Effect of a Novel Food Rich in Miraculin on the Oral Microbiome of Malnourished Oncologic Patients with Dysgeusia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design and Participants
2.3. Biological Samples and DNA Sequencing
2.3.1. Saliva DNA Extraction
2.3.2. Whole 16S rRNA Gene Sequencing and Taxonomic Assignment
2.4. Plasma Cytokines
2.5. Electrical Taste Perception
2.6. Dietary Pattern Assessment
2.7. Mucositis
2.8. Statistical Analysis
3. Results
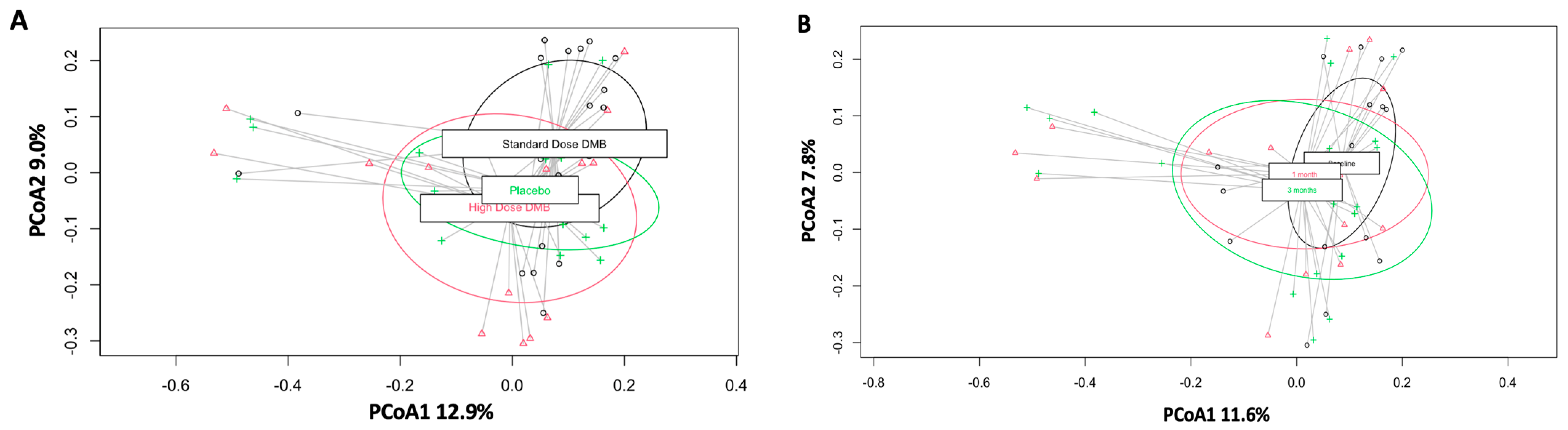
3.1. Beta Diversity
3.2. Phylum and Family Levels
3.3. Genus Level
3.4. Species Level
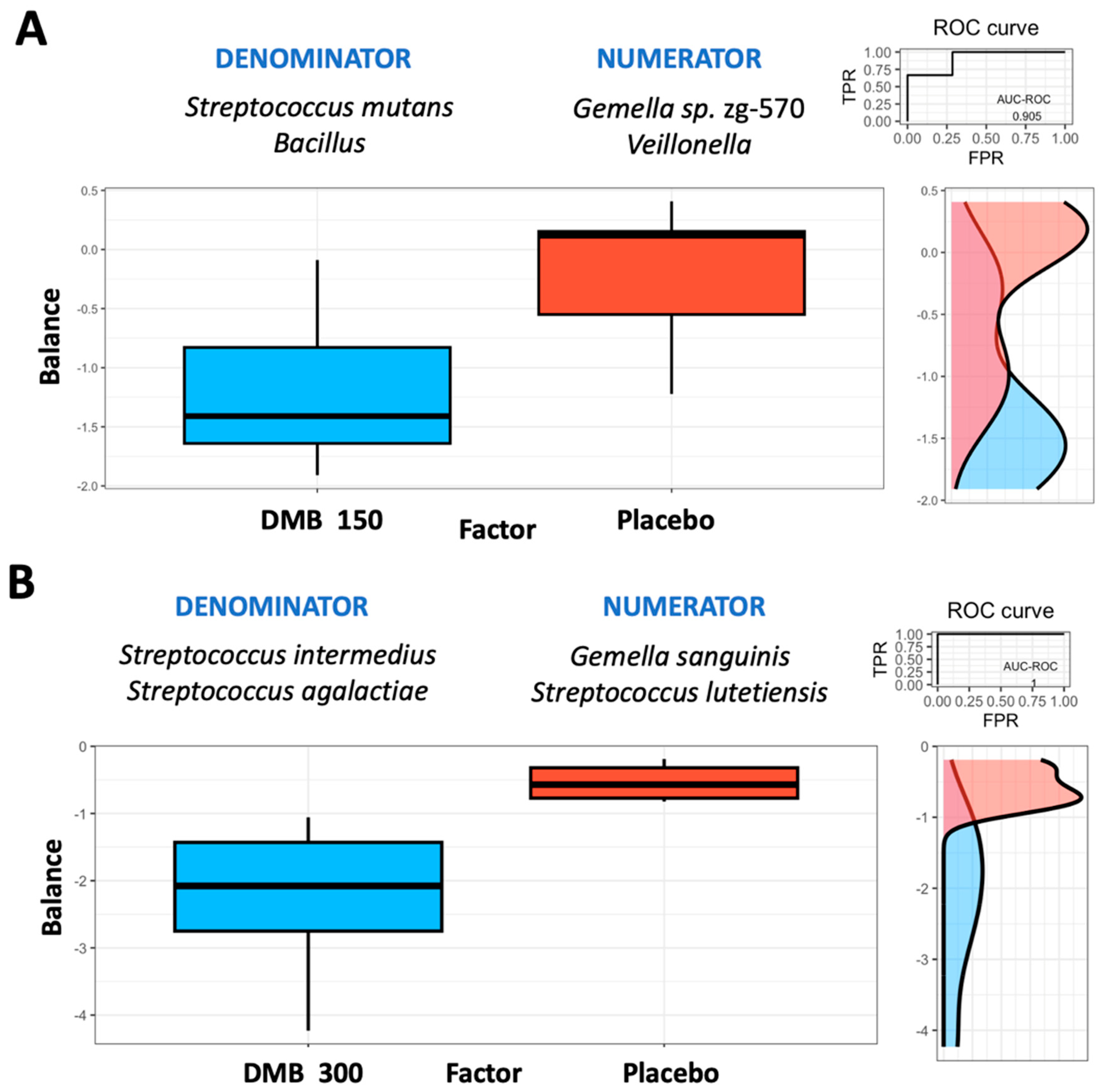
3.5. Microbiome Balance
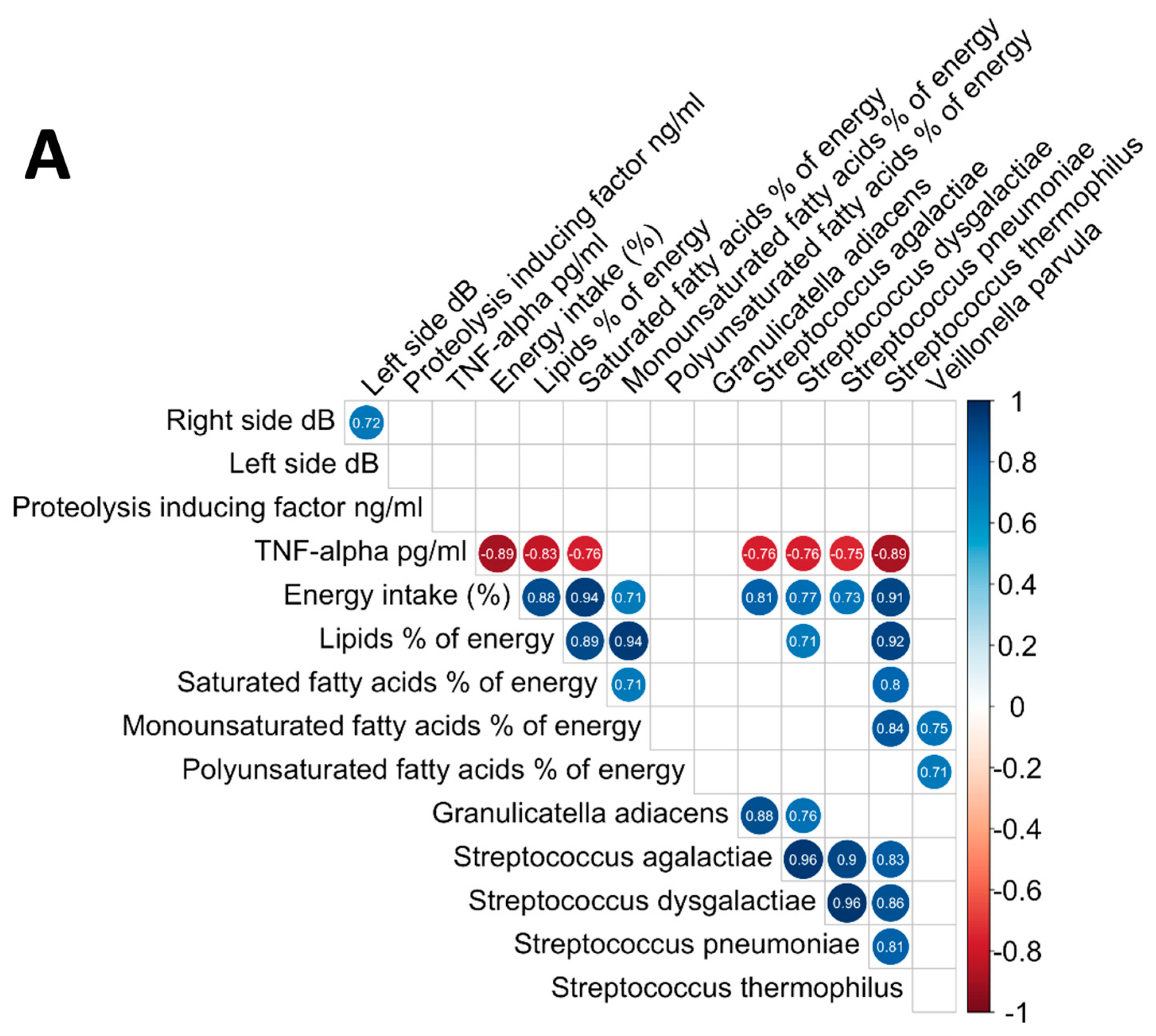
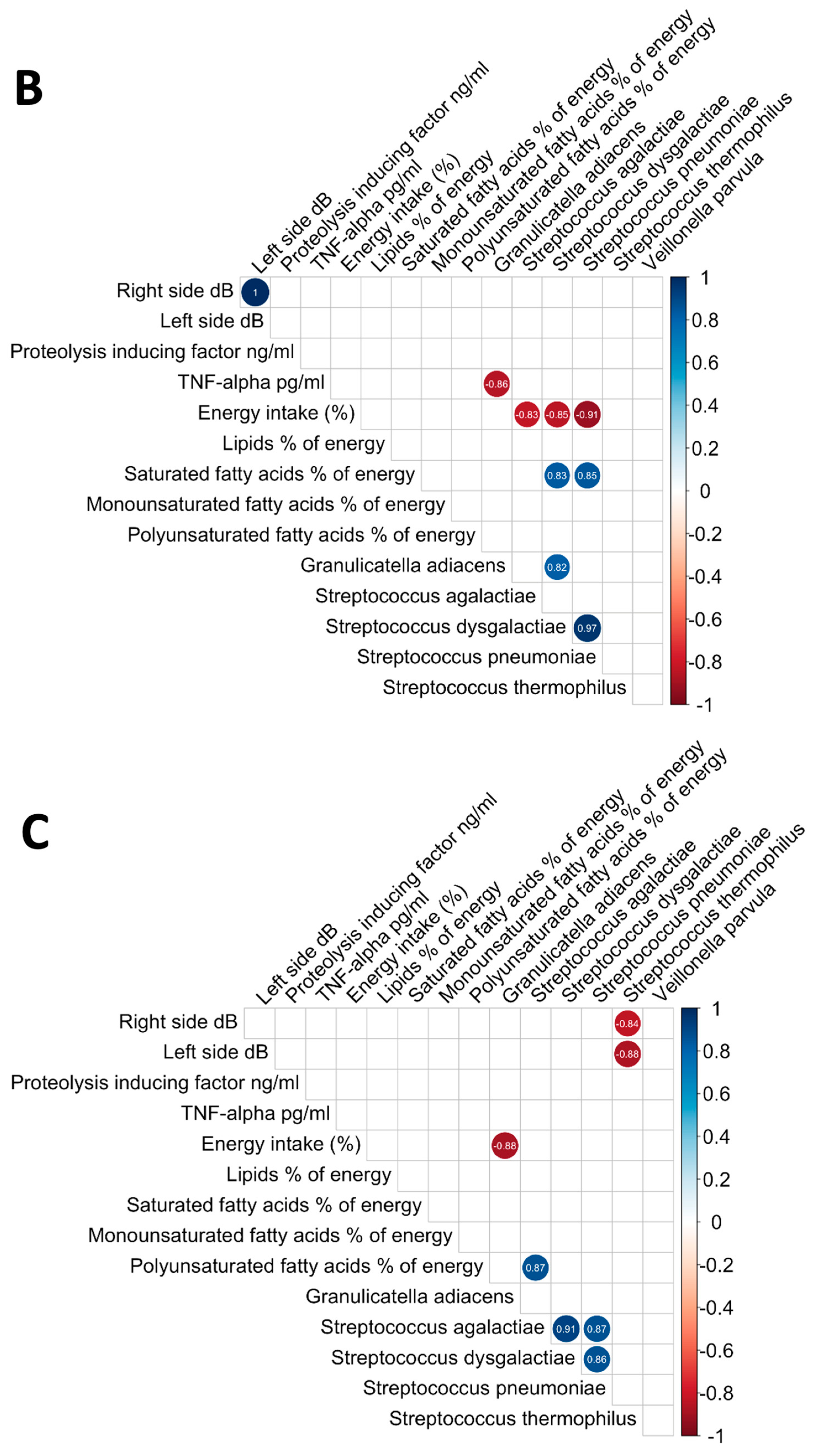
3.6. Analysis of the Relationships between Oral Microbiome, Inflammatory Cytokines, Nutritional Status, and Electrical Taste Perception in the Three Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Dolu, S.; Menekli, T. The Effects of Taste Changes on the Quality of Life of Patients Receiving Chemotherapy Treatment. Med. Rec. 2023, 5, 210–216. [Google Scholar] [CrossRef]
- Ponticelli, E.; Clari, M.; Frigerio, S.; De Clemente, A.; Bergese, I.; Scavino, E.; Bernardini, A.; Sacerdote, C. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur. J. Cancer Care 2017, 26, e12633. [Google Scholar] [CrossRef] [PubMed]
- IJpma, I.; Timmermans, E.R.; Renken, R.J.; Ter Horst, G.J.; Reyners, A.K. Metallic taste in cancer patients treated with systemic therapy: A questionnaire-based study. Nutr. Cancer 2017, 69, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zabernigg, A.; Gamper, E.-M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef]
- Kano, T.; Kanda, K. Development and validation of a chemotherapy-induced taste alteration scale. Proc. Oncol. Nurs. Forum 2013, 40, E79–E85. [Google Scholar] [CrossRef]
- Sevryugin, O.; Kasvis, P.; Vigano, M.; Vigano, A. Taste and smell disturbances in cancer patients: A scoping review of available treatments. Support. Care Cancer 2021, 29, 49–66. [Google Scholar] [CrossRef]
- Sözeri, E.; Kutlutürkan, S. Taste alteration in patients receiving chemotherapy. J. Breast Health 2015, 11, 81. [Google Scholar] [CrossRef]
- Gamper, E.-M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Wintner, L.M.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B.; Zabernigg, A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: Prevalence, course of severity, and quality of life correlates. Acta Oncol. 2012, 51, 490–496. [Google Scholar] [CrossRef]
- Hovan, A.J.; Williams, P.M.; Stevenson-Moore, P.; Wahlin, Y.B.; Ohrn, K.E.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T. A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 2010, 18, 1081–1087. [Google Scholar] [CrossRef]
- Erkurt, E.; Erkisi, M.; Tunali, C. Supportive treatment in weight-losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. J. Exp. Clin. Cancer Res. 2000, 19, 431–439. [Google Scholar]
