Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics in the Overall Cohort
3.2. Patient and Tumor Characteristics According to the Site of Origin
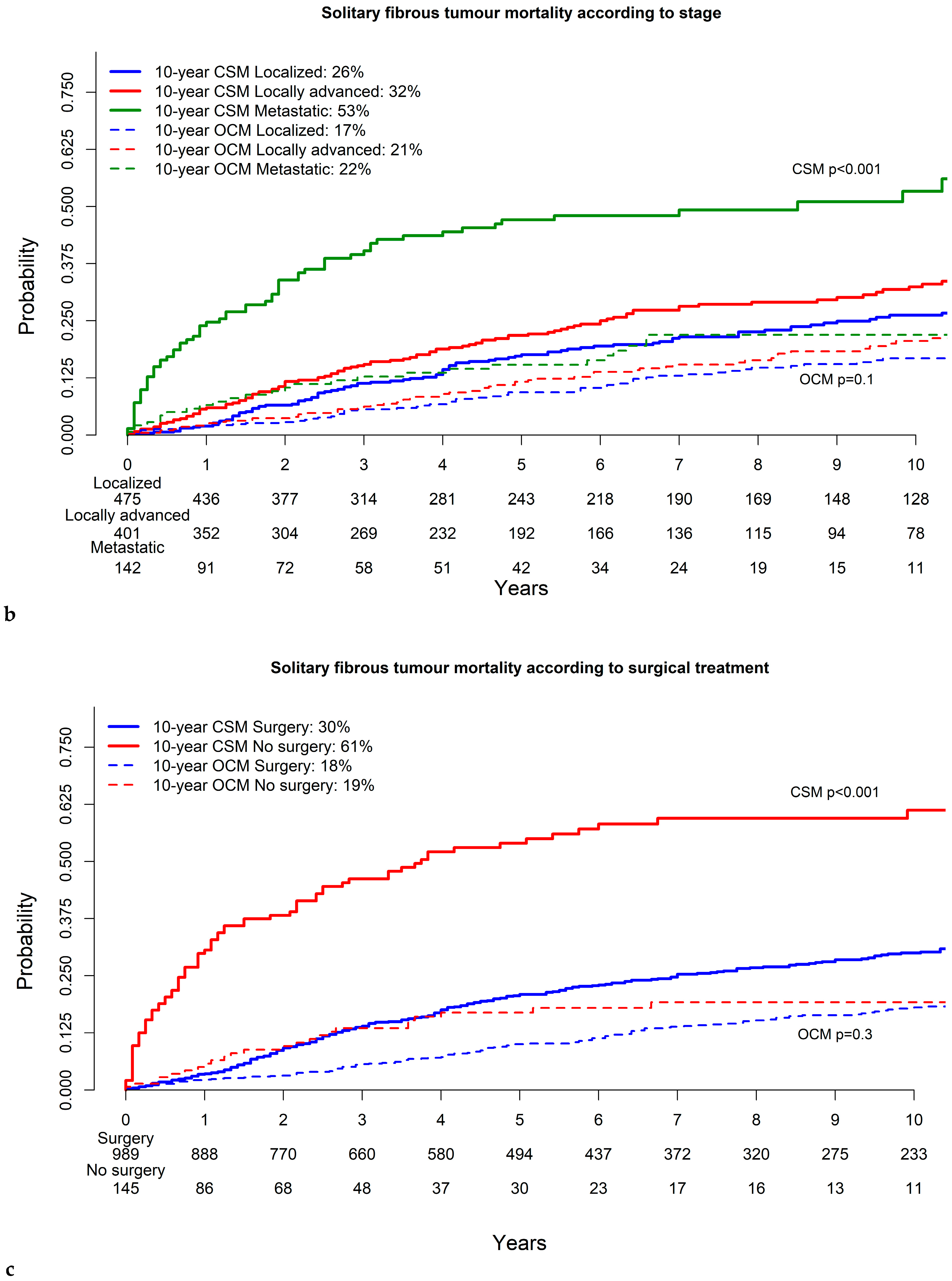
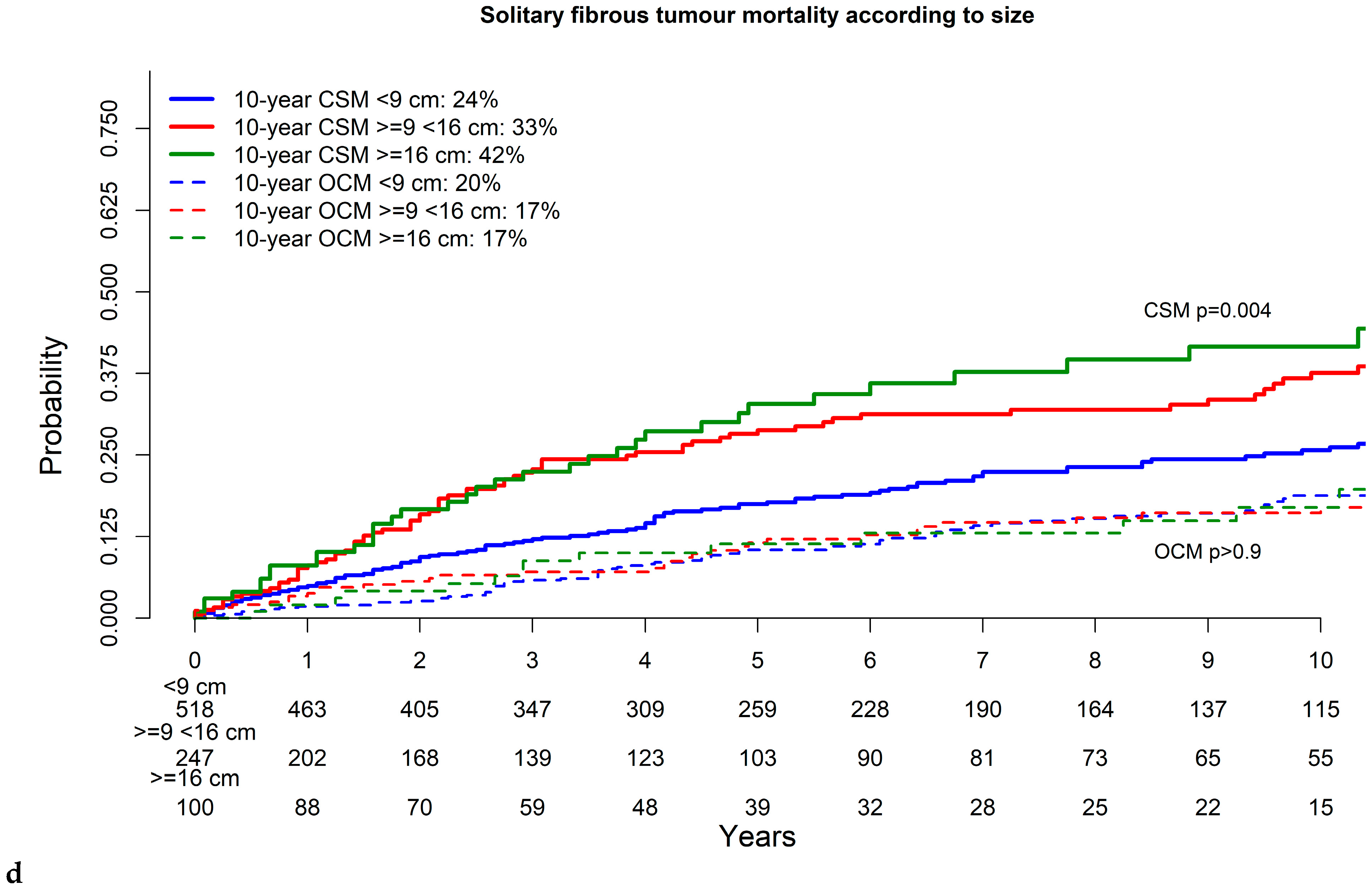
3.3. Cancer-Specific and Other-Cause Mortality in Solitary Fibrous Tumor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gold, J.S.; Antonescu, C.R.; Hajdu, C.; Ferrone, C.R.; Hussain, M.; Lewis, J.J.; Brennan, M.F.; Coit, D.G. Clinicopathologic Correlates of Solitary Fibrous Tumors. Cancer 2002, 94, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- de Bernardi, A.; Dufresne, A.; Mishellany, F.; Blay, J.Y.; Ray-Coquard, I.; Brahmi, M. Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond. Cancers 2022, 14, 1064. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Giner, F.; Cruz, J.; Lavernia, J.; Marhuenda-Fluixa, A.; Claramunt, R.; López-Guerrero, J.A.; Navarro, S.; Ferrandez, A.; Bujeda, Á.B.; et al. Extra-Meningeal Solitary Fibrous Tumor: An Evolving Entity with Chameleonic Morphological Diversity, a Hallmark Molecular Alteration and Unresolved Issues in Risk Stratification Assessment. Histol. Histopathol. 2023, 38, 1079–1097. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. The Evolving Classification of Soft Tissue Tumours—An Update Based on the New 2013 WHO Classification. Histopathology 2014, 64, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Frith, A.E.; Hirbe, A.C.; Van