Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Tumor Characteristics
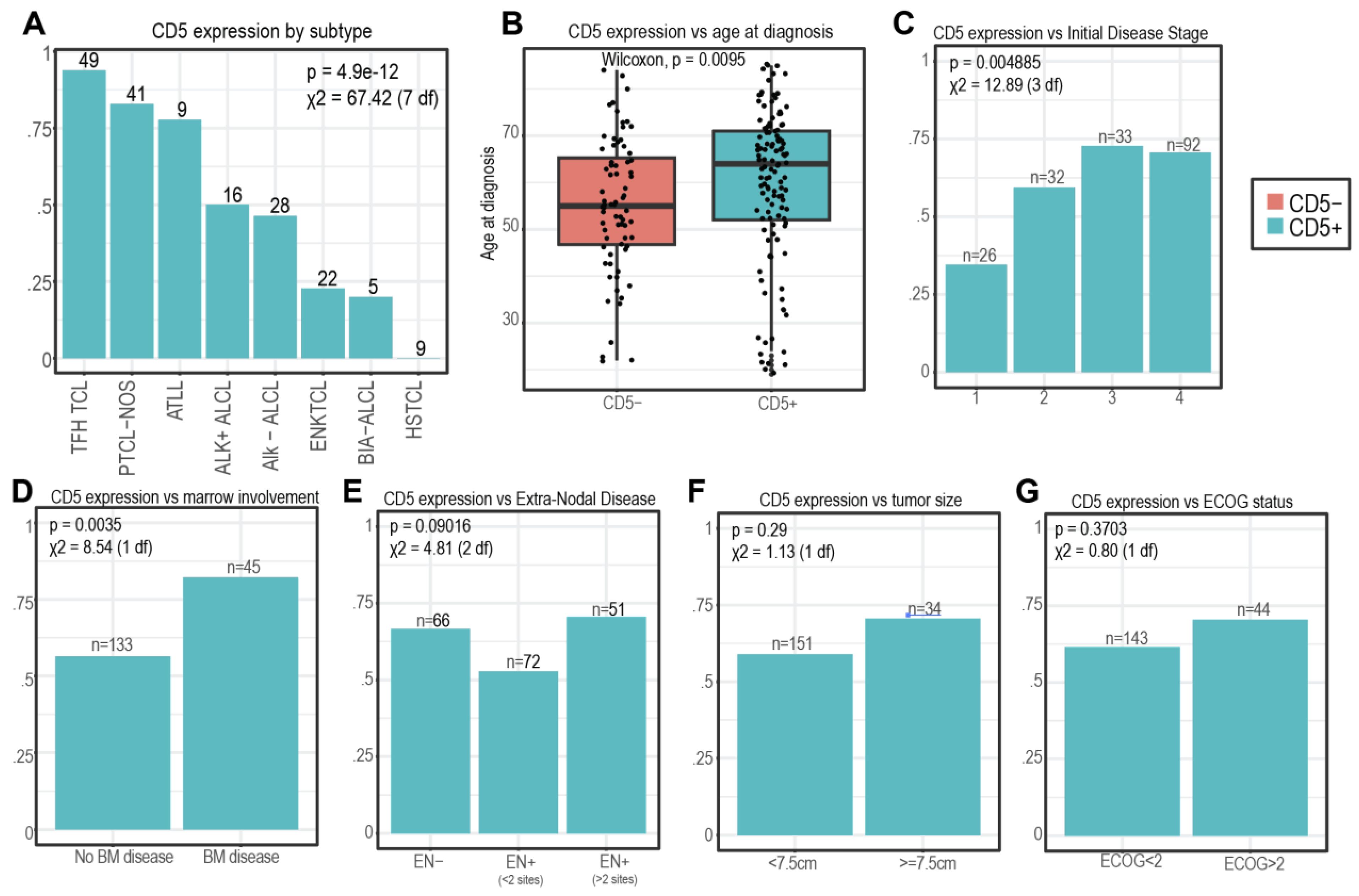
3.2. CD5 Expression and Patient/Disease Characteristics
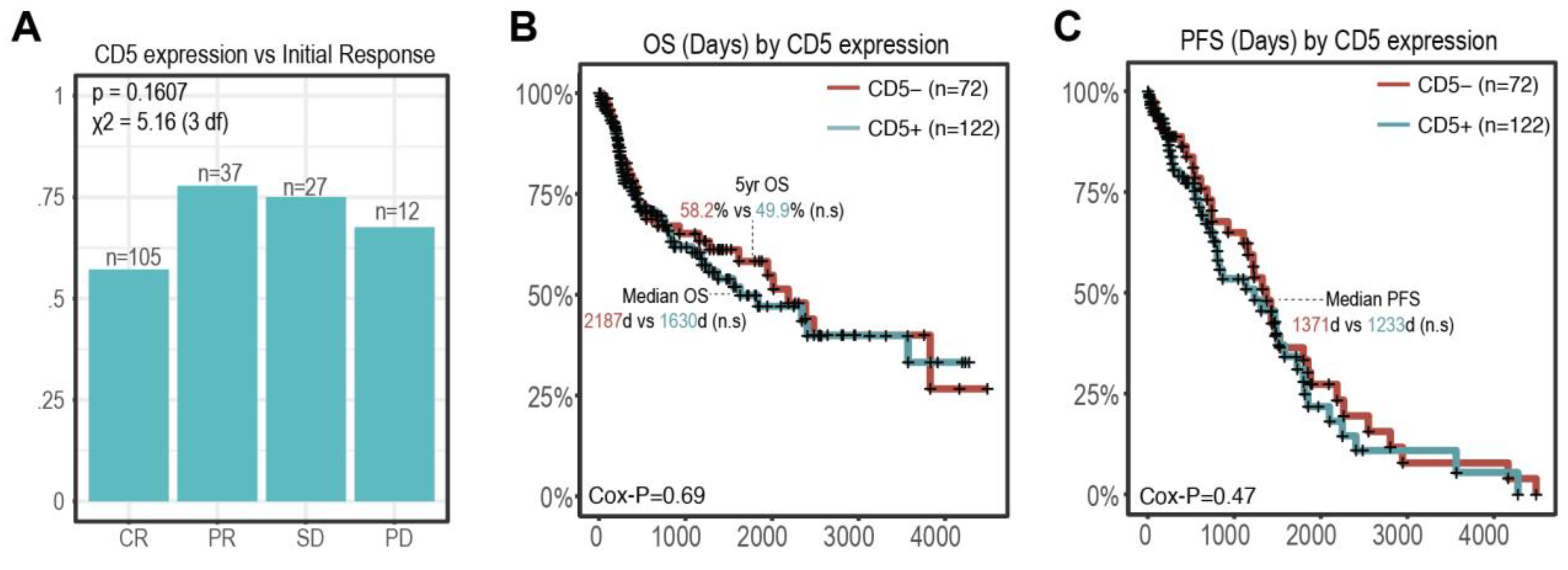
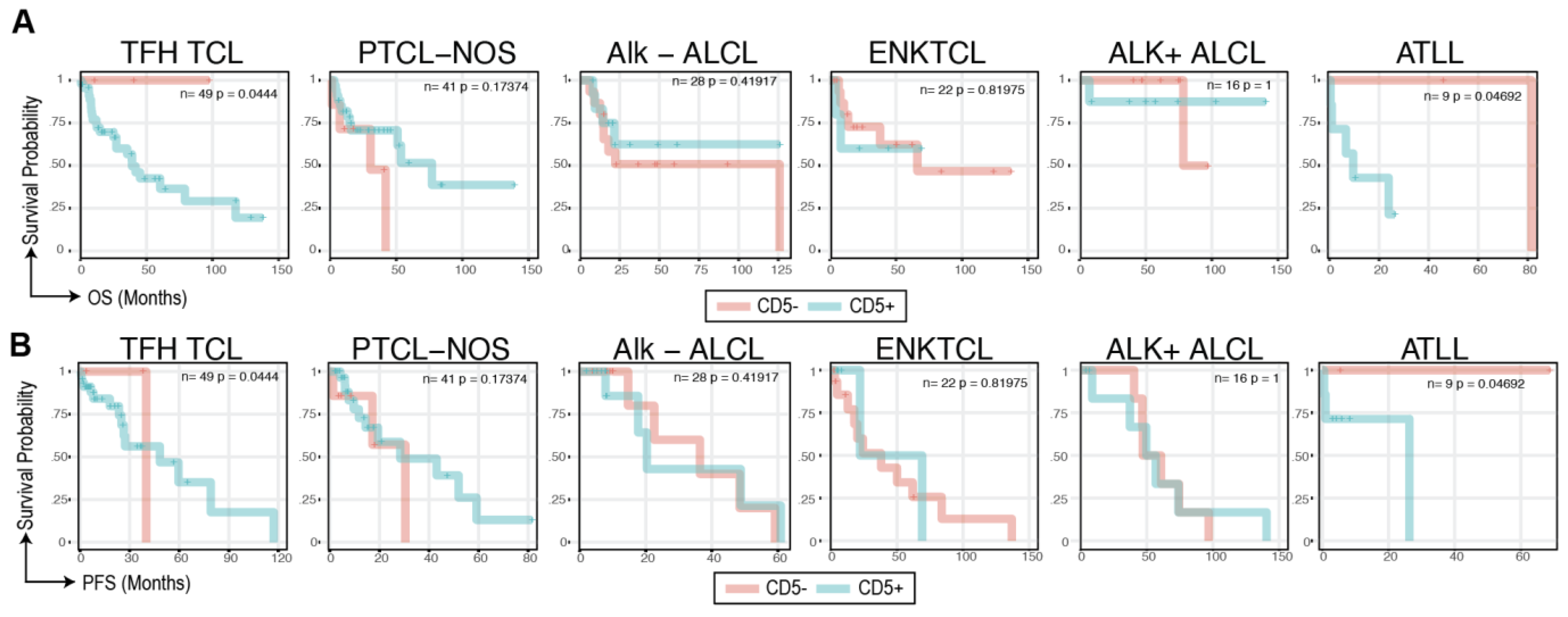
3.3. CD5 Status and Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchi, E.; O’Connor, O.A. The rapidly changing landscape in mature T-cell lymphoma (MTCL) biology and management. CA A Cancer J. Clin. 2020, 70, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Mak, V.; Hamm, J.; Chhanabhai, M.; Shenkier, T.; Klasa, R.; Sehn, L.H.; Villa, D.; Gascoyne, R.D.; Connors, J.M.; Savage, K.J. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and Rare Long-Term Survivors. J. Clin. Oncol. 2013, 31, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Li, W. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. In Leukemia; Li, W., Ed.; Exon Publications: Brisbane City, Australia, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK586208/ (accessed on 24 July 2024).
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Tarakhovsky, A.; Kanner, S.B.; Hombach, J.; Ledbetter, J.A.; Müller, W.; Killeen, N.; Rajewsky, K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 1995, 269, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Soldevila, G.; Raman, C.; Lozano, F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr. Opin. Immunol. 2011, 23, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Taher, T.E.; Debant, M.; Burgos, M.; Melayah, S.; Berthou, C.; Parikh, K.; Pers, J.-O.; Luque-Paz, D.; Chiocchia, G.; et al. CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes. Cell. Mol. Immunol. 2018, 15, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.M.T.; Johnson, D.K.; Weber, K.S. T Cell Calcium Signaling Regulation by the Co-Receptor CD5. Int. J. Mol. Sci. 2018, 19, 1295. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef]
- Taher, T.E.; Bystrom, J.; Mignen, O.; Pers, J.O.; Renaudineau, Y.; Mageed, R.A. CD5 and B lymphocyte responses: Multifaceted effects through multitudes of pathways and channels. Cell. Mol. Immunol. 2020, 17, 1201–1203. [Google Scholar] [CrossRef]