- Laura McLaughlin, R.; Mahon, S.M. Understanding taste dysfunction in patients with cancer. Clin. J. Oncol. Nurs. 2012, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Ambaldhage, V.K.; Puttabuddi, J.H.; Nunsavath, P.N.; Tummuru, Y.R. Taste disorders: A review. J. Indian Acad. Oral Med. Radiol. 2014, 26, 69–76. [Google Scholar] [CrossRef]
- Yagi, T.; Asakawa, A.; Ueda, H.; Ikeda, S.; Miyawaki, S.; Inui, A. The role of zinc in the treatment of taste disorders. Recent Pat. Food Nutr. Agric. 2013, 5, 44–51. [Google Scholar] [CrossRef]
- van Oort, S.; Kramer, E.; de Groot, J.-W.; Visser, O. Taste alterations and cancer treatment. Curr. Opin. Support. Palliat. Care 2018, 12, 162–167. [Google Scholar] [CrossRef]
- Granot, M.; Nagler, R.M. Association between regional idiopathic neuropathy and salivary involvement as the possible mechanism for oral sensory complaints. J. Pain 2005, 6, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Togni, L.; Mascitti, M.; Vignini, A.; Alia, S.; Sartini, D.; Barlattani, A.; Emanuelli, M.; Santarelli, A. Treatment-related dysgeusia in oral and oropharyngeal cancer: A comprehensive review. Nutrients 2021, 13, 3325. [Google Scholar] [CrossRef] [PubMed]
- Wilken, M.K.; Satiroff, B.A. Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin. J. Oncol. Nurs. 2012, 16, E173–E177. [Google Scholar] [CrossRef]
- Dominiak, H.S.; Hasselsteen, S.D.; Nielsen, S.W.; Andersen, J.R.; Herrstedt, J. Prevention of taste alterations in patients with cancer receiving Paclitaxel-or Oxaliplatin-based chemotherapy—A pilot trial of cannabidiol. Nutrients 2023, 15, 3014. [Google Scholar] [CrossRef]
- Epstein, J.B.; de Andrade e Silva, S.M.; Epstein, G.L.; Leal, J.H.S.; Barasch, A.; Smutzer, G. Taste disorders following cancer treatment: Report of a case series. Support. Care Cancer 2019, 27, 4587–4595. [Google Scholar] [CrossRef]
- Pellegrini, M.; Merlo, F.D.; Agnello, E.; Monge, T.; Devecchi, A.; Casalone, V.; Montemurro, F.; Ghigo, E.; Sapino, A.; Bo, S. Dysgeusia in Patients with Breast Cancer Treated with Chemotherapy-A Narrative Review. Nutrients 2023, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.W.; Daskalou, D.; Macario, S.; Voruz, F.; Landis, B.N. How to Manage Taste Disorders. Curr. Otorhinolaryngol. Rep. 2022, 10, 385–392. [Google Scholar] [CrossRef]
- Hug, L.A.; Baker, B.J.; Anantharaman, K.; Brown, C.T.; Probst, A.J.; Castelle, C.J.; Butterfield, C.N.; Hernsdorf, A.W.; Amano, Y.; Ise, K.; et al. A new view of the tree of life. Nat. Microbiol. 2016, 1, 16048. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Pasto, A.; Ng, T.; Patel, V.; Urbano, T.G.; Sears, C.; Wade, W.G. The microbiota and radiotherapy for head and neck cancer: What should clinical oncologists know? Cancer Treat. Rev. 2022, 109, 102442. [Google Scholar] [CrossRef]
- Mukherjee, C.; Moyer, C.O.; Steinkamp, H.M.; Hashmi, S.B.; Beall, C.J.; Guo, X.; Ni, A.; Leys, E.J.; Griffen, A.L. Acquisition of oral microbiota is driven by environment, not host genetics. Microbiome 2021, 9, 54. [Google Scholar] [CrossRef]
