, W.T.; Chewning, J.; Abramson, S.; Rosen, N.; Gholizadeh, M.; Healey, J.; Meyers, P.; La Quaglia, M.P. Surgical management and outcome of osteosarcoma patients with unilateral pulmonary metastases. J. Pediatr. Surg. 2004, 39, 418–423; discussion 418–423. [Google Scholar] [CrossRef] [PubMed]
- Heaton, T.E.; Hammond, W.J.; Farber, B.A.; Pallos, V.; Meyers, P.A.; Chou, A.J.; Price, A.P.; LaQuaglia, M.P. A 20-year retrospective analysis of CT-based pre-operative identification of pulmonary metastases in patients with osteosarcoma: A single-center review. J. Pediatr. Surg. 2017, 52, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Carrle, D.; Bielack, S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat. Res. 2009, 152, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Ciernik, I.F.; Niemierko, A.; Harmon, D.C.; Kobayashi, W.; Chen, Y.L.; Yock, T.I.; Ebb, D.H.; Choy, E.; Raskin, K.A.; Liebsch, N.; et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 2011, 117, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Seidensaal, K.; Mattke, M.; Haufe, S.; Rathke, H.; Haberkorn, U.; Bougatf, N.; Kudak, A.; Blattmann, C.; Oertel, S.; Kirchner, M.; et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother. Oncol. 2021, 159, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Bruland, O.; Cassoni, A.; Schomberg, P.; Bielack, S. The role of radiotherapy in oseosarcoma. Pediatr. Adolesc. Osteosarcoma 2009, 152, 147–164. [Google Scholar] [CrossRef]
- Matsunobu, A.; Imai, R.; Kamada, T.; Imaizumi, T.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Tatezaki, S.; Working Group for Bone; et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012, 118, 4555–4563. [Google Scholar] [CrossRef]
- Zhang, W.; Tanaka, M.; Sugimoto, Y.; Takigawa, T.; Ozaki, T. Carbon-ion radiotherapy of spinal osteosarcoma with long-term follow. Eur. Spine J. 2016, 25 (Suppl. S1), 113–117. [Google Scholar] [CrossRef]
- Anderson, P.M.; Subbiah, V.; Rohren, E. Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: Samarium-153-EDTMP and radium-223. Adv. Exp. Med. Biol. 2014, 804, 291–304. [Google Scholar] [CrossRef]
- Berger, M.; Grignani, G.; Giostra, A.; Ferrari, S.; Ferraresi, V.; Tamburini, A.; Cefalo, G.; Carnevale-Schianca, F.; Vassallo, E.; Picci, P.; et al. 153Samarium-EDTMP administration followed by hematopoietic stem cell support for bone metastases in osteosarcoma patients. Ann. Oncol. 2012, 23, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Mathoulin-Pelissier, S.; Penel, N.; Piperno-Neumann, S.; Bompas, E.; Chevreau, C.; Duffaud, F.; Entz-Werle, N.; Saada, E.; et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Reed, D.; Keedy, V.; et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Dileo, P.; Asaftei, S.D.; D’Ambrosio, L.; Pignochino, Y.; Mercuri, M.; Picci, P.; Fagioli, F.; Casali, P.G.; et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann. Oncol. 2012, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015, 16, 98–107. [Google Scholar] [CrossRef]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef]
- Rothzerg, E.; Pfaff, A.L.; Koks, S. Innovative approaches for treatment of osteosarcoma. Exp. Biol. Med. 2022, 247, 310–316. [Google Scholar] [CrossRef]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef]
- Pilavaki, P.; Gahanbani Ardakani, A.; Gikas, P.; Constantinidou, A. Osteosarcoma: Current Concepts and Evolutions in Management Principles. J. Clin. Med. 2023, 12, 2785. [Google Scholar] [CrossRef]