- Ceuppens, J.L.; Baroja, M.L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J. Immunol. 1986, 137, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, P.; Ceuppens, J.L. Immobilized anti-CD5 together with prolonged activation of protein kinase C induce interleukin 2-dependent T cell growth: Evidence for signal transduction through CD5. Eur. J. Immunol. 1991, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Voisinne, G.; García-Blesa, A.; Chaoui, K.; Fiore, F.; Bergot, E.; Girard, L.; Malissen, M.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Malissen, B.; et al. Co-recruitment analysis of the CBL and CBLB signalosomes in primary T cells identifies CD5 as a key regulator of TCR-induced ubiquitylation. Mol. Syst. Biol. 2016, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Brossard, C.; Semichon, M.; Trautmann, A.; Bismuth, G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J. Immunol. 2003, 170, 4623–4629. [Google Scholar] [CrossRef]
- Peña-Rossi, C.; Zuckerman, L.A.; Strong, J.; Kwan, J.; Ferris, W.; Chan, S.; Tarakhovsky, A.; Beyers, A.D.; Killeen, N. Negative regulation of CD4 lineage development and responses by CD5. J. Immunol. 1999, 163, 6494–6501. [Google Scholar] [CrossRef]
- Tabbekh, M.; Mokrani-Hammani, M.; Bismuth, G.; Mami-Chouaib, F. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2013, 2, e22841. [Google Scholar] [CrossRef]
- Voisinne, G.; Gonzalez de Peredo, A.; Roncagalli, R. CD5, an Undercover Regulator of TCR Signaling. Front. Immunol. 2018, 9, 2900. [Google Scholar] [CrossRef]
- McGuire, D.J.; Rowse, A.L.; Li, H.; Peng, B.J.; Sestero, C.M.; Cashman, K.S.; De Sarno, P.; Raman, C. CD5 enhances Th17-cell differentiation by regulating IFN-γ response and RORγt localization. Eur. J. Immunol. 2014, 44, 1137–1142. [Google Scholar] [CrossRef]
- Durani, U.; Ansell, S.M. CD5+ diffuse large B-cell lymphoma: A narrative review. Leuk Lymphoma 2021, 62, 3078–3086. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, W.; Li, F. De Novo CD5+ Diffuse Large B-Cell Lymphoma: Biology, Mechanism, and Treatment Advances. Clin. Lymphoma Myeloma Leuk. 2020, 20, e782–e790. [Google Scholar] [CrossRef]