- Gomez, A.; Nelson, K.E. The Oral Microbiome of Children: Development, Disease, and Implications Beyond Oral Health. Microb. Ecol. 2017, 73, 492–503. [Google Scholar] [CrossRef]
- Mougeot, J.-L.C.; Stevens, C.B.; Morton, D.S.; Brennan, M.T.; Mougeot, F.B. Oral microbiome and cancer therapy-induced oral mucositis. JNCI Monogr. 2019, 2019, lgz002. [Google Scholar]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van de Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, M.E.; Pérez-Sayáns, M.; Chauca-Bajaña, L.A.; Barbeito-Castiñeiras, G.; del Molino-Bernal, M.L.P.; López-López, R. Oral microbiome and systemic antineoplastics in cancer treatment: A systematic review. Med. Oral Patol. Oral y Cirugía Bucal 2022, 27, e248. [Google Scholar] [CrossRef]
- Egan, J.M. Physiological Integration of Taste and Metabolism. N. Engl. J. Med. 2024, 390, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in oral health: Homeostasis maintenance, disease prevention, and therapeutic applications. Nutrients 2023, 15, 4384. [Google Scholar] [CrossRef] [PubMed]
- Bashraheel, S.S.; Domling, A.; Goda, S.K. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed. Pharmacother. 2020, 125, 110009. [Google Scholar] [CrossRef] [PubMed]
- Misaka, T. Molecular mechanisms of the action of miraculin, a taste-modifying protein. Semin. Cell Dev. Biol. 2013, 24, 222–225. [Google Scholar] [CrossRef]
- Kurihara, K.; Beidler, L.M. Mechanism of the Action of Taste-modifying Protein. Nature 1969, 222, 1176–1179. [Google Scholar] [CrossRef]
- Gómez de Cedrón, M.; Wagner, S.; Reguero, M.; Menéndez-Rey, A.; de Molina, A.R. Miracle Berry as a Potential Supplement in the Control of Metabolic Risk Factors in Cancer. Antioxidants 2020, 9, 1282. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried fruits of Synsepalum dulcificum as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06600. [Google Scholar] [CrossRef]
- Osabor, V.N.; Etiuma, R.A.; Ntinya, M.U. Chemical Profile of Leaves and Roots of Miracle Fruit (Synsepalum dulcificum). Am. Chem. Sci. J. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- He, Z.; Tan, J.S.; Abbasiliasi, S.; Lai, O.M.; Tam, Y.J.; Ariff, A.B. Phytochemicals, nutritionals and antioxidant properties of miracle fruit Synsepalum dulcificum. Ind. Crops Prod. 2016, 86, 87–94. [Google Scholar] [CrossRef]
- Soares, H.P.; Cusnir, M.; Schwartz, M.A.; Pizzolato, J.F.; Lutzky, J.; Campbell, R.J.; Beaumont, J.L.; Eton, D.; Stonick, S.; Lilenbaum, R. Treatment of taste alterations in chemotherapy patients using the “miracle fruit”: Preliminary analysis of a pilot study. J. Clin. Oncol. 2010, 28, e19523. [Google Scholar] [CrossRef]
- Lopez-Plaza, B.; Alvarez-Mercado, A.I.; Arcos-Castellanos, L.; Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Brandimonte-Hernandez, M.; Feliu-Batlle, J.; Hummel, T.; Gil, A.; Palma Milla, S. Efficacy and Safety of Habitual Consumption of a Food Supplement Containing Miraculin in Malnourished Cancer Patients: The CLINMIR Pilot Study. Nutrients 2024, 16, 1905. [Google Scholar] [CrossRef] [PubMed]