- Gazouli, I.; Kyriazoglou, A.; Kotsantis, I.; Anastasiou, M.; Pantazopoulos, A.; Prevezanou, M.; Chatzidakis, I.; Kavourakis, G.; Economopoulou, P.; Kontogeorgakos, V.; et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers 2021, 13, 1757. [Google Scholar] [CrossRef] [PubMed]


| Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | % | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | ||||
| All eligible patients | 83 | 100 | 0.540 | 0.056 | 0.232 | 0.049 | 0.087 | 0.033 | ||
| Age at initial diagnosis, years | ||||||||||
| <14.8 | 41 | 49 | 0.455 | 0.079 | 0.278 | 0.071 | 0.111 | 0.052 | 0.960 | |
| ≥14.8 | 42 | 51 | 0.627 | 0.076 | 0.177 | 0.064 | 0.059 | 0.040 | ||
| Sex | ||||||||||
| Female | 40 | 48 | 0.479 | 0.081 | 0.160 | 0.060 | 0.096 | 0.050 | 0.311 | |
| Male | 43 | 52 | 0.597 | 0.076 | 0.304 | 0.074 | 0.083 | 0.046 | ||
| Duration of pain until initial diagnosis | ||||||||||
| <47 days | 35 | 50 | 0.460 | 0.087 | 0.246 | 0.075 | 0.140 | 0.063 | 0.773 | |
| ≥47 days | 35 | 50 | 0.591 | 0.084 | 0.217 | 0.076 | 0.072 | 0.049 | ||
| No pain/unknown | 13 | |||||||||
| Duration of swelling until initial diagnosis | ||||||||||
| <38 days | 27 | 50 | 0.622 | 0.095 | 0.311 | 0.091 | 0.133 | 0.070 | 0.559 | |
| ≥38 days | 27 | 50 | 0.542 | 0.098 | 0.152 | 0.079 | 0.051 | 0.049 | ||
| No swelling/unknown | 29 | |||||||||
| Pathological fracture at initial diagnosis | ||||||||||
| No | 70 | 89 | 0.537 | 0.060 | 0.248 | 0.053 | 0.099 | 0.038 | 0.402 | |
| Yes | 9 | 11 | 0.292 | 0.173 | 0.146 | 0.135 | 0.000 | 0.000 | ||
| Unknown | 4 | |||||||||
| Alkaline phosphatase before start of chemotherapy | ||||||||||
| Normal | 12 | 18 | 0.750 | 0.125 | 0.536 | 0.156 | 0.214 | 0.133 | 0.019 | |
| Elevated | 56 | 82 | 0.456 | 0.068 | 0.152 | 0.049 | 0.057 | 0.032 | ||
| Unknown | 15 | |||||||||
| Lactate dehydrogenase before start of chemotherapy | ||||||||||
| Normal | 14 | 22 | 0.929 | 0.069 | 0.314 | 0.129 | 0.079 | 0.075 | 0.078 | |
| Elevated | 50 | 78 | 0.388 | 0.071 | 0.186 | 0.058 | 0.093 | 0.044 | ||
| Unknown | 19 | |||||||||
| Primary tumour site | ||||||||||
| Extremity | 72 | 88 | 0.503 | 0.060 | 0.241 | 0.053 | 0.069 | 0.033 | 0.525 | |
| Trunk | 10 | 12 | 0.778 | 0.139 | 0.111 | 0.105 | 0.111 | 0.105 | ||
| Head and neck | 1 | 1.000 | 1.000 | 1.000 | ||||||
| Tumour size at initial diagnosis (limb only) | ||||||||||
| Small (<1/3 of the involved bone’s length) | 23 | 42 | 0.595 | 0.104 | 0.250 | 0.095 | 0.062 | 0.059 | 0.364 | |
| Large (≥1/3 of the involved bone’s length) | 32 | 58 | 0.488 | 0.090 | 0.199 | 0.076 | 0.079 | 0.053 | ||
| Other site/unknown | 28 | |||||||||
| Number of metastatic sites involved at initial diagnosis | ||||||||||
| Two | 57 | 70 | 0.529 | 0.066 | 0.232 | 0.058 | 0.077 | 0.037 | 0.839 | |
| Three | 25 | 30 | 0.438 | 0.103 | 0.246 | 0.093 | 0.123 | 0.077 | ||
| Unknown | 1 | |||||||||
| Metastatic sites involved at initial diagnosis | ||||||||||
| Lung and bone | 42 | 74 | 0.468 | 0.078 | 0.133 | 0.055 | 0.027 | 0.026 | 0.005 | |
| Lung and other | 13 | 23 | 0.923 | 0.074 | 0.508 | 0.144 | 0.169 | 0.109 | ||
| Bone and other | 2 | 4 | 1.000 | 0.500 | 0.354 | 0.500 | 0.354 | |||
| Other and other | 0 | 0 | ||||||||
| Three sites/unknown | 26 | |||||||||
| Pulmonary metastases at initial diagnosis | ||||||||||
| No | 2 | 2 | 1.000 | 0.500 | 0.354 | 0.500 | 0.354 | 0.428 | ||
| Yes | 81 | 98 | 0.529 | 0.056 | 0.225 | 0.049 | 0.075 | 0.032 | ||
| Laterality of pulmonary metastases | ||||||||||
| Unilateral | 4 | 5 | 0.750 | 0.217 | 0.500 | 0.250 | 0.000 | 0.000 | 0.637 | |
| Bilateral | 71 | 95 | 0.519 | 0.061 | 0.215 | 0.052 | 0.083 | 0.035 | ||