Tine, B.A. Novel Pathways and Molecular Targets for the Treatment of Sarcoma. Curr. Oncol. Rep. 2013, 15, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary Fibrous Tumor: A Clinicopathological Study of 110 Cases and Proposed Risk Assessment Model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef]
- England, D.M.; Hochholzer, L.; McCarthy, M.J. Localized Benign and Malignant Fibrous Tumors of the Pleura. A Clinicopathologic Review of 223 Cases. Am. J. Surg. Pathol. 1989, 13, 640–658. [Google Scholar] [CrossRef]
- Hassani, M.; Jung, S.; Ghodsi, E.; Seddigh, L.; Kooner, P.; Aoude, A.; Turcotte, R. Value of Cellular Components and Focal Dedifferentiation to Predict the Risk of Metastasis in a Benign-Appearing Extra-Meningeal Solitary Fibrous Tumor: An Original Series from a Tertiary Sarcoma Center. Cancers 2023, 15, 1441. [Google Scholar] [CrossRef]
- Tolstrup, J.; Loya, A.; Aggerholm-Pedersen, N.; Preisler, L.; Penninga, L. Risk Factors for Recurrent Disease after Resection of Solitary Fibrous Tumor: A Systematic Review. Front. Surg. 2024, 11, 1–11. [Google Scholar] [CrossRef]
- Medina-Ceballos, E.; Machado, I.; Giner, F.; Bujeda, Á.B.; Navarro, S.; Ferrandez, A.; Lavernia, J.; Ruíz-Sauri, A.; Llombart-Bosch, A. Solitary Fibrous Tumor: Can the New Huang Risk Stratification System for Orbital Tumors Improve Prognostic Accuracy in Other Tumor Locations? Pathol. Res. Pract. 2024, 254, 6–11. [Google Scholar] [CrossRef]