- López-Plaza, B.; Gil, Á.; Menéndez-Rey, A.; Bensadon-Naeder, L.; Hummel, T.; Feliú-Batlle, J.; Palma-Milla, S. Effect of Regular Consumption of a Miraculin-Based Food Supplement on Taste Perception and Nutritional Status in Malnourished Cancer Patients: A Triple-Blind, Randomized, Placebo-Controlled Clinical Trial-CLINMIR Pilot Protocol. Nutrients 2023, 15, 4639. [Google Scholar] [CrossRef] [PubMed]
- Baber, N. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Br. J. Clin. Pharmacol. 1994, 37, 401. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mercado, A.I.; López Plaza, B.; Plaza-Diaz, J.; Arcos Castellanos, L.; Ruiz-Ojeda, F.J.; Brandimonte-Hernández, M.; Feliú-Batlle, J.; Hummel, T.; Palma Milla, S.; Gil, Á. The Regular Consumption of a Food Supplement Containing Miraculin Can Contribute to Reducing Biomarkers of Inflammation and Cachexia in Malnourished Patients with Cancer and Taste Disorders: The CLINMIR Pilot Study. medRxiv 2024. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Mora-Gonzalez, J.; Migueles, J.H.; Esteban-Cornejo, I.; Cadenas-Sanchez, C.; Pastor-Villaescusa, B.; Molina-Garcia, P.; Rodriguez-Ayllon, M.; Rico, M.C.; Gil, A.; Aguilera, C.M.; et al. Sedentarism, Physical Activity, Steps, and Neurotrophic Factors in Obese Children. Med. Sci. Sports Exerc. 2019, 51, 2325–2333. [Google Scholar] [CrossRef]
- Frank, M.E.; Smith, D.V. Electrogustometry-a simple way to test taste. In Smell and Taste in Health and Disease, 1st ed.; Getchell, T.V., Doty, R.L., Bartoshuk, L.M., Snow, J.B., Jr., Eds.; Raven Press: New York, NY, USA, 1991; pp. 503–514. [Google Scholar]
- Barry, M.A.; Gatenby, J.C.; Zeiger, J.D.; Gore, J.C. Hemispheric dominance of cortical activity evoked by focal electrogustatory stimuli. Chem. Senses 2001, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Dietary Reference Values for Nutrients Summary Report; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 2397–8325. [Google Scholar]
- Bartrina, J.A.; Majem, L.S. Objetivos nutricionales para la población española: Consenso de la Sociedad Española de Nutrición Comunitaria 2011. Rev. Española Nutr. Comunitaria = Span. J. Community Nutr. 2011, 17, 178–199. [Google Scholar]
- National Cancer institute. National Cancer Institute Common Terminology Criteria for Adverse Events, NCI-CTCAE v.5. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 25 June 2024).
- Sonis, S.T.; Eilers, J.P.; Epstein, J.B.; LeVeque, F.G.; Liggett, W.H., Jr.; Mulagha, M.T.; Peterson, D.E.; Rose, A.H.; Schubert, M.M.; Spijkervet, F.K. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer 1999, 85, 2103–2113. [Google Scholar] [CrossRef]
- Wang, T.; Graves, B.; Rosseel, Y.; Merkle, E.C. Computation and application of generalized linear mixed model derivatives using lme4. Psychometrika 2022, 87, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/about.html (accessed on 28 May 2024).