- Cabeçadas, J.; Nava, V.E.; Ascensao, J.L.; Gomes da Silva, M. How to Diagnose and Treat CD5-Positive Lymphomas Involving the Spleen. Curr. Oncol. 2021, 28, 4611–4633. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Xu, J.; Chen, C.; Vincent, T.; Pölönen, P.; Hu, J.; Yoshimura, S.; Yu, W.; Sussman, J.; Chen, C.H.; et al. Identification and targeting of treatment resistant progenitor populations in T-cell Acute Lymphoblastic Leukemia. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Demir, C.; Kara, E.; Ekinci, Ö.; Ebinç, S. Clinical and Laboratory Features of CD5-Negative Chronic Lymphocytic Leukemia. Med. Sci. Monit. 2017, 23, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.R.; Guadalupe, E.; Volkheimer, A.; Moore, J.O.; Brice Weinberg, J. Clinical outcomes in chronic lymphocytic leukaemia associated with expression of CD5, a negative regulator of B-cell receptor signalling. Br. J. Haematol. 2018, 183, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lin, P.; Saksena, A.; Xu, J.; Wang, M.; Romaguera, J.; Yin, C.C.; Medeiros, L.J.; Li, S. CD5-negative Mantle Cell Lymphoma: Clinicopathologic Correlations and Outcome in 58 Patients. Am. J. Surg. Pathol. 2019, 43, 1052–1060. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Zuo, Z.; Hong, M.; Lin, P.; Li, S.; Konoplev, S.; Wang, Z.; Khoury, J.D.; Young, K.H.; et al. CD5-positive follicular lymphoma: Clinicopathologic correlations and outcome in 88 cases. Mod. Pathol. 2015, 28, 787–798. [Google Scholar] [CrossRef]
- Miyoshi, H.; Sato, K.; Yoshida, M.; Kimura, Y.; Kiyasu, J.; Ichikawa, A.; Ishibashi, Y.; Arakawa, F.; Nakamura, Y.; Nakashima, S.; et al. CD5-positive follicular lymphoma characterized by CD25, MUM1, low frequency of t (14; 18) and poor prognosis. Pathol. Int. 2014, 64, 95–103. [Google Scholar] [CrossRef]
- Mayson, E.; Saverimuttu, J.; Cartwright, K. CD5-positive follicular lymphoma: Prognostic significance of this aberrant marker? Intern. Med. J. 2014, 44, 417–422. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Imai, H.; Wakabayashi, M.; Sawada, T.; Ichikawa, K.; Komatsu, N.; Noguchi, M. CD5-positive follicular lymphoma: A case report and literature review. Intern. Med. 2011, 50, 899–904. [Google Scholar] [CrossRef][Green Version]
- Xia, Y.; Ge, J.; Sun, Z.; Nan, F.; Wan, W.; Xu, D.; Zhang, M.; Fu, X. CD5-positive marginal zone lymphoma: Clinicopathological features and survival outcomes. Leuk. Res. 2022, 117, 106840. [Google Scholar] [CrossRef]