| None/unknown | 8 | |||||||||
| Pleural effusion at initial diagnosis | ||||||||||
| No | 35 | 80 | 0.441 | 0.085 | 0.173 | 0.069 | 0.069 | 0.047 | 0.010 | |
| Yes | 9 | 20 | 0.222 | 0.139 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Unknown | 39 | |||||||||
| Bone metastases at initial diagnosis | ||||||||||
| No | 13 | 16 | 0.923 | 0.074 | 0.508 | 0.144 | 0.169 | 0.109 | 0.010 | |
| Yes | 69 | 84 | 0.473 | 0.061 | 0.182 | 0.049 | 0.073 | 0.034 | ||
| Unknown | 1 | |||||||||
| Number of bone metastases at initial diagnosis | ||||||||||
| One | 14 | 21 | 0.701 | 0.126 | 0.390 | 0.136 | 0.156 | 0.101 | 0.028 | |
| At least two | 53 | 79 | 0.393 | 0.068 | 0.133 | 0.051 | 0.053 | 0.035 | ||
| None/unknown | 16 | |||||||||
| Other metastases at initial diagnosis | ||||||||||
| No | 42 | 51 | 0.468 | 0.078 | 0.133 | 0.055 | 0.027 | 0.026 | 0.018 | |
| Yes | 41 | 49 | 0.617 | 0.078 | 0.338 | 0.078 | 0.154 | 0.062 | ||
| Number of other metastases at initial diagnosis | ||||||||||
| One | 17 | 43 | 0.765 | 0.103 | 0.499 | 0.128 | 0.166 | 0.105 | 0.476 | |
| At least two | 23 | 58 | 0.525 | 0.109 | 0.239 | 0.093 | 0.143 | 0.077 | ||
| None/unknown | 43 | |||||||||
| Complete surgical resection assessed as feasible at initial diagnosis | ||||||||||
| No | 45 | 74 | 0.387 | 0.073 | 0.137 | 0.052 | 0.091 | 0.043 | 0.010 | |
| Yes | 16 | 26 | 0.938 | 0.061 | 0.554 | 0.138 | 0.092 | 0.087 | ||
| Unknown | 22 | |||||||||
| Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | % | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | ||||
| Resection of the primary tumour | ||||||||||
| None or macroscopically incomplete | 43 | 55 | 0.326 | 0.071 | 0.051 | 0.035 | 0.026 | 0.025 | <0.001 | |
| Macroscopically complete, microscopically incomplete | 3 | 4 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||
| Microscopically complete | 32 | 41 | 0.775 | 0.075 | 0.495 | 0.093 | 0.152 | 0.069 | ||
| Unknown | 5 | |||||||||
| Response to first-line chemotherapy † | ||||||||||
| Good (grades 1–3) | 11 | 42 | 1.000 | 0.727 | 0.134 | 0.364 | 0.145 | 0.044 | ||
| Poor (grades 4–6) | 15 | 58 | 0.786 | 0.110 | 0.397 | 0.136 | 0.000 | 0.000 | ||
| Unknown/primary or no tumour resection | 57 | |||||||||
| Macroscopically complete resection of all metastases | ||||||||||
| No | 72 | 89 | 0.481 | 0.059 | 0.135 | 0.042 | 0.068 | 0.032 | 0.001 | |
| Yes | 9 | 11 | 1.000 | 1.000 | 0.250 | 0.153 | ||||
| Unknown | 2 | |||||||||
| Macroscopically complete resection of all pulmonary metastases | ||||||||||
| No | 68 | 87 | 0.450 | 0.061 | 0.111 | 0.041 | 0.056 | 0.030 | <0.001 | |
| Yes | 10 | 13 | 1.000 | 1.000 | 0.222 | 0.139 | ||||
| No pulmonary metastases/unknown | 5 | |||||||||
| Macroscopically complete resection of all bone metastases | ||||||||||
| No | 57 | 86 | 0.424 | 0.065 | 0.129 | 0.046 | 0.064 | 0.035 | 0.062 | |
| Yes | 9 | 14 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| No bone metastases/unknown | 17 | |||||||||
| Macroscopically complete resection of all other metastases | ||||||||||
| No | 25 | 68 | 0.400 | 0.098 | 0.040 | 0.039 | 0.000 | 0.000 | <0.001 | |
| Yes | 12 | 32 | 1.000 | 0.900 | 0.095 | 0.450 | 0.166 | |||
| No other metastases/unknown | 36 | |||||||||
| Radiotherapy | ||||||||||
| No | 46 | 63 | 0.478 | 0.074 | 0.205 | 0.061 | 0.046 | 0.031 | 0.352 | |
| Yes | 27 | 37 | 0.593 | 0.095 | 0.244 | 0.085 | 0.163 | 0.074 | ||
| Unknown | 10 | |||||||||
| Therapeutic radioactive medication | ||||||||||
| No | 63 | 88 | 0.551 | 0.063 | 0.273 | 0.058 | 0.109 | 0.042 | 0.011 | |
