Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
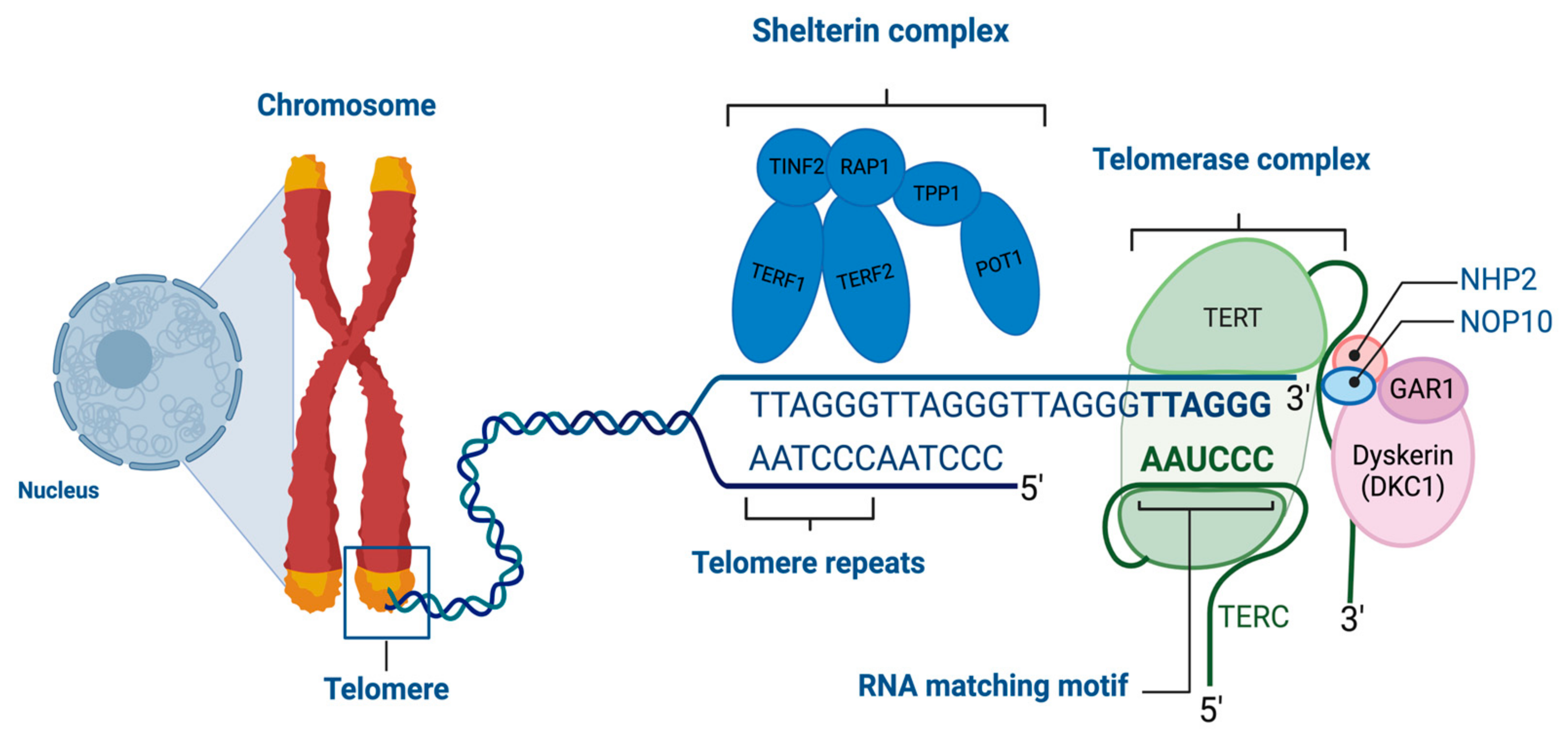
1.1. Telomeres
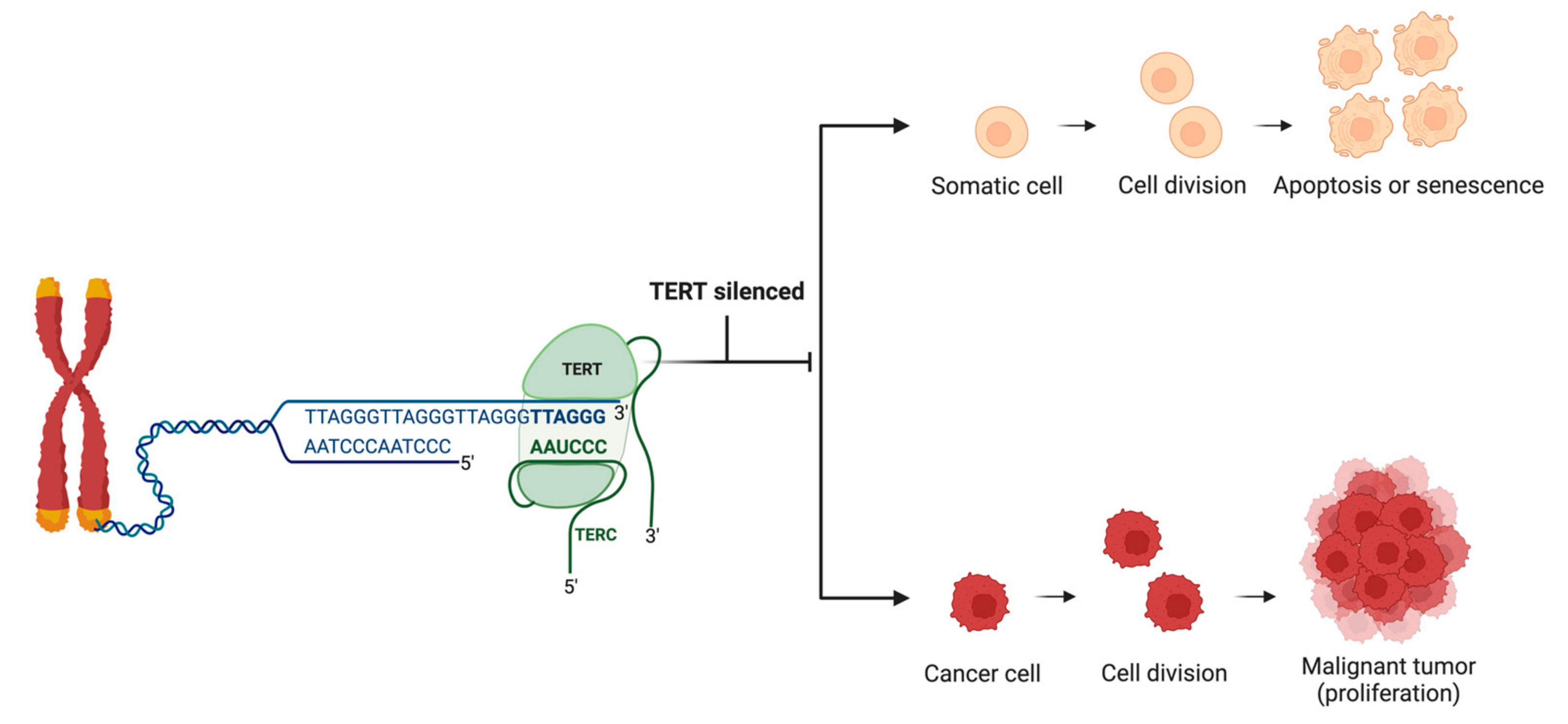
1.2. Telomerase
2. Telomere Length and Telomerase Activity in Gastrointestinal Cancers
2.1. Esophageal Cancer
2.2. Gastric Cancer
2.3. Colorectal Cancer
2.4. Liver Cancer
2.5. Pancreatic Cancer
3. Telomere Length and Anticancer Therapeutic Strategies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [PubMed]
- Sewpersad, S.; Pillay, T.S. Historical perspectives in clinical pathology: Bence Jones protein—Early urine chemistry and the impact on modern day diagnostics. J. Clin. Pathol. 2021, 74, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S.; Xie, W.; Wang, Q.; Luo, Q.; Huang, M.; Gu, M.; Lan, P.; Chen, D. MCCC2 is a novel mediator between mitochondria and telomere and functions as an oncogene in colorectal cancer. Cell. Mol. Biol. Lett. 2023, 28, 80. [Google Scholar] [CrossRef]
- Wentzensen, I.M.; Mirabello, L.; Pfeiffer, R.M.; Savage, S.A. The Association of Telomere Length and Cancer: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, C.M.; Meeker, A.K. The potential utility of telomere-related markers for cancer diagnosis. J. Cell. Mol. Med. 2011, 15, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Son, N.H.; Murray, S.; Yanovski, J.; Hodes, R.J.; Weng, N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J. Immunol. 2000, 165, 1191–1196. [Google Scholar] [CrossRef]
- Shay, J.; Wright, W. Hallmarks of telomeres in ageing research. J. Pathol. 2007, 211, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Pooley, K.A.; Sandhu, M.S.; Tyrer, J.; Shah, M.; Driver, K.E.; Luben, R.N.; Bingham, S.A.; Ponder, B.A.J.; Pharoah, P.D.P.; Khaw, K.-T.; et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010, 70, 3170–3176. [Google Scholar] [CrossRef]
- Vakonaki, E.; Tsiminikaki, K.; Plaitis, S.; Fragkiadaki, P.; Tsoukalas, D.; Katsikantami, I.; Vaki, G.; Tzatzarakis, M.N.; Spandidos, D.A.; Tsatsakis, A.M. Common mental disorders and association with telomere length (Review). Biomed. Rep. 2018, 8, 111–116. [Google Scholar] [CrossRef]
- Sharma, G.; Hall, E.; Dhar, S.; Gupta, A.; Rao, P.; Pandita, T. Telomere stability correlates with longevity of human beings exposed to ionizing radiations. Oncol. Rep. 2003, 10, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Andrew, T.; Gardner, J.; Kimura, M.; Oelsner, E.; Cherkas, L.; Aviv, A.; Spector, T. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Starodubova, A.V.; Orekhov, A.N. Role of Telomeres Shortening in Atherogenesis: An Overview. Cells 2021, 10, 395. [Google Scholar] [CrossRef]
- Starr, J.; McGurn, B.; Harris, S.; Whalley, L.; Deary, I.; Shiels, P. Association between telomere length and heart disease in a narrow age cohort of older people. Exp. Gerontol. 2007, 42, 571–573. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Oikonomopoulou, T.; Nikolouzakis, T.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Renieri, E.; Fragkiadaki, P.; Iliaki, E.; Bachlitzanaki, M.; et al. Role of telomere length in human carcinogenesis (Review). Int. J. Oncol. 2023, 63, 78. [Google Scholar] [CrossRef] [PubMed]
- Diotti, R.; Loayza, D. Shelterin complex and associated factors at human telomeres. Nucleus 2011, 2, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.N.; Bronner, M.P.; Brentnall, T.A.; Finley, J.C.; Shen, W.T.; Emerson, S.; Emond, M.J.; Gollahon, K.A.; Moskovitz, A.H.; Crispin, D.A.; et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 2002, 32, 280–284. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Risques, R.A.; Mandelson, M.T.; Chen, L.; Brentnall, T.A.; Bronner, M.P.; Macmillan, M.P.; Feng, Z.; Siebert, J.R.; Potter, J.D.; et al. Telomere Length in the Colon Declines with Age: A Relation to Colorectal Cancer? Cancer Epidemiol. Biomark. Prev. 2006, 15, 573–577. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Fan, H.C.; Chang, F.W.; Tsai, J.D.; Lin, K.M.; Chen, C.M.; Lin, S.Z.; Liu, C.A.; Harn, H.J. Telomeres and Cancer. Life 2021, 11, 1405. [Google Scholar] [CrossRef]