- Ghione, P.; Bantilan, K.S.; Joffe, E.; Palomba, M.L.; Noy, A.; Caron, P.; Hamlin, P.; Kumar, A.; Matasar, M.; Owens, C.; et al. CD5 expression in marginal zone lymphoma does not predict inferior outcome and has similarities to indolent lymphomas. Blood Neoplasia 2024, 1, 100031. [Google Scholar] [CrossRef]
- Ma, D.; Ma, Y.; Ma, Y.; Liu, J.; Gu, Y.; Liu, N.; Xiang, C.; Liu, H.; Sang, W. Molecular subtyping of CD5+ diffuse large B-cell lymphoma based on DNA-targeted sequencing and Lymph2Cx. Front. Oncol. 2022, 12, 941347. [Google Scholar] [CrossRef] [PubMed]
- Nato, Y.; Miyazaki, K.; Maruyama, D.; Takahashi, H.; Sunami, K.; Murakami, S.; Negoro, E.; Miyazawa, Y.; Choi, I.; Okada, T.; et al. Survival and CNS Relapse in Patients with CD5-Positive Diffuse Large B-Cell Lymphoma: A Multi-Institutional Observational Study in Japan. Blood 2023, 142, 1762. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Zhang, L.; Li, X.; Fu, X.; Wang, X.; Wu, J.; Sun, Z.; Kong, F.; Ren, L.; et al. Prognostic analysis of CD5 expression in double-hit diffuse large B-cell lymphoma and effectiveness comparison in patients treated with dose-adjusted EPOCH plus rituximab/R-CHOP regimens. Blood Lymphat. Cancer 2019, 9, 33–43. [Google Scholar] [CrossRef]
- Reimer, P.; Rüdiger, T.; Geissinger, E.; Weissinger, F.; Nerl, C.; Schmitz, N.; Engert, A.; Einsele, H.; Müller-Hermelink, H.K.; Wilhelm, M. Autologous Stem-Cell Transplantation as First-Line Therapy in Peripheral T-Cell Lymphomas: Results of a Prospective Multicenter Study. JCO 2009, 27, 106–113. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician’s choice in cutaneous T-cell lymphoma: Final data. Blood Adv. 2021, 5, 5098–5106. [Google Scholar] [CrossRef]
- Dearden, C.E.; Khot, A.; Else, M.; Hamblin, M.; Grand, E.; Roy, A.; Hewamana, S.; Matutes, E.; Catovsky, D. Alemtuzumab therapy in T-cell prolymphocytic leukemia: Comparing efficacy in a series treated intravenously and a study piloting the subcutaneous route. Blood 2011, 118, 5799–5802. [Google Scholar] [CrossRef]