- Salas, S.; Resseguier, N.; Blay, J.Y.; Le Cesne, A.; Italiano, A.; Chevreau, C.; Rosset, P.; Isambert, N.; Soulie, P.; Cupissol, D.; et al. Prediction of Local and Metastatic Recurrence in Solitary Fibrous Tumor: Construction of a Risk Calculator in a Multicenter Cohort from the French Sarcoma Group (FSG) Database. Ann. Oncol. 2017, 28, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Cassidy, M.R.; Kirane, A.; Kuk, D.; Zanchelli, B.; Antonescu, C.R.; Singer, S.; Brennan, M. Size and Location Are the Most Important Risk Factors for Malignant Behavior in Resected Solitary Fibrous Tumors. Ann. Surg. Oncol. 2017, 24, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Gronchi, A.; Strauss, D.; Bonvalot, S.; Jeys, L.; Stacchiotti, S.; Hayes, A.; Honore, C.; Collini, P.; Renne, S.L.; et al. Resectable Extra-Pleural and Extra-Meningeal Solitary Fibrous Tumours: A Multi-Centre Prognostic Study. Eur. J. Surg. Oncol. 2016, 42, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Wushou, A.; Jiang, Y.Z.; Liu, Y.R.; Shao, Z.M. The Demographic Features, Clinicopathologic Characteristics, Treatment Outcome and Disease-Specific Prognostic Factors of Solitary Fibrous Tumor: A Population-Based Analysis. Oncotarget 2015, 6, 41875–41883. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mei, F.; Wu, S.; Tan, Z. Hemangiopericytoma: Incidence, Treatment, and Prognosis Analysis Based on SEER Database. Biomed. Res. Int. 2020, 2020, 2468320. [Google Scholar] [CrossRef]