- Gong, P.; Wang, H.; Zhang, J.; Fu, Y.; Zhu, Z.; Wang, J.; Yin, Y.; Wang, H.; Zhou, Z.; Yang, J.; et al. Telomere Maintenance-Associated PML Is a Potential Specific Therapeutic Target of Human Colorectal Cancer. Transl. Oncol. 2019, 12, 1164–1176. [Google Scholar] [CrossRef]
- Kibriya, M.G.; Raza, M.; Kamal, M.; Haq, Z.; Paul, R.; Mareczko, A.; Pierce, B.L.; Ahsan, H.; Jasmine, F. Relative Telomere Length Change in Colorectal Carcinoma and Its Association with Tumor Characteristics, Gene Expression and Microsatellite Instability. Cancers 2022, 14, 2250. [Google Scholar] [CrossRef]
- Shay, J.W.; Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.L.; Westra, W.H.; et al. Prevalence of the Alternative Lengthening of Telomeres Telomere Maintenance Mechanism in Human Cancer Subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Nikolouzakis, T.K.; Chrysos, E.; Docea, A.O.; Fragkiadaki, P.; Souglakos, J.; Tsiaoussis, J.; Tsatsakis, A. Current and Future Trends of Colorectal Cancer Treatment: Exploring Advances in Immunotherapy. Cancers 2024, 16, 1995. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Willeit, J.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Brandstätter, A.; Kronenberg, F.; Kiechl, S. Telomere Length and Risk of Incident Cancer and Cancer Mortality. JAMA 2010, 304, 69. [Google Scholar] [CrossRef]
- Skinner, H.G.; Gangnon, R.E.; Litzelman, K.; Johnson, R.A.; Chari, S.T.; Petersen, G.M.; Boardman, L.A. Telomere Length and Pancreatic Cancer: A Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2095–2100. [Google Scholar] [CrossRef]
- Tsiaoussis, J.; Vassilopoulou, L.; Nikolouzakis, T.; Rakitskii, V.N.; Vakonaki, E.; Fragkiadaki, P.; Stivaktakis, P.; Tsatsakis, A.M. Biomolecular Profile of Colorectal Cancer—The Role of Telomerase as a Potent Biomarker. Farmacia 2017, 66, 643–659. [Google Scholar]
- Calado, R.T.; Young, N.S. Telomere diseases. N. Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, T.; Zhang, N.; Zheng, Y.; Kou, P.; Shang, S.; Yang, M. Leukocyte telomere length and risk of gastric cardia adenocarcinoma. Sci. Rep. 2018, 8, 14584. [Google Scholar] [CrossRef]
- Risques, R.A.; Vaughan, T.L.; Li, X.; Odze, R.D.; Blount, P.L.; Ayub, K.; Gallaher, J.L.; Reid, B.J.; Rabinovitch, P.S. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2649–2655. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, H.C.; Wang, Q.; Yang, H.I.; Chen, C.J.; Santella, R.M.; Shen, J. Telomere Length and Risk of Hepatocellular Carcinoma: A Nested Case–Control Study in Taiwan Cancer Screening Program Cohort. Anticancer. Res. 2017, 37, 637–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.; Han, W.; Xue, W.; Zou, Y.; Xie, C.; Du, J.; Jin, G. The association between telomere length and cancer risk in population studies. Sci. Rep. 2016, 6, 22243. [Google Scholar] [CrossRef] [PubMed]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Lu, R.; Pickett, H.A. Telomeric replication stress: The beginning and the end for alternative lengthening of telomeres cancers. Open Biol. 2022, 12, 220011. [Google Scholar] [CrossRef]
- Okayasu, I.; Mitomi, H.; Yamashita, K.; Mikami, T.; Fujiwara, M.; Kato, M.; Oshimura, M. Telomerase activity significantly correlates with cell differentiation, proliferation and lymph node metastasis in colorectal carcinomas. J. Cancer Res. Clin. Oncol. 1998, 124, 444–449. [Google Scholar] [CrossRef]