| Yes | 9 | 13 | 0.222 | 0.139 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Unknown | 11 | |||||||||
| Duration until start of chemotherapy, days | ||||||||||
| <21 | 57 | 69 | 0.500 | 0.067 | 0.206 | 0.055 | 0.056 | 0.031 | 0.289 | |
| ≥21 | 26 | 31 | 0.636 | 0.097 | 0.300 | 0.099 | 0.180 | 0.089 | ||
| Tumour progression under first-line treatment | ||||||||||
| No | 18 | 27 | 0.941 | 0.057 | 0.627 | 0.121 | 0.314 | 0.116 | <0.001 | |
| Yes | 48 | 73 | 0.396 | 0.071 | 0.090 | 0.043 | 0.000 | 0.000 | ||
| Unknown | 17 | |||||||||
| Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | |||
| All eligible patients with bone metastases | 69 | 0.473 | 0.061 | 0.182 | 0.049 | 0.073 | 0.034 | ||
| Complete local treatment of all bone metastases | |||||||||
| No | 46 | 0.370 | 0.071 | 0.095 | 0.045 | 0.024 | 0.023 | 0.010 | |
| Yes (radiotherapy and/or surgery) | 12 | 0.667 | 0.136 | 0.476 | 0.150 | 0.190 | 0.120 | ||
| Unknown | 11 | ||||||||
| Type of local treatment of all bone metastases | |||||||||
| No | 46 | 0.370 | 0.071 | 0.095 | 0.045 | 0.024 | 0.023 | 0.037 | |
| Surgery only | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| Radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Unknown | 11 | ||||||||
| Type of local treatment of all bone metastases | |||||||||
| Surgery only | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | 0.913 | |
| Radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Not all bone metastases treated locally/unknown | 47 | ||||||||
| Macroscopically complete resection of all bone metastases | |||||||||
| No | 57 | 0.424 | 0.065 | 0.129 | 0.046 | 0.064 | 0.035 | 0.062 | |
| Yes | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| Unknown | 3 | ||||||||
| Radiotherapy as part of local treatment of all unresected bone metastases | |||||||||
| No | 55 | 0.436 | 0.067 | 0.163 | 0.052 | 0.041 | 0.028 | 0.250 | |
| Yes, radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Unknown | 11 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mettmann, V.L.; Blattmann, C.; Friedel, G.; Harrabi, S.; von Kalle, T.; Kager, L.; Kevric, M.; Kühne, T.; Nathrath, M.; Sorg, B.; et al. Primary Multi-Systemic Metastases in Osteosarcoma: Presentation, Treatment, and Survival of 83 Patients of the Cooperative Osteosarcoma Study Group. Cancers 2024, 16, 275. https://doi.org/10.3390/cancers16020275
Mettmann VL, Blattmann C, Friedel G, Harrabi S, von Kalle T, Kager L, Kevric M, Kühne T, Nathrath M, Sorg B, et al. Primary Multi-Systemic Metastases in Osteosarcoma: Presentation, Treatment, and Survival of 83 Patients of the Cooperative Osteosarcoma Study Group. Cancers. 2024; 16(2):275. https://doi.org/10.3390/cancers16020275
Chicago/Turabian StyleMettmann, Vanessa L., Claudia Blattmann, Godehard Friedel, Semi Harrabi, Thekla von Kalle, Leo Kager, Matthias Kevric, Thomas Kühne, Michaela Nathrath, Benjamin Sorg, and et al. 2024. "Primary Multi-Systemic Metastases in Osteosarcoma: Presentation, Treatment, and Survival of 83 Patients of the Cooperative Osteosarcoma Study Group" Cancers 16, no. 2: 275. https://doi.org/10.3390/cancers16020275
APA StyleMettmann, V. L., Blattmann, C., Friedel, G., Harrabi, S., von Kalle, T., Kager, L., Kevric, M., Kühne, T., Nathrath, M., Sorg, B., Werner, M., Bielack, S. S., & Hecker-Nolting, S. (2024). Primary Multi-Systemic Metastases in Osteosarcoma: Presentation, Treatment, and Survival of 83 Patients of the Cooperative Osteosarcoma Study Group. Cancers, 16(2), 275. https://doi.org/10.3390/cancers16020275