- Foss, F.M.; Zinzani, P.L.; Vose, J.M.; Gascoyne, R.D.; Rosen, S.T.; Tobinai, K. Peripheral T-cell lymphoma. Blood 2011, 117, 6756–6767. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.C.; Rouce, R.H.; Wu, M.J.; Wang, T.; Ma, R.; Zhang, H.; Mehta, B.; Lapteva, N.; Mei, Z.; Smith, T.S.; et al. Antitumor efficacy and safety of unedited autologous CD5.CAR T cells in relapsed/refractory mature T-cell lymphomas. Blood 2024, 143, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tan, Y.; Shan, L.; Deng, B.; Ling, Z.; Song, W.; Feng, X.; Hu, G. Phase I study of donor-derived CD5 CAR T cells in patients with relapsed or refractory T-cell acute lymphoblastic leukemia. JCO 2022, 40 (Suppl. 16), 7028. [Google Scholar] [CrossRef]
- Alotaibi, F.M.; Min, W.P.; Koropatnick, J. CD5 blockade, a novel immune checkpoint inhibitor, enhances T cell anti-tumour immunity and delays tumour growth in mice harbouring poorly immunogenic 4T1 breast tumour homografts. Front. Immunol. 2024, 15, 1256766. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Mu, W.; Zhao, Y.; Cheng, J.; Lin, H.; Ouyang, K.; Jia, X.; Liu, J.; Wei, Q.; Wang, M.; et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct. Target. Ther. 2022, 7, 85. [Google Scholar] [CrossRef]
- Schwarz, S.; Linnebacher, M. CD5: From antiquated T cell marker to immunotherapy’s new hope. Sig. Transduct. Target Ther. 2023, 8, 216. [Google Scholar] [CrossRef]
- Patel, R.P.; Ghilardi, G.; Zhang, Y.; Chiang, Y.-H.; Xie, W.; Guruprasad, P.; Kim, K.H.; Chun, I.; Angelos, M.G.; Pajarillo, R.; et al. CD5 deletion enhances the antitumor activity of adoptive T cell therapies. Sci. Immunol. 2024, 9, eadn6509. [Google Scholar] [CrossRef]
- AbouYabis, A.N.; Shenoy, P.J.; Flowers, C.; Lechowicz Mary, J. Response and Survival Rates in Patients with Peripheral T-Cell Lymphoma Treated with Anthracycline-Based Regimens: A Comprehensive Meta-Analysis. Blood 2007, 110, 3452. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Pinz, K.G.; Liu, H.; Lin, K.W.; Jares, A.; Firor, A.E.; Shuai, X.; Salman, H.; Golightly, M.; et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017, 31, 2151–2160. [Google Scholar] [CrossRef]

| Characteristic | Value (%) | CD5+ | CD5− |
|---|---|---|---|
| n = 194 | n = 122 | n = 72 | |
| Sex | |||
| Male | 107 (55.2%) | 59 | 48 |
| Female | 87 (44.8%) | 63 | 24 |
| Race | |||
| Caucasian | 136 (70.1%) | 83 | 53 |
| African American | 37 (19.1%) | 25 | 12 |