- Yoshida, R.; Kiyozuka, Y.; Ichiyoshi, H.; Senzaki, H.; Takada, H.; Hioki, K.; Tsubura, A. Change in telomerase activity during human colorectal carcinogenesis. Anticancer. Res. 1999, 19, 2167–2172. [Google Scholar] [PubMed]
- Bertorelle, R.; Briarava, M.; Rampazzo, E.; Biasini, L.; Agostini, M.; Maretto, I.; Lonardi, S.; Friso, M.L.; Mescoli, C.; Zagonel, V.; et al. Telomerase is an independent prognostic marker of overall survival in patients with colorectal cancer. Br. J. Cancer 2013, 108, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Ishida, M.; Motoi, F.; Yamaguchi, T.; Naitoh, T.; Katayose, Y.; Egawa, S.; Unno, M. Telomerase activity in pancreatic juice differentiates pancreatic cancer from chronic pancreatitis: A meta-analysis. Pancreatology 2016, 16, 372–381. [Google Scholar] [CrossRef]
- Liu, X.; Bao, G.; Huo, T.; Wang, Z.; He, X.; Dong, G. Constitutive telomere length and gastric cancer risk: Case-control analysis in Chinese Han population. Cancer Sci. 2009, 100, 1300–1305. [Google Scholar] [CrossRef]
- Hou, L.; Savage, S.A.; Blaser, M.J.; Perez-Perez, G.; Hoxha, M.; Dioni, L.; Pegoraro, V.; Dong, L.M.; Zatonski, W.; Lissowska, J.; et al. Telomere Length in Peripheral Leukocyte DNA and Gastric Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3103–3109. [Google Scholar]
- Shi, Y.; Zhang, Y.; Zhang, L.; Ma, J.L.; Zhou, T.; Li, Z.X.; Liu, W.-D.; Li, W.-Q.; Deng, D.-J.; You, W.-C.; et al. Telomere Length of Circulating Cell-Free DNA and Gastric Cancer in a Chinese Population at High-Risk. Front. Oncol. 2019, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koh, W.P.; Jin, A.; Wang, R.; Yuan, J.M. Telomere length and risk of developing gastric adenocarcinoma: The Singapore Chinese Health Study. Gastric Cancer 2018, 21, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Bhat, G.R.; Rah, B.; Besina, S.; Zahoor, S.; Wani, M.A.; Shah, M.A.; Bashir, S.; Farooq, M.; Rather, R.A.; et al. Telomere Attrition with Concomitant hTERT Overexpression Involved in the Progression of Gastric Cancer May Have Prognostic and Clinical Implications in High-Risk Population Group from North India. Front. Oncol. 2022, 12, 919351. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Shiota, G.; Oshimura, M.; Kawasaki, H. Clinical usefulness of telomerase activity and telomere length in the preoperative diagnosis of gastric and colorectal cancer. J. Cancer Res. Clin. Oncol. 1999, 125, 405–410. [Google Scholar] [CrossRef]
- Rathi, A.; Hur, K.; Gazdar, A.F.; Bae, J.S.; Jang, J.J.; Kim, D.Y. Telomerase RNA expression during progression of gastric cancer. Hum. Pathol. 1999, 30, 1302–1308. [Google Scholar] [CrossRef]
- Hu, X.; Wu, H.; Zhang, S.; Yuan, H.; Cao, L. Clinical Significance of Telomerase Activity in Gastric Carcinoma and Peritoneal Dissemination. J. Int. Med. Res. 2009, 37, 1127–1138. [Google Scholar] [CrossRef]
- Svinareva, L.V.; Glukhov, A.I.; Zimnik, O.V.; Bykov, I.I.; Khorobrykh, T.V.; Shvets, V.I. The study of telomerase activity in gastric cancer. Biochem. Mosc. Suppl. B Biomed. Chem. 2011, 5, 188–192. [Google Scholar] [CrossRef]
- Xing, J.; Ajani, J.A.; Chen, M.; Izzo, J.; Lin, J.; Chen, Z.; Gu, J.; Wu, X. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev. Res. 2009, 2, 459–465. [Google Scholar] [CrossRef]
- Wennerström, E.C.M.; Risques, R.A.; Prunkard, D.; Giffen, C.; Corley, D.A.; Murray, L.J.; Whiteman, D.C.; Wu, A.H.; Bernstein, L.; Ye, W.; et al. Leukocyte telomere length in relation to the risk of Barrett’s esophagus and esophageal adenocarcinoma. Cancer Med. 2016, 5, 2657–2665. [Google Scholar] [CrossRef][Green Version]