- Wei, T.S.V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Package ‘Corrplot’. 2022. Available online: https://github.com/taiyun/corrplot (accessed on 30 August 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Rivera-Pinto, J.; Egozcue, J.J.; Pawlowsky-Glahn, V.; Paredes, R.; Noguera-Julian, M.; Calle, M.L. Balances: A New Perspective for Microbiome Analysis. mSystems 2018, 3, 10-1128. [Google Scholar] [CrossRef]
- L’Heureux, J.E.; van der Giezen, M.; Winyard, P.G.; Jones, A.M.; Vanhatalo, A. Localisation of nitrate-reducing and highly abundant microbial communities in the oral cavity. PLoS ONE 2023, 18, e0295058. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Vollaard, E.; Clasener, H. Colonization resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef]
- Frey-Furtado, L.; Magalhães, I.; Sampaio-Maia, B.; Azevedo, M.J. Oral microbiome characterization in oral mucositis patients—A systematic review. J. Oral Pathol. Med. 2023, 52, 911–918. [Google Scholar] [CrossRef]
- Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A review of the role of oral microbiome in the development, detection, and management of head and neck squamous cell cancers. Cancers 2022, 14, 4116. [Google Scholar] [CrossRef]
- Triarico, S.; Agresti, P.; Rinninella, E.; Mele, M.C.; Romano, A.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Oral microbiota during childhood and its role in chemotherapy-induced oral mucositis in children with cancer. Pathogens 2022, 11, 448. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, C.; Hua, H.; Hu, X. Compositional and functional changes in the salivary microbiota related to oral leukoplakia and oral squamous cell carcinoma: A case control study. BMC Oral Health 2023, 23, 1021. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhu, H.; Mou, Q.; Wong, P.Y.; Lan, L.; Ng, C.W.; Lei, P.; Cheung, M.K.; Wang, D.; Wong, E.W. Integrative analysis reveals associations between oral microbiota dysbiosis and host genetic and epigenetic aberrations in oral cavity squamous cell carcinoma. Npj Biofilms Microbiomes 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Marzhoseyni, Z.; Shojaie, L.; Tabatabaei, S.A.; Movahedpour, A.; Safari, M.; Esmaeili, D.; Mahjoubin-Tehran, M.; Jalili, A.; Morshedi, K.; Khan, H.; et al. Streptococcal bacterial components in cancer therapy. Cancer Gene Ther. 2022, 29, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Crean, S.J.; Lewis, M.A.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006, 44, 1719–1725. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef]
- Iqbal, N.T.; Chen, R.Y.; Griffin, N.W.; Hibberd, M.C.; Khalid, A.; Sadiq, K.; Jamil, Z.; Ahmed, K.; Iqbal, J.; Hotwani, A. A shared group of bacterial taxa in the duodenal microbiota of undernourished Pakistani children with environmental enteric dysfunction. Msphere 2024, e00196-24. [Google Scholar] [CrossRef]
- Nibali, L.; Henderson, B. The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Steffen, M.; Holt, S.; Ebersole, J.L. Porphyromonas gingivalis induction of mediator and cytokine secretion by human gingival fibroblasts. Oral Microbiol. Immunol. 2000, 15, 172–180. [Google Scholar] [CrossRef]
- Colucci, F. An oral commensal associates with disease: Chicken, egg, or red herring? Immunity 2015, 42, 208–210. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017, 170, 548–563.e516. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Xiao, H.; Zhang, S.; Wang, B.; Wang, H.; Li, Y.; Fan, S.; Cui, M. Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep. 2021, 37, 109886. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Li, M.; Zhang, Y.; Sun, M.; Wang, L.; Lin, J.; Cui, Y.; Chen, Q.; Jin, C. Fusobacterium nucleatum reduces METTL3-mediated m6A modification and contributes to colorectal cancer metastasis. Nat. Commun. 2022, 13, 1248. [Google Scholar] [CrossRef]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell. Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef]
- Higham, J.; Scannapieco, F.A. Epidemiological associations between periodontitis and cancer. Periodontol 2000 2024. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, S.; Gunda, V.; Reinartz, D.M.; Pond, K.W.; Thorne, C.A.; Santiago Raj, P.V.; Johnson, M.D.; Wilson, J.E. Oral streptococci S. anginosus and S. mitis induce distinct morphological, inflammatory, and metabolic signatures in macrophages. Infect. Immun. 2024, 92, e00536-23. [Google Scholar] [CrossRef]
- Baraniya, D.; Chitrala, K.N.; Al-Hebshi, N.N. Global transcriptional response of oral squamous cell carcinoma cell lines to health-associated oral bacteria—An in vitro study. J. Oral Microbiol. 2022, 14, 2073866. [Google Scholar] [CrossRef]