- Yamada, Y.; Kohashi, K.; Kinoshita, I.; Yamamoto, H.; Iwasaki, T.; Yoshimoto, M.; Ishihara, S.; Toda, Y.; Itou, Y.; Koga, Y.; et al. Clinicopathological Review of Solitary Fibrous Tumors: Dedifferentiation Is a Major Cause of Patient Death. Virchows Arch. 2019, 475, 467–477. [Google Scholar] [CrossRef]
- Hall, W.A.; Ali, A.N.; Gullett, N.; Crocker, I.; Landry, J.C.; Shu, H.K.; Prabhu, R.; Curran, W. Comparing Central Nervous System (CNS) and Extra-CNS Hemangiopericytomas in the Surveillance, Epidemiology, and End Results Program: Analysis of 655 Patients and Review of Current Literature. Cancer 2012, 118, 5331–5338. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 1 September 2024).
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; van Houdt, W.J.; Strauss, D.; Schrage, Y.; Hayes, A.J.; Raut, C.P.; Fairweather, M.; Baldini, E.H.; et al. Extrameningeal Solitary Fibrous Tumors—Surgery Alone or Surgery plus Perioperative Radiotherapy: A Retrospective Study from the Global Solitary Fibrous Tumor Initiative in Collaboration with the Sarcoma Patients EuroNet. Cancer 2020, 126, 3002–3012. [Google Scholar] [CrossRef]
- Localized/Regional/Distant Stage Adjustments. Available online: https://seer.cancer.gov/seerstat/variables/seer/yr1975_2017/lrd_stage/index.html (accessed on 15 January 2023).
- Wilson, A.; Norden, N. The R Project for Statistical Computing the R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 September 2024).