- Lv, Y.; Zhang, Y.; Li, X.; Ren, X.; Wang, M.; Tian, S.; Hou, P.; Shi, B.; Yang, Q. Long telomere length predicts poor clinical outcome in esophageal cancer patients. Pathol. Res. Pr. 2017, 213, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, G.; Song, J.; Tan, G.; Wu, X. Telomerase inhibition decreases esophageal squamous carcinoma cell migration and invasion. Oncol. Lett. 2020, 20, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Unate, H.; Maeta, M.; Kaibara, N. Detection of telomerase activity in esophageal squamous cell carcinoma and normal esophageal epithelium. Langenbecks Arch. Surg. 1999, 384, 550–555. [Google Scholar] [CrossRef]
- Takubo, K.; Nakamura, K.; Izumiyama, N.; Mafune, K.; Tanaka, Y.; Miyashita, M.; Sasajima, K.; Kato, M.; Oshimura, M. Telomerase activity in esophageal carcinoma. J. Surg. Oncol. 1997, 66, 88–92. [Google Scholar] [CrossRef]
- Mitsui, A.; Kuwabara, Y.; Iwase, H.; Mitani, M.; Shinoda, N.; Sato, A.; Toyama, T.; Sugiura, M.; Suzuki, T.; Kato, J.; et al. Telomerase activity in esophageal squamous cell carcinoma: Down-regulation by chemotherapeutic agent. J. Surg. Oncol. 2002, 79, 37–45. [Google Scholar] [CrossRef]
- Raynaud, C.M.; Jang, S.J.; Nuciforo, P.; Lantuejoul, S.; Brambilla, E.; Mounier, N.; Olaussen, K.A.; André, F.; Morat, L.; Sabatier, L.; et al. Telomere shortening is correlated with the DNA damage response and telomeric protein down-regulation in colorectal preneoplastic lesions. Ann. Oncol. 2008, 19, 1875–1881. [Google Scholar] [CrossRef]
- Garcia-Aranda, C.; de Juan, C.; Diaz-Lopez, A.; Sanchez-Pernaute, A.; Torres, A.; Diaz-Rubio, E.; Balibrea, J.-L.; Benito, M.; Iniesta, P. Correlations of telomere length, telomerase activity, and telomeric-repeat binding factor 1 expression in colorectal carcinoma. Cancer 2006, 106, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Gertler, R.; Rosenberg, R.; Stricker, D.; Friederichs, J.; Hoos, A.; Werner, M.; Ulm, K.; Holzmann, B.; Nekarda, H.; Siewert, J.-R. Telomere Length and Human Telomerase Reverse Transcriptase Expression As Markers for Progression and Prognosis of Colorectal Carcinoma. J. Clin. Oncol. 2004, 22, 1807–1814. [Google Scholar] [CrossRef]
- Tatsumoto, N.; Hiyama, E.; Murakami, Y.; Imamura, Y.; Shay, J.W.; Matsuura, Y.; Yokoyama, T. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin. Cancer Res. 2000, 6, 2696–2701. [Google Scholar]
- Engelhardt, M.; Drullinsky, P.; Guillem, J.; Moore, M.A. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin. Cancer Res. 1997, 3, 1931–1941. [Google Scholar]
- Engelhardt, M.; Albanell, J.; Drullinsky, P.; Han, W.; Guillem, J.; Scher, H.I.; Reuter, V.; Moore, M.A. Relative contribution of normal and neoplastic cells determines telomerase activity and telomere length in primary cancers of the prostate, colon, and sarcoma. Clin. Cancer Res. 1997, 3, 1849–1857. [Google Scholar] [PubMed]
- Hastie, N.D.; Dempster, M.; Dunlop, M.G.; Thompson, A.M.; Green, D.K.; Allshire, R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990, 346, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Zee, R.Y.L.; Castonguay, A.J.; Barton, N.S.; Buring, J.E. Mean telomere length and risk of incident colorectal carcinoma: A prospective, nested case-control approach. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Y.; Lai, M.-D. Colorectal cancer, one entity or three. J. Zhejiang Univ. Sci. B 2009, 10, 219–229. [Google Scholar] [CrossRef]
- Fernández-Marcelo, T.; Sánchez-Pernaute, A.; Pascua, I.; De Juan, C.; Head, J.; Torres-García, A.J.; Iniesta, P. Clinical Relevance of Telomere Status and Telomerase Activity in Colorectal Cancer. PLoS ONE 2016, 11, e0149626. [Google Scholar] [CrossRef]
- Rampazzo, E.; Bertorelle, R.; Serra, L.; Terrin, L.; Candiotto, C.; Pucciarelli, S.; Del Bianco, P.; Nitti, D.; De Rossi, A. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br. J. Cancer 2010, 102, 1300–1305. [Google Scholar] [CrossRef]