| Asian | 7 (3.6%) | 4 | 3 |
| Hispanic/Latino | 6 (3.1%) | 5 | 1 |
| Other | 1 (0.5%) | 1 | 0 |
| Unknown | 7 (3.6%) | 4 | 3 |
| ECOG | |||
| <2 | 143 (73.7%) | 88 | 55 |
| ≥2 | 44 (22.7%) | 31 | 13 |
| Unknown | 7 (3.6%) | 3 | 4 |
| Stage | |||
| I | 26 (13.4%) | 9 | 17 |
| II | 32 (16.4%) | 19 | 13 |
| III | 33 (17%) | 24 | 9 |
| IV | 92 (47%) | 65 | 27 |
| Unknown | 11 (5.6%) | 5 | 6 |
| B-Symptoms at presentation | |||
| Yes | 105 (54.1%) | 70 | 35 |
| No | 82 (42.3%) | 46 | 36 |
| Unknown | 7 (3.6%) | 6 | 1 |
| Subtype | |||
| T-follicular-helper (TFH) TCL (including angioimmunoblastic T-cell lymphoma [AITL]) | 49 (25.3%) | 46 | 3 |
| Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) | 41 (21.1%) | 34 | 7 |
| Anaplastic lymphoma kinase negative anaplastic large cell lymphoma (ALK- ALCL) | 28 (14.4%) | 13 | 15 |
| Extranodal NK/T-cell lymphoma (ENK TCL) | 22 (11.3%) | 5 | 17 |
| Anaplastic lymphoma kinase positive anaplastic large cell lymphoma (ALK+ ALCL) | 16 (8.2%) | 8 | 8 |
| Adult T-cell leukemia/lymphoma (ATLL) | 9 (4.6%) | 7 | 2 |
| Hepatosplenic T-cell lymphoma (HSTCL) | 9 (4.6%) | 0 | 9 |
| Breast implant-associated lymphoma (BIA-ALCL) | 5 (2.6%) | 1 | 4 |
| Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) | 4 (2.1%) | 2 | 2 |
| Primary cutaneous gamma-delta T-cell lymphoma (PCGD-TCL) | 3 (1.5%) | 2 | 1 |
| Enteropathy-associated T-cell lymphoma disease (EATL) | 3 (1.5%) | 2 | 2 |
| Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) | 2 (1%) | 0 | 2 |
| Primary cutaneous CD30-positive T-cell lymphoproliferative disorder (CD30+ CTCL/LPD) | 1 (0.5%) | 1 | 0 |
| T-cell prolymphocytic leukemia (T-PLL) | 1 (0.5%) | 1 | 0 |
| Characteristic | Value (%) | CD5+ | CD5− |
|---|---|---|---|
| Bulky disease | |||
| Yes | 34 (17.5%) | 24 | 10 |
| No | 151 (77.8%) | 89 | 62 |
| Unknown | 9 (4.6%) | 9 | 0 |
| LDH | |||
| ≥ULN | 106 (54.6%) | 74 | 32 |
| <ULN | 60 (30.9%) | 34 | 26 |
| Unknown | 28 (12.9%) | 14 | 14 |
| CD5 status | |||
| Positive * | 122 (62.9%) | ||
| CD5 > Dim | 103 (53.1%) | ||
| Negative | 72 (37.1%) | ||
| B-symptoms at presentation | |||
| Yes | 105 (54.1%) | 70 | 35 |
| No | 82 (42.3%) | 46 | 36 |
| Unknown | 7 (3.6%) | 6 | 1 |
| Extra-nodal disease | |||
| Yes | 123 (63.4%) | 74 | 49 |