- Georgiesh, T.; Boye, K.; Bjerkehagen, B. A Novel Risk Score to Predict Early and Late Recurrence in Solitary Fibrous Tumour. Histopathology 2020, 77, 123–132. [Google Scholar] [CrossRef]
- Demicco, E.G.; Griffin, A.M.; Gladdy, R.A.; Dickson, B.C.; Ferguson, P.C.; Swallow, C.J.; Wunder, J.S.; Wang, W.L. Comparison of Published Risk Models for Prediction of Outcome in Patients with Extrameningeal Solitary Fibrous Tumour. Histopathology 2019, 75, 723–737. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Tirumani, S.H.; Do, W.S.; Keraliya, A.R.; Hornick, J.L.; Shinagare, A.B.; Ramaiya, N.H. Metastatic Patterns of Solitary Fibrous Tumors: A Single-Institution Experience. Am. J. Roentgenol. 2017, 208, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Luzzago, S.; Palumbo, C.; Rosiello, G.; Knipper, S.; Pecoraro, A.; Mistretta, F.A.; Tian, Z.; Musi, G.; Montanari, E.; Soulières, D.; et al. Association Between Systemic Therapy and/or Cytoreductive Nephrectomy and Survival in Contemporary Metastatic Non–Clear Cell Renal Cell Carcinoma Patients. Eur. Urol. Focus. 2021, 7, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Würnschimmel, C.; Wenzel, M.; Collà Ruvolo, C.; Nocera, L.; Tian, Z.; Saad, F.; Briganti, A.; Shariat, S.F.; Mirone, V.; Chun, F.K.H.; et al. Life Expectancy in Metastatic Prostate Cancer Patients According to Racial/Ethnic Groups. Int. J. Urol. 2021, 28, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Cena, T.; Zilli, T.; Jereczek-Fossa, B.A.; De Bari, B.; Villa Freixa, S.; Kaanders, J.H.A.M.; Torrente, S.; Pasquier, D.; Sole, C.V.; et al. Radiotherapy in the Treatment of Extracranial Hemangiopericytoma/Solitary Fibrous Tumor: Study from the Rare Cancer Network. Radiother. Oncol. 2020, 144, 114–120. [Google Scholar] [CrossRef]
- Bishop, A.J.; Zagars, G.K.; Demicco, E.G.; Wang, W.L.; Feig, B.W.; Guadagnolo, B.A. Soft Tissue Solitary Fibrous Tumor Combined Surgery and Radiation Therapy Results in Excellent Local Control. Am. J. Clin. Oncol. 2018, 41, 81–85. [Google Scholar] [CrossRef]
- Vaz Salgado, M.A.; Soto, M.; Reguero, M.E.; Muñoz, G.; Cabañero, A.; Gallego, I.; Resano, S.; Longo, F.; Madariaga, A.; Gomez, A.; et al. Clinical Behavior of Solitary Fibrous Tumor: A Retrospective Review of 30 Patients. Clin. Transl. Oncol. 2017, 19, 357–363. [Google Scholar] [CrossRef]
- Demicco, E.G.; Wagner, M.J.; Maki, R.G.; Gupta, V.; Iofin, I.; Lazar, A.J.; Wang, W.L. Risk Assessment in Solitary Fibrous Tumors: Validation and Refinement of a Risk Stratification Model. Mod. Pathol. 2017, 30, 1433–1442. [Google Scholar] [CrossRef]
- Colditz, G.A. American Joint Committee on Cancer. In The SAGE Encyclopedia of Cancer and Society; Springer: New York, NY, USA, 2015; pp. 1–2. [Google Scholar]



| Malignant Solitary Fibrous Tumor | Overall n = 1134 |
|---|---|
| Age at diagnosis (years) Median (IQR) | 60 (50–69) |
| Sex—Male | 551 (49%) |
| Race/ethnicity | |
| Caucasian | 771 (68%) |
| African American | 95 (8%) |
| Hispanic | 149 (13%) |
| Asian/Pacific Islander | 102 (9%) |
| Other | 17 (2%) |
| Surgical resection | 989 (87%) |
| Site of origin | |
| Extremities and head | 500 (44%) |
| Central nervous system | 261 (23%) |
| Head and neck | 120 (11%) |
| Extremities | 119 (10%) |
| Chest | 322 (29%) |