- Zöchmeister, C.; Brezina, S.; Hofer, P.; Baierl, A.; Bergmann, M.M.; Bachleitner-Hofmann, T.; Karner-Hanusch, J.; Stift, A.; Gerger, A.; Leeb, G.; et al. Leukocyte telomere length throughout the continuum of colorectal carcinogenesis. Oncotarget 2018, 9, 13582–13592. [Google Scholar] [CrossRef]
- Peacock, S.D.; Massey, T.E.; Vanner, S.J.; King, W.D. Telomere length in the colon is related to colorectal adenoma prevalence. PLoS ONE 2018, 13, e0205697. [Google Scholar] [CrossRef]
- Nikolouzakis, T.; Vassilopoulou, L.; Fragkiadaki, P.; Mariolis Sapsakos, T.; Papadakis, G.; Spandidos, D.; Tsatsakis, A.M.; Tsiaoussis, J. Improving diagnosis, prognosis and prediction by using biomarkers in CRC patients (Review). Oncol Rep. 2018, 39, 2455–2472. [Google Scholar] [CrossRef]
- Kroupa, M.; Rachakonda, S.K.; Liska, V.; Srinivas, N.; Urbanova, M.; Jiraskova, K.; Schneiderova, M.; Vycital, O.; Vymetalkova, V.; Vodickova, L.; et al. Relationship of telomere length in colorectal cancer patients with cancer phenotype and patient prognosis. Br. J. Cancer 2019, 121, 344–350. [Google Scholar] [CrossRef]
- Kroupa, M.; Kubecek, O.; Tomasova, K.; Hanak, P.; Krupova, M.; Cervena, K.; Siskova, A.; Rosendorf, J.; Hosek, P.; Vodickova, L.; et al. The dynamics of telomere length in primary and metastatic colorectal cancer lesions. Sci. Rep. 2023, 13, 9097. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, J.; Song, C.; Chen, W. Telomere in colorectal cancer associated with distant metastases and predicted a poor prognosis. Transl. Cancer Res. 2021, 10, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Bertorelle, R. Telomeres, telomerase and colorectal cancer. World J. Gastroenterol. 2014, 20, 1940. [Google Scholar] [CrossRef]
- Urabe, Y.; Nouso, K.; Higashi, T.; Nakatsukasa, H.; Hino, N.; Ashida, K.; Kinugasa, N.; Yoshida, K.; Uematsu, S.; Tsuji, T. Telomere length in human liver diseases. Liver 1996, 16, 293–297. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, C.; Huang, M.; Du, F.; Song, C.; Ma, Z.; Zhai, X.; Yang, Y.; Liu, J.; Bei, J.-X.; et al. Genetic association of telomere length with hepatocellular carcinoma risk: A Mendelian randomization analysis. Cancer Epidemiol. 2017, 50 Pt A, 39–45. [Google Scholar] [CrossRef]
- Ma, L.J.; Wang, X.Y.; Duan, M.; Liu, L.Z.; Shi, J.Y.; Dong, L.Q.; Yang, L.-X.; Wang, Z.-C.; Ding, Z.-B.; Ke, A.-W.; et al. Telomere length variation in tumor cells and cancer-associated fibroblasts: Potential biomarker for hepatocellular carcinoma. J. Pathol. 2017, 243, 407–417. [Google Scholar] [CrossRef]
- Rashid, S.; Sun, Y.; Ali Khan Saddozai, U.; Hayyat, S.; Usman Munir, M.; Usman Akbar, M.; Khawar, M.B.; Ren, Z.; Ji, X.; Khan, M.I.U. Circulating tumor DNA and its role in detection, prognosis and therapeutics of hepatocellular carcinoma. Chin. J. Cancer Res. 2024, 36, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Choi, J.Y.; Byun, B.H.; Cho, C.H.; Kim, H.S.; Kim, B.S. Telomerase is strongly activated in hepatocellular carcinoma but not in chronic hepatitis and cirrhosis. Exp. Mol. Med. 1998, 30, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.I.; Tahara, H.; Tahara, E.; Saito, M.; Ito, K.; Nakamura, H.; Nakanishi, T.; Tahara, E.; Ide, T.; Ishikawa, F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat. Genet. 1998, 18, 65–68. [Google Scholar] [CrossRef]
- Satyanarayana, A.; Manns, M.P.; Rudolph, K.L. Telomeres and telomerase: A dual role in hepatocarcinogenesis. Hepatology 2004, 40, 276–283. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kubota, K.; Takayama, T.; Makuuchi, M. Telomerase activity as a predictive marker for recurrence of hepatocellular carcinoma after hepatectomy. Am. J. Surg. 2001, 181, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sugawara, Y.; Shi, Y.Z.; Makuuchi, M. Telomerase Expression and p53 Status in Hepatocellular Carcinoma. Am. J. Gastroenterol. 2002, 97, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J. Telomere length and pancreatic cancer risk: Breaking down the evidence. Gut 2017, 66, 1. [Google Scholar] [CrossRef]