- Moynihan, P. Sugars and Dental Caries: Evidence for Setting a Recommended Threshold for Intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Newby, E.E.; Martinez-Mier, E.A.; Zero, D.T.; Kelly, S.A.; Fleming, N.; North, M.; Bosma, M.L. A randomised clinical study to evaluate the effect of brushing duration on fluoride levels in dental biofilm fluid and saliva in children aged 4-5 years. Int. Dent. J. 2013, 63 (Suppl. S2), 39–47. [Google Scholar] [CrossRef]
- Holt, S.; Ebersole, J. Porphyromonas gingivalis, Treponema denticola, andTannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72. [Google Scholar] [CrossRef]
- Takahashi, N. Oral microbiome metabolism: From “who are they?” to “what are they doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Pitts, N.; Zero, D.; Marsh, P.; Ekstrand, K.; Weintraub, J.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Banas, J.A.; Drake, D.R. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Morton, J.T.; Dinis, M.; Alvarez, R.; Tran, N.C.; Knight, R.; Edlund, A. Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 2021, 31, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.-Y.; Pratap, S.; Xie, H. Oral microbiome associated with differential ratios of Porphyromonas gingivalis and Streptococcus cristatus. Microbiol. Spectr. 2024, 12, e03482-23. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US adults: National health and nutrition examination survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e576. [Google Scholar] [CrossRef]
| Standard-Dose DMB (150 mg) (n = 8) | High-Dose DMB (300 mg) (n = 6) | Placebo (n = 7) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | Baseline | 1 Month | 3 Months | Baseline | 1 Month | 3 Months | Baseline | 1 Month | 3 Months | Treatment (T) | Time (t) | T × t |
| Actinomyces | 0.1 (0.02–0.3) | 0.2 (0.03–0.3) | 0.08 (0.02–0.3) | 0.09 (0.04–0.2) | 0.08 (0.04–0.2) | 0.07 (0.04–0.1) | 0.1 (0.07–0.6) | 0.1 (0.04–0.7) | 0.1 (0.05–0.6) | 0.238 | 0.308 | 0.846 |
| Aminipila | 0.1 (0.01–0.5) | 0.1 (0.02–0.8) | 0.2 (0.03–0.5) | 0.1 (0.06–0.4) | 0.1 (0.03–0.3) | 0.08 (0.07–0.2) | 0.2 (0.006–1.6) | 0.5 (0.2–2.8) | 0.2 (0.1–4.7) | 0.138 | 0.229 | 0.237 |
| Atopobium | 0.4 (0.1–0.7) | 0.4 (0.1–0.7) | 0.6 (0.1–1.1) | 0.06 (0.02–0.1) | 0.3 (0.3–0.3) | 0.1 (0.07–0.1) | 0.01 (0.01–0.01) | 0.02 (0.02–0.02) | 0.03 (0.03–0.03) | 0.319 | 0.913 | 0.962 |
| Bacillus | 0.68 (0.3–1.6) | 0.5 (0.4–1.1) | 0.9 (0.5–1.2) | 0.3 (0.1–0.9) | 0.3 (0.2–0.5) | 0.4 (0.2–2.5) | 0.4 (0.3–1.1) | 0.6 (0.4–0.8) | 0.4 (0.3–0.4) | 0.179 | 0.26 | 0.061 |
| Bulleidia | 0.1 (0.06–0.4) | 0.1 (0.04–0.3) | 0.07 (0.02–0.2) | 0.06 (0.02–0.5) | 0.07 (0.03–0.2) | 0.1 (0.01–0.2) | 0.08 (0.02–0.5) | 0.2 (0.1–0.5) | 0.1 (0.02–0.3) | 0.328 | 0.752 | 0.74 |
| Clostridioides | 0.4 (0.02–1.0) | 0.5 (0.01–3.1) | 0.8 (0.02–2.9) | 0.4 (0.01–4.8) | 1.2 (0.7–1.9) | 0.4 (0.06–0.7) | 1.3 (0.1–2.9) | 2.3 (0.1–6.4) | 1.2 (0.1–4.4) | 0.076 | 0.37 | 0.388 |
| Enterococcus | 0.5 (0.2–1.4) | 0.4 (0.3–0.8) | 0.5 a (0.4–0.9) | 0.2 (0.03–0.7) | 0.1 (0.04–0.4) | 0.3 b (0.08–2.4) | 0.3 (0.1–0.8) | 0.5 (0.2–0.5) | 0.3 b (0.1–0.3) | 0.251 | 0.234 | 0.044 |
| Gemella | 4.8 (2.1–13.3) | 5.9 (1.0–17.5) | 8.6 (1.6–15.0) | 6.3 (0.5–11.7) | 3.7 (1.3–6.7) | 3.4 (0.6–11.6) | 4.4 (1.9–12.5) | 6.0 (4.1–13.0) | 8.6 (4.6–14.2) | 0.605 | 0.512 | 0.309 |
| Granulicatella | 4.8 (1.6–14.7) | 3.2 (2.2–8.0) | 4.8 (1.4–9.2) | 1.6 (0.09–7.4) | 1.6 (0.3–4.8) | 4.0 (0.8–23.4) | 3.0 (1.4–8.8) | 4.7 (1.3–7.4) | 2.7 (1.3–3.1) | 0.411 | 0.468 | 0.066 |
| Herbinix | 0.4 (0.1–1.0) | 0.2 (0.07–1.6) | 1.0 (0.3–1.3) | 0.2 (0.01–0.9) | 0.4 (0.3–1.9) | 0.2 (0.03–0.5) | 0.9 (0.07–1.7) | 0.3 (0.09–4.2) | 0.9 (0.1–2.5) | 0.246 | 0.436 | 0.575 |
| Lachnoanaerobaculum | 1.3 (0.06–4.0) | 1.4 (0.2–3.8) | 0.9 (0.1–8.9) | 0.1 (0.04–3.9) | 0.9 (0.1–1.7) | 0.5 (0.03–5.4) | 1.1 (0.5–2.5) | 2.0 (0.2–5.1) | 1.3 (0.2–4.6) | 0.464 | 0.632 | 0.835 |
| Lachnoclostridium | 0.5 (0.3–0.5) | 0.4 (0.2–1.7) | 1.2 (0.1–3.2) | 0.6 (0.008–1.0) | 0.5 (0.03–1.8) | 0.3 (0.01–0.7) | 0.3 (0.2–0.5) | 0.5 (0.1–2.3) | 0.4 (0.2–1.0) | 0.376 | 0.304 | 0.172 |
| Listeria | 0.4 (0.1–1.1) | 0.3 (0.2–0.6) | 0.5 (0.3–0.8) | 0.1 (0.01–0.7) | 0.2 (0.06–0.3) | 0.5 (0.5–0.5) | 0.2 (0.08–0.7) | 0.3 (0.1–0.4) | 0.2 (0.07–0.2) | 0.159 | 0.242 | 0.103 |
| Mediterraneibacter | 0.9 (0.2–2.1) | 1.1 (0.3–2.5) | 1.1 (0.2–9.7) | 0.6 (0.02–4.2) | 1.0 (0.2–1.7) | 0.9 (0.09–6.0) | 0.9 (0.5–3.4) | 1.5 (0.7–2.7) | 1.6 (0.3–2.7) | 0.783 | 0.213 | 0.706 |
| Megasphaera | 0.3 (0.02–1.9) | 0.2 (0.03–2.7) | 0.1 (0.03–2.0) | 0.4 (0.05–2.1) | 0.8 (0.3–2.2) | 1.0 (0.2–4.5) | 0.6 (0.3–3.9) | 0.5 (0.07–1.5) | 0.3 (0.1–1.3) | 0.709 | 0.724 | 0.129 |
| Mogibacterium | 0.4 (0.05–1.1) | 0.3 (0.06–1.8) | 0.5 (0.07–6.3) | 0.3 (0.04–0.7) | 0.3 (0.08–0.7) | 0.2 (0.05–0.4) | 1.0 (0.2–1.9) | 0.4 (0.06–2.6) | 0.6 (0.03–3.0) | 0.405 | 0.7 | 0.705 |