| Infradiaphragmatic | 312 (28%) |
| Pelvis | 128 (11%) |
| Abdomen | 114 (10%) |
| Retroperitoneum | 70 (6%) |
| Size (cm) | |
| Median (IQR) | 75 (46–120) |
| <9 cm | 518 (46%) |
| 9–15.9 cm | 247 (22%) |
| ≥16 cm | 100 (9%) |
| Unknown | 269 (24%) |
| Stage | |
| Localized | 475 (42%) |
| Locally advanced | 401 (35%) |
| Metastatic | 142 (13%) |
| Unstaged | 116 (10%) |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables Tested | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| Age at diagnosis (years) | 1.01 | (1.01–1.02) | <0.001 | 1.01 | (1–1.02) | 0.08 |
| Sex—Female | 0.92 | (0.7–1.2) | 0.5 | |||
| Race/ethnicity | ||||||
| Caucasian | Ref | |||||
| African American | 1.2 | (0.7–1.9) | 0.5 | |||
| Hispanic | 0.7 | (0.4–1.1) | 0.1 | |||
| Asian or Pacific Islander | 0.9 | (0.6–1.4) | 0.7 | |||
| Surgical resection status—No | 4 | (2.7–6.1) | <0.001 | 3.6 | (2.3–5.6) | <0.001 |
| Site of origin | ||||||
| Central nervous system | Ref | Ref | ||||
| Extremities | 1.6 | (0.98–2.7) | 0.06 | 1.6 | (0.9–2.9) | 0.1 |
| Head and neck | 1.3 | (0.7–2.2) | 0.4 | 1.2 | (0.7–2.1) | 0.6 |
| Chest | 1.7 | (1.1–2.5) | 0.01 | 0.97 | (0.6–1.7) | 0.9 |
| Pelvis | 1.5 | (0.9–2.5) | 0.11 | 0.9 | (0.5–1.7) | 0.8 |
| Abdomen | 1.7 | (0.99–3) | 0.06 | 1.3 | (0.7–2.5) | 0.5 |
| Retroperitoneum | 1.3 | (0.6–2.5) | 0.52 | 0.8 | (0.3–1.6) | 0.5 |
| Size | ||||||
| <9 cm | Ref | Ref | ||||
| 9–15.9 cm | 1.5 | (1.1–2.0) | 0.01 | 1.6 | (1.1–2.4) | 0.01 |
| ≥16 cm | 1.8 | (1.2–2.6) | <0.001 | 1.9 | (1.1–3.1) | 0.01 |
| Stage | ||||||
| Localized | Ref | Ref | ||||
| Locally advanced | 1.5 | (1.1–2) | 0.02 | 1.6 | (1.2–2.3) | <0.001 |
| Metastatic | 3.4 | (2.3–5) | <0.001 | 2.9 | (2.0–4.4) | <0.001 |
| Localized | Locally Advanced | Metastatic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables Tested | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| Surgical resection status—No | 1.8 | (0.6–5.1) | 0.03 | 2.6 | (1.3–5.3) | 0.01 | 5.1 | (2.6–9.8) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccinelli, M.L.; Law, K.; Incesu, R.-B.; Tappero, S.; Cano Garcia, C.; Barletta, F.; Morra, S.; Scheipner, L.; Baudo, A.; Tian, Z.; et al. Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis. Cancers 2024, 16, 3331. https://doi.org/10.3390/cancers16193331
Piccinelli ML, Law K, Incesu R-B, Tappero S, Cano Garcia C, Barletta F, Morra S, Scheipner L, Baudo A, Tian Z, et al. Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis. Cancers. 2024; 16(19):3331. https://doi.org/10.3390/cancers16193331
Chicago/Turabian StylePiccinelli, Mattia Luca, Kyle Law, Reha-Baris Incesu, Stefano Tappero, Cristina Cano Garcia, Francesco Barletta, Simone Morra, Lukas Scheipner, Andrea Baudo, Zhe Tian, and et al. 2024. "Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis" Cancers 16, no. 19: 3331. https://doi.org/10.3390/cancers16193331
APA StylePiccinelli, M. L., Law, K., Incesu, R.-B., Tappero, S., Cano Garcia, C., Barletta, F., Morra, S., Scheipner, L., Baudo, A., Tian, Z., Luzzago, S., Mistretta, F. A., Ferro, M., Saad, F., Shariat, S. F., Carmignani, L., Ahyai, S., Longo, N., Briganti, A., ... Karakiewicz, P. I. (2024). Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis. Cancers, 16(19), 3331. https://doi.org/10.3390/cancers16193331