- Lynch, S.M.; Major, J.M.; Cawthon, R.; Weinstein, S.J.; Virtamo, J.; Lan, Q.; Rothman, N.; Albanes, D.; Stolzenberg-Solomon, R.Z. A prospective analysis of telomere length and pancreatic cancer in the alpha-tocopherol beta-carotene cancer (ATBC) prevention study. Int. J. Cancer 2013, 133, 2672–2680. [Google Scholar] [CrossRef]
- Luu, H.N.; Huang, J.Y.; Wang, R.; Adams-Haduch, J.; Jin, A.; Koh, W.P.; Yuan, J.-M. Association between leukocyte telomere length and the risk of pancreatic cancer: Findings from a prospective study. PLoS ONE 2019, 14, e0221697. [Google Scholar] [CrossRef]
- Campa, D.; Mergarten, B.; De Vivo, I.; Boutron-Ruault, M.C.; Racine, A.; Severi, G.; Nieters, A.; Katzke, V.A.; Trichopoulou, A.; Yiannakouris, N.; et al. Leukocyte Telomere Length in Relation to Pancreatic Cancer Risk: A Prospective Study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2447–2454. [Google Scholar] [CrossRef]
- Mizumoto, K.; Suehara, N.; Muta, T.; Kitajima, S.; Hamasaki, N.; Tominaga, Y.; Shimura, H.; Tanaka, M. Semi-quantitative analysis of telomerase in pancreatic ductal adenocarcinoma. J. Gastroenterol. 1996, 31, 894–897. [Google Scholar] [CrossRef]
- Hiyama, E.; Kodama, T.; Shinbara, K.; Iwao, T.; Itoh, M.; Hiyama, K.; Shay, J.W.; Matsuura, Y.; Yokoyama, T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997, 57, 326–331. [Google Scholar] [PubMed]
- Inoue, H.; Tsuchida, A.; Kawasaki, Y.; Fujimoto, Y.; Yamasaki, S.; Kajiyama, G. Preoperative diagnosis of intraductal papillary-mucinous tumors of the pancreas with attention to telomerase activity. Cancer 2001, 91, 35–41. [Google Scholar] [CrossRef]
- Nakashima, A.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Hashimoto, Y.; Ohge, H.; Oda, M.C.; Sueda, T.; Hiyama, E. Usefulness of human telomerase reverse transcriptase in pancreatic juice as a biomarker of pancreatic malignancy. Pancreas 2009, 38, 527–533. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Ohge, H.; Fukuda, E.; Sueda, T.; Hiyama, E. Detection of human telomerase reverse transcriptase (hTERT) expression in tissue and pancreatic juice from pancreatic cancer. Surgery 2008, 143, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Srinivasan, R.; Vasishta, R.K.; Wig, J.D. Positive regulation of human telomerase reverse transcriptase gene expression and telomerase activity by DNA methylation in pancreatic cancer. Ann. Surg. Oncol. 2009, 16, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, M.Y.; Liang, Y.R.; Wu, X.Y. Correlation between expression of human telomerase subunits and telomerase activity in esophageal squamous cell carcinoma. World J. Gastroenterol. 2003, 9, 2395–2399. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, Q.; Mei, L.; Liu, X.; Yang, W.; Yu, J. Telomere shortening occurs early during gastrocarcinogenesis. Med. Oncol. 2012, 29, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Pascua, I.; Fernández-Marcelo, T.; Sánchez-Pernaute, A.; de Juan, C.; Head, J.; Torres-García, A.J.; Iniesta, P. Prognostic value of telomere function in gastric cancers with and without microsatellite instability. Eur. J. Gastroenterol. Hepatol. 2015, 27, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rufer, N.; Brümmendorf, T.H.; Kolvraa, S.; Bischoff, C.; Christensen, K.; Wadsworth, L.; Schulzer, M.; Lansdorp, P.M. Telomere Fluorescence Measurements in Granulocytes and T Lymphocyte Subsets Point to a High Turnover of Hematopoietic Stem Cells and Memory T Cells in Early Childhood. J. Exp. Med. 1999, 190, 157–168. [Google Scholar] [CrossRef]