| Novisyntrophococcus | 0.1 (0.01–0.4) | 1.2 (0.02–1.9) | 1.8 (0.07–4.0) | 3.2 (0.01–6.3) | 1.1 (0.01–2.2) | 5.4 (5.4–5.4) | 0.6 (0.04–2.3) | 2.8 (0.3–5.5) | 0.6 (0.06–4.4) | 0.317 | 0.366 | 0.249 |
| Parvimonas | 0.4 (0.04–2.8) | 0.3 (0.06–3.4) | 1.4 (0.02–5.2) | 1.4 (0.3–2.0) | 0.8 (0.3–2.5) | 0.7 (0.2–1.6) | 0.2 (0.01–6.1) | 0.2 (0.003–6.9) | 1.5 (0.05–17.7) | 0.406 | 0.427 | 0.581 |
| Romboutsia | 0.2 (0.07–0.6) | 0.09 (0.05–0.9) | 0.5 (0.2–0.8) | 0.3 (0.09–0.6) | 0.3 (0.2–1.0) | 0.2 (0.02–0.3) | 0.4 (0.03–1.0) | 0.1 (0.04–2.8) | 0.5 (0.05–1.6) | 0.385 | 0.536 | 0.557 |
| Rothia | 0.2 (0.02–0.3) | 0.06 (0.01–0.2) | 0.08 (0.03–0.3) | 0.06 (0.02–0.5) | 0.05 (0.02–0.1) | 0.04 (0.009–0.1) | 0.08 (0.01–0.3) | 0.2 (0.01–0.3) | 0.07 (0.01–0.7) | 0.627 | 0.661 | 0.147 |
| Schaalia | 0.1 (0.07–0.8) | 0.1 (0.02–0.9) | 0.3 (0.2–0.4) | 0.1 (0.02–0.4) | 0.1 (0.03–0.3) | 0.2 (0.07–0.2) | 0.2 (0.08–1.1) | 0.2 (0.07–0.5) | 0.1 (0.05–0.2) | 0.553 | 0.211 | 0.52 |
| Staphylococcus | 0.2 (0.1–0.5) | 0.2 (0.1–0.4) | 0.3 (0.3–0.3) | 0.1 (0.03–0.5) | 0.1 (0.07–0.3) | 0.6 (0.6–0.6) | 0.2 (0.07–0.3) | 0.3 (0.2–1.6) | 0.1 (0.1–0.3) | 0.693 | 0.478 | 0.053 |
| Streptococcus | 69.5 (61.3–71.2) | 64.4 (56.6–75.3) | 68.7 (37.2–77.3) | 72.0 (53.1–94.0) | 76.3 (60.0–84.6) | 69.0 (53.3–84.9) | 65.0 (49.7–85.3) | 63.0 (33.7–76.9) | 68.0 (41.3–72.6) | 0.236 | 0.525 | 0.789 |
| Veillonella | 9.3 (3.3–19.6) | 6.6 (3.5–24.8) | 2.4 a (0.06–5.5) | 4.3 (1.4–5.6) | 7.9 (3.8–23.7) | 6.6 b (2.7–10.6) | 10.5 (3.1–20.1) | 5.4 (2.3–13.3) | 5.6 b (4.1–8.0) | 0.956 | 0.052 | 0.015 |
| Shannon index | 1.4 (1.2–1.5) | 1.5 (1.1–2.0) | 1.4 (1.1–2.6) | 1.2 (0.4–2.1) | 0.8 (0.6–1.7) | 1.3 (0.8–1.7) | 1.5 (0.8–1.9) | 1.6 (1.1–2.6) | 1.4 (1.1–2.2) | 0.516 | 0.373 | 0.591 |
| Simpson’s index | 0.5 (0.5–0.6) | 0.6 (0.4–0.7) | 0.5 (0.4–0.8) | 0.5 (0.1–0.7) | 0.4 (0.3–0.6) | 0.5 (0.3–0.7) | 0.6 (0.3–0.7) | 0.6 (0.4–0.9) | 0.5 (0.5–0.8) | 0.673 | 0.428 | 0.714 |
| Chao1 index | 41.0 (22.8–60.0) | 39.9 (32.0–57.2) | 38.0 (24.3–64.0) | 37.5 (21.0–47.0) | 32.4 (4.0–79.0) | 38.6 (30.2–52.0) | 36.2 (22.0–49.0) | 39.6 (24.8–66.0) | 41.5 (23.8–54.7) | 0.575 | 0.411 | 0.405 |
| Standard-Dose DMB (150 mg) (n = 8) | High-Dose DMB (300 mg) (n= 6) | Placebo (n = 7) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Baseline | 1 Month | 3 Months | Baseline | 1 Month | 3 Months | Baseline | 1 Month | 3 Months | Treatment (T) | Time (t) | T × t |
| Gemella haemolysans | 0.3 (0.06–1.4) | 0.2 (0.03–0.6) | 0.3 (0.07–1.2) | 0.6 (0.03–7.6) | 0.5 (0.2–0.7) | 0.5 (0.03–2.7) | 0.7 (0.1–6.1) | 0.7 (0.1–2.2) | 1.8 (0.3–6.7) | 0.105 | 0.297 | 0.517 |
| Gemella morbillorum | 0.1 (0.06–0.9) | 0.2 (0.07–0.6) | 0.3 (0.07–1.2) | 0.4 (0.04–2.2) | 0.4 (0.03–1.7) | 0.08 (0.06–0.5) | 0.3 (0.03–3.9) | 0.6 (0.08–9.4) | 2.5 (0.2–7.2) | 0.103 | 0.17 | 0.054 |
| Gemella sanguinis | 3.7 (1.4–12.8) | 5.3 (0.6–16.8) | 7.5 (1.3–15.1) | 1.0 (0.009–8.0) | 1.9 (0.1–6.0) | 1.1 (0.06–9.7) | 2.2 (1.2–6.1) | 3.2 (1.8–6.2) | 4.0 (0.4–7.0) | 0.312 | 0.352 | 0.769 |
| Gemella sp. zg-570 | 0.06 (0.01–0.5) | 0.06 (0.02–0.2) | 0.1 (0.04–0.3) | 0.3 (0.04–1.8) | 0.3 (0.2–0.4) | 0.4 (0.01–0.6) | 0.2 (0.04–1.3) | 0.4 (0.06–0.5) | 0.4 (0.06–0.8) | 0.102 | 0.3 | 0.584 |
| Granulicatella adiacens | 4.8 (1.6–14.7) | 3.7 (2.2–8.0) | 4.8 (1.4–9.2) | 1.4 (0.09–7.4) | 1.6 (0.3–4.1) | 4.0 (0.8–23.6) | 3.0 (1.4–8.8) | 4.7 (1.3–7.5) | 2.6 (1.3–3.1) | 0.396 | 0.484 | 0.069 |
| Granulicatella elegans | 0.01 (0.004–0.1) | 0.02 (0.01–0.1) | 0.03 a (0.01–0.07) | 0.02 (0.009–0.5) | 0.6 (0.6–0.6) | 0.01 b (0.006–0.02) | 0.03 (0.01–0.2) | 0.01 (0.01–0.3) | 0.02 b (0.005–0.05) | <0.001 | <0.001 | <0.001 |
| Streptococcus agalactiae | 10.5 (5.1–15.4) | 10.0 (5.6–14.2) | 9.8 (6.0–13.9) | 10.9 (5.5–14.1) | 7.8 (6.6–11.9) | 8.0 (5.9–11.4) | 9.2 (7.3–12.6) | 8.7 (6.2–14.2) | 7.9 (7.3–16.3) | 0.878 | 0.385 | 0.586 |
| Streptococcus australis | 0.1 (0.01–0.7) | 0.07 (0.02–0.2) | 0.06 (0.006–0.2) | 0.03 (0.02–0.03) | 0.03 (0.03–0.03) | 0.06 (0.06–0.06) | 0.01 (0.01–0.07) | 0 (0–0) | 0.6 (0.6–0.6) | 0.218 | 0.301 | 0.063 |
| Streptococcus cristatus | 0.4 (0.04–1.6) | 0.3 (0.06–1.5) | 0.1 (0.03–3.0) | 0.4 (0.1–4.0) | 1.2 (0.1–1.5) | 0.2 (0.04–0.7) | 0.7 (0.06–3.4) | 0.4 (0.06–0.7) | 0.3 (0.05–2.2) | 0.733 | 0.149 | 0.087 |
| Streptococcus dysgalactiae | 5.0 (3.3–6.3) | 4.8 (2.9–7.5) | 4.2 (2.5–5.8) | 4.5 (3.3–5.6) | 3.8 (3.3–6.0) | 3.6 (2.7–5.3) | 3.9 (2.8–6.2) | 3.6 (3.1–5.5) | 3.9 (3.1–6.3) | 0.583 | 0.261 | 0.666 |
| Streptococcus infantis | 1.0 (0.7–2.4) | 1.0 (0.6–2.8) | 0.9 (0.4–2.3) | 0.9 (0.5–1.2) | 0.7 (0.5–1.3) | 0.7 (0.6–1.0) | 0.9 (0.7–1.6) | 0.9 (0.6–1.7) | 1.1 (0.7–1.2) | 0.321 | 0.844 | 0.307 |