| >2 sites | 51 (26.3%) | 36 | 15 |
| No | 66 (34%) | 44 | 22 |
| Unknown | 5 (2.5%) | 4 | 1 |
| Bone marrow disease | |||
| Yes | 45 (23.2%) | 37 | 8 |
| No | 133 (68.6%) | 75 | 58 |
| Unknown | 16 (8.2%) | 10 | 6 |
| Characteristic | Value (%) | CD5+ | CD5− |
|---|---|---|---|
| Initial Response | |||
| CR | 105 (54.1%) | 60 | 45 |
| PR | 27 (13.9%) | 21 | 6 |
| SD | 12 (6.2%) | 9 | 3 |
| PD | 37 (19.1%) | 25 | 12 |
| Unknown | 13 (0.7%) | 4 | 3 |
| Time of Progression | |||
| None | 65 (33.5%) | 35 | 30 |
| Refractory * | 73 (37.6%) | 58 | 20 |
| Relapse/Progression | 42 (21.6%) | 21 | 16 |
| Unknown | 5 (2.6%) | 3 | 2 |
| Alive at Last Follow-Up | |||
| Alive | 111 (57.2%) | 71 | 40 |
| Deceased | 83 (42.8%) | 51 | 32 |
| Survival Outcomes | |||
| 1-year OS | 154 (76.3%) | 96 | 58 |
| 2-year OS | 137 (66.4%) | 87 | 50 |
| Cause of death | |||
| Lymphoma | 49 (25.3%) | 33 | 16 |
| Treatment related | 17 (8.8%) | 5 | 7 |
| Within 30 days | 12 (6.2%) | 1 | 6 |
| >30 days | 5 (2.6%) | 4 | 1 |
| Other | 4 (2.1%) | 3 | 1 |
| Unknown | 118 (60.8%) | 73 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghawy, O.; Cao, M.; Xu, J.; Landsburg, D.J.; Svoboda, J.; Nasta, S.D.; Chong, E.A.; Schuster, S.J.; Thomas, C.J.; Carter, J.S.; et al. Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas. Cancers 2024, 16, 3430. https://doi.org/10.3390/cancers16193430
Elghawy O, Cao M, Xu J, Landsburg DJ, Svoboda J, Nasta SD, Chong EA, Schuster SJ, Thomas CJ, Carter JS, et al. Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas. Cancers. 2024; 16(19):3430. https://doi.org/10.3390/cancers16193430
Chicago/Turabian StyleElghawy, Omar, Miao Cao, Jason Xu, Daniel J. Landsburg, Jakub Svoboda, Sunita D. Nasta, Elise A. Chong, Stephen J. Schuster, Colin J. Thomas, Jordan S. Carter, and et al. 2024. "Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas" Cancers 16, no. 19: 3430. https://doi.org/10.3390/cancers16193430
APA StyleElghawy, O., Cao, M., Xu, J., Landsburg, D. J., Svoboda, J., Nasta, S. D., Chong, E. A., Schuster, S. J., Thomas, C. J., Carter, J. S., Tavakkoli, M., Ruella, M., & Barta, S. K. (2024). Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas. Cancers, 16(19), 3430. https://doi.org/10.3390/cancers16193430