- Friedrich, U.; Griese, E.U.; Schwab, M.; Fritz, P.; Thon, K.P.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef]
- Fern, L.; Pallis, M.; Ian Carter, G.; Seedhouse, C.; Russell, N.; Byrne, J. Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br. J. Haematol. 2004, 126, 63–71. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Zorov, D.B.; Plotnikov, E.Y.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Zorov, S.D.; Babenko, V.A.; Jankauskas, S.S.; Popkov, V.A.; Savina, P.S. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochemistry 2014, 79, 1017–1031. [Google Scholar] [CrossRef]
- Aeby, E.; Ahmed, W.; Redon, S.; Simanis, V.; Lingner, J. Peroxiredoxin 1 Protects Telomeres from Oxidative Damage and Preserves Telomeric DNA for Extension by Telomerase. Cell Rep. 2016, 17, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Gagnon, M.; Weckx, S.; Roy, D.; De Vuyst, L. Mutual Cross-Feeding Interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 Explain the Bifidogenic and Butyrogenic Effects of Arabinoxylan Oligosaccharides. Appl Environ Microbiol. 2015, 81, 7767–7781. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- in der Stroth, L.; Tharehalli, U.; Günes, C.; Lechel, A. Telomeres and Telomerase in the Development of Liver Cancer. Cancers 2020, 12, 2048. [Google Scholar] [CrossRef]
- Kitada, T.; Seki, S.; Kawakita, N.; Kuroki, T.; Monna, T. Telomere Shortening in Chronic Liver Diseases. Biochem. Biophys. Res. Commun. 1995, 211, 33–39. [Google Scholar] [CrossRef]
- Ivancich, M.; Schrank, Z.; Wojdyla, L.; Leviskas, B.; Kuckovic, A.; Sanjali, A.; Puri, N. Treating cancer by targeting telomeres and telomerase. Antioxidants 2017, 6, 15. [Google Scholar] [CrossRef]
- Gallicchio, L.; Gadalla, S.M.; Murphy, J.D.; Simonds, N.I. The Effect of Cancer Treatments on Telomere Length: A Systematic Review of the Literature. J. Natl. Cancer Inst. 2018, 110, 1048–1058. [Google Scholar] [CrossRef]
- Lee, J.J.; Nam, C.E.; Cho, S.H.; Park, K.S.; Chung, I.J.; Kim, H.J. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann. Hematol. 2003, 82, 492–495. [Google Scholar] [CrossRef]
- Chiappori, A.A.; Kolevska, T.; Spigel, D.R.; Hager, S.; Rarick, M.; Gadgeel, S.; Blais, N.; Von Pawel, J.; Hart, L.; Reck, M.; et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann. Oncol. 2015, 26, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Oshima, Y.; Yamamoto, Y.; Uochi, T.A.; Kusaka, H.; Akinaga, S.; Yamashita, Y.; Pongracz, K.; Pruzan, R.; Wunder, E.; et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003, 63, 3931–3939. [Google Scholar] [PubMed]
- Ali, J.H.; Walter, M. Combining old and new concepts in targeting telomerase for cancer therapy: Transient, immediate, complete and combinatory attack (TICCA). Cancer Cell Int. 2023, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- Ruden, M.; Puri, N. Novel anticancer therapeutics targeting telomerase. Cancer Treat. Rev. 2013, 39, 444–456. [Google Scholar] [CrossRef]
- Crees, Z.; Girard, J.; Rios, Z.; Botting, G.M.; Harrington, K.; Shearrow, C.; Wojdyla, L.; Stone, A.L.; Uppada, S.B.; Devito, J.T.; et al. Oligonucleotides and G-quadruplex stabilizers: Targeting telomeres and telomerase in cancer therapy. Curr. Pharm. Des. 2014, 20, 6422–6437. [Google Scholar] [CrossRef]
- Maestroni, L.; Matmati, S.; Coulon, S. Solving the Telomere Replication Problem. Genes 2017, 8, 55. [Google Scholar] [CrossRef]
- Wojdyla, L.; Stone, A.L.; Sethakorn, N.; Uppada, S.B.; Devito, J.T.; Bissonnette, M.; Puri, N. T-oligo as an anticancer agent in colorectal cancer. Biochem. Biophys. Res. Commun. 2014, 446, 596–601. [Google Scholar] [CrossRef]
- Uppada, S.B.; Erickson, T.; Wojdyla, L.; Moravec, D.N.; Song, Z.; Cheng, J.; Puri, N. Novel delivery system for T-oligo using a nanocomplex formed with an alpha helical peptide for melanoma therapy. Int. J. Nanomed. 2014, 9, 43–53. [Google Scholar]