| Streptococcus mutans | 0.3 (0.2–2.9) | 0.3 (0.2–3.5) | 0.4 a (0.2–3.4) | 1.9 (0.3–2.7) | 2.2 (0.4–7.2) | 0.6 b (0.3–1.8) | 0.4 (0.2–1.2) | 0.3 (0.2–0.3) | 0.6 b (0.2–0.9) | 0.022 | 0.125 | 0.012 |
| Streptococcus parasanguinis | 1.2 (0.09–3.9) | 1.2 (0.5–1.6) | 0.8 a (0.2–2.2) | 0.3 (0.09–1.4) | 0.6 (0.09–1.8) | 1.5 b (0.1–2.5) | 0.5 (0.2–1.3) | 0.8 (0.09–3.6) | 0.5 c (0.1–2.3) | 0.78 | 0.746 | 0.018 |
| Streptococcus pneumoniae | 13.5 (5.5–17.3) | 13.1 (6.1–24.2) | 11.3 (6.8–20.3) | 12.2 (4.8–17.4) | 9.6 (5.3–15.5) | 8.9 (5.2–14.5) | 10.4 (6.8–15.5) | 9.5 (6.8–14.0) | 10.7 (6.8–21.5) | 0.577 | 0.734 | 0.429 |
| Streptococcus pyogenes | 2.9 (2.5–3.4) | 2.8 (2.1–3.3) | 2.8 (1.4–3.1) | 2.8 (2.0–4.4) | 2.6 (2.1–3.6) | 2.5 (2.1–3.6) | 2.4 (2.0–3.5) | 2.6 (1.6–3.7) | 2.6 (2.3–3.4) | 0.948 | 0.363 | 0.727 |
| Streptococcus salivarius | 2.9 (1.3–22.1) | 2.6 (1.4–11.7) | 2.4 (1.0–22.8) | 3.1 (1.6–35.2) | 4.2 (2.0–34.8) | 12.6 (1.3–23.5) | 3.8 (1.0–26.5) | 6.3 (1.4–12.4) | 10.0 (1.7–10.6) | 0.57 | 0.758 | 0.713 |
| Streptococcus sp. HSISS1 | 5.9 (1.0–12.6) | 7.1 (0.1–12.7) | 4.3 (0.5–11.5) | 4.6 (0.4–12.8) | 4.3 (1.3–6.5) | 3.8 (1.8–9.7) | 5.0 (2.1–10.0) | 4.5 (0.3–9.4) | 3.5 (1.5–8.8) | 0.809 | 0.814 | 0.681 |
| Streptococcus sp. LPB0220 | 1.8 (0.6–10.7) | 3.3 (0.9–6.8) | 1.3 (0.5–9.8) | 3.5 (0.5–13.0) | 5.9 (1.4–10.8) | 8.0 (2.7–11.1) | 5.9 (0.3–12.4) | 0.8 (0.1–11.3) | 1.6 (0.08–7.2) | 0.446 | 0.802 | 0.308 |
| Streptococcus suis | 3.4 (2.0–6.3) | 3.5 (1.9–4.8) | 2.9 (1.5–4.9) | 3.5 (1.6–8.4) | 3.6 (2.0–7.6) | 4.1 (1.3–6.2) | 2.6 (1.4–7.0) | 3.5 (1.1–4.9) | 3.2 (1.6–4.5) | 0.635 | 0.885 | 0.927 |
| Streptococcus thermophilus | 8.9 (7.7–10.1) | 8.5 (7.3–10.3) | 8.6 (3.9–9.5) | 9.6 (6.4–16.7) | 9.5 (6.9–16.8) | 9.2 (6.4–13.5) | 7.4 (6.4–12.9) | 8.3 (4.4–10.1) | 8.6 (5.6–10.2) | 0.401 | 0.329 | 0.243 |
| Lachnoanaerobaculum umeaense | 1.4 (0.06–4.0) | 1.4 (0.2–3.8) | 0.9 (0.1–9.0) | 0.1 (0.05–3.9) | 0.9 (0.1–1.7) | 0.5 (0.03–5.4) | 1.1 (0.5–2.5) | 2.0 (0.2–5.2) | 1.6 (0.2–4.6) | 0.458 | 0.62 | 0.868 |
| Veillonella atypica | 1.5 (0.01–10.2) | 1.6 (0.02–13.7) | 0.3 (0.03–3.0) | 0.5 (0.07–3.3) | 0.6 (0.6–6.2) | 2.5 (1.0–4.0) | 4.1 (0.3–10.7) | 1.4 (0.1–8.4) | 2.8 (1.0–4.3) | 0.437 | 0.64 | 0.129 |
| Veillonella nakazawae | 0.7 (0.1–1.0) | 0.5 (0.1–1.7) | 0.3 (0.2–0.3) | 0.1 (0.1–0.3) | 0.3 (0.2–0.5) | 0.3 (0.1–0.4) | 0.1 (0.04–0.6) | 0.4 (0.4–0.4) | 0.05 (0.05–0.08) | 0.158 | 0.683 | 0.946 |
| Veillonella parvula | 6.9 (2.8–9.6) | 4.8 (2.9–15.8) | 2.1 a (0.03–4.9) | 3.4 (1.3–3.9) | 5.1 (3.1–7.1) | 4.7 b (0.5–7.9) | 4.6 (1.3–9.6) | 3.6 (0.5–8.9) | 2.9 b (1.8–5.2) | 0.608 | 0.053 | 0.043 |
| Shannon index | 3.1 (2.8–3.3) | 3.1 (2.7–3.5) | 3.0 (2.9–3.6) | 3.1 (2.6–3.5) | 3.1 (2.6–3.4) | 3.0 (2.9–3.2) | 3.2 (2.7–3.5) | 3.2 (2.9–3.7) | 3.3 (2.8–3.4) | 0.473 | 0.851 | 0.948 |
| Simpson’s index | 0.9 (0.9–0.9) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.9 (0.9–0.9) | 0.9 (0.9–0.9) | 0.9 (0.9–1.0) | 0.9 (0.9–0.9) | 0.584 | 0.998 | 0.895 |
| Chao1 index | 48.0 (21.0–89.0) | 40.1 (21.9–80.0) | 42.6 (26.6–65.7) | 39.2 (27.2–51.8) | 46.5 (26.0–69.3) | 33.1 (24.1–52.3) | 37.0 (26.6–55.7) | 36.7 (30.0–59.0) | 36.3 (26.5–59.0) | 0.339 | 0.339 | 0.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; López-Plaza, B.; Brandimonte-Hernández, M.; Álvarez-Mercado, A.I.; Arcos-Castellanos, L.; Feliú-Batlle, J.; Hummel, T.; Palma-Milla, S.; Gil, A. Effect of a Novel Food Rich in Miraculin on the Oral Microbiome of Malnourished Oncologic Patients with Dysgeusia. Cancers 2024, 16, 3414. https://doi.org/10.3390/cancers16193414
Plaza-Diaz J, Ruiz-Ojeda FJ, López-Plaza B, Brandimonte-Hernández M, Álvarez-Mercado AI, Arcos-Castellanos L, Feliú-Batlle J, Hummel T, Palma-Milla S, Gil A. Effect of a Novel Food Rich in Miraculin on the Oral Microbiome of Malnourished Oncologic Patients with Dysgeusia. Cancers. 2024; 16(19):3414. https://doi.org/10.3390/cancers16193414
Chicago/Turabian StylePlaza-Diaz, Julio, Francisco Javier Ruiz-Ojeda, Bricia López-Plaza, Marco Brandimonte-Hernández, Ana Isabel Álvarez-Mercado, Lucía Arcos-Castellanos, Jaime Feliú-Batlle, Thomas Hummel, Samara Palma-Milla, and Angel Gil. 2024. "Effect of a Novel Food Rich in Miraculin on the Oral Microbiome of Malnourished Oncologic Patients with Dysgeusia" Cancers 16, no. 19: 3414. https://doi.org/10.3390/cancers16193414
APA StylePlaza-Diaz, J., Ruiz-Ojeda, F. J., López-Plaza, B., Brandimonte-Hernández, M., Álvarez-Mercado, A. I., Arcos-Castellanos, L., Feliú-Batlle, J., Hummel, T., Palma-Milla, S., & Gil, A. (2024). Effect of a Novel Food Rich in Miraculin on the Oral Microbiome of Malnourished Oncologic Patients with Dysgeusia. Cancers, 16(19), 3414. https://doi.org/10.3390/cancers16193414