- Wan, C.; Allen, T.M.; Cullis, P.R. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv. Transl. Res. 2014, 4, 74–83. [Google Scholar] [CrossRef]
- Apetroaei, M.M.; Fragkiadaki, P.; Velescu, B.Ș.; Baliou, S.; Renieri, E.; Dinu-Pirvu, C.E.; Drăgănescu, D.; Vlăsceanu, A.M.; Nedea, M.I.; Udeanu, D.I.; et al. Pharmacotherapeutic considerations on telomere biology: The positive effect of pharmacologically active substances on telomere length. Int. J. Mol. Sci. 2024, 25, 7694. [Google Scholar] [CrossRef]
- Keith, W.N.; Bilsland, A.; Hardie, M.; Evans, T.R. Drug insight: Cancer cell immortality-telomerase as a target for novel cancer gene therapies. Nat. Clin. Pract. Oncol. 2004, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
| Tumor Site | References | |
|---|---|---|
| Telomere Length | Telomerase Activity | |
| Stomach | Wentzensen et al., 2011 [11] Liu et al., 2018 [38] Liu et al., 2009 [49] Hou et al., 2009 [50] Shi et al., 2019 [51] Wang et al., 2018 [52] Cesare et al., 2010 [53] Mushtaq et al., 2022 [54] | Katayama et al., 1999 [55] Rathi et al. [56] Hu et al. [57] Svinareva et al. [58] |
| Esophagus | Wentzensen et al., 2011 [11] O’Sullivan et al., 2006 [26] Risques et al., 2007 [39] Zeng et al., 2017 [41] Xing et al., 2009 [59] Wennerström et al., 2016 [60] Lv et al., 2017 [61] Li et al., 2020 [62] | Ikeguchi et al. [63] Takubo et al. [64] Mitsui et al. [65] |
| Colorectum | Pooley et al., 2010 [16] O’Sullivan et al., 2006 [26] Wennerström et al., 2016 [60] Raynaud et al., 2008 [66] Garcia-Aranda et al., 2006 [67] Gertler et al., 2004 [68] Tatsumoto et al., 2000 [69] Engelhardt et al., 1997 [70,71] Hastie et al., 1990 [72] Zee et al., 2009 [73] Li and Lai 2009 [74] Fernández-Marcelo et al., 2016 [75] Rampazzo et al., 2010 [76] Zöchmeister et al., 2018 [77] Peacock et al., 2018 [78] Nikolouzakis et al., 2018 [79] Kroupa et al. [80] Kroupa et al. [81] Ye et al. [82] | Nikolouzakis et al., 2024 [33] Katayama et al., 1999 [55] Garcia-Aranda et al., 2006 [67] Tatsumoto et al., 2000 [69] Engelhardt et al., 1997 [70,71] Bertorelle 2013 [83] |
| Liver | Tsatsakis et al., 2023 [22] Zeng et al., 2017 [41] Urabe et al., 1996 [84] Cheng et al., 2017 [85] Ma et al., 2017 [86] Rashid et al., 2024 [87] | Park et al., 1998 [88] Nakayama et al., 1998 [89] Satyanarayana et al., 2004 [90] Kobayashi et al., 2001 [91] Kobayashi et al., 2002 [92] |
| Pancreas | Skinner et al., 2012 [35] Zeng et al., 2017 [41] Ma et al., 2017 [86] Duell 2017 [93] Lynch et al., 2013 [94] Luu et al., 2019 [95] Campa et al., 2014 [96] | Lynch et al., 2013 [94] Luu et al., 2019 [95] Campa et al., 2014 [96] Mizumoto et al., 1996 [97] Hiyama et al., 1997 [98] Inoue et al., 2001 [99] Nakashima et al., 2009 [100] Hashimoto et al., 2008 [101] Kumari et al., 2009 [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukopoulou, C.; Nikolouzakis, T.; Koliarakis, I.; Vakonaki, E.; Tsiaoussis, J. Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer. Cancers 2024, 16, 3370. https://doi.org/10.3390/cancers16193370
Loukopoulou C, Nikolouzakis T, Koliarakis I, Vakonaki E, Tsiaoussis J. Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer. Cancers. 2024; 16(19):3370. https://doi.org/10.3390/cancers16193370
Chicago/Turabian StyleLoukopoulou, Christina, Taxiarchis Nikolouzakis, Ioannis Koliarakis, Elena Vakonaki, and John Tsiaoussis. 2024. "Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer" Cancers 16, no. 19: 3370. https://doi.org/10.3390/cancers16193370
APA StyleLoukopoulou, C., Nikolouzakis, T., Koliarakis, I., Vakonaki, E., & Tsiaoussis, J. (2024). Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer. Cancers, 16(19), 3370. https://doi.org/10.3390/cancers16193370






