Risk Stratification by Combination of Heart and Lung Dose in Locally Advanced Non-Small-Cell Lung Cancer after Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Radiation Dose Parameters for Heart and Lungs
3.2. Univariate and Multivariable Models for Overall Survival
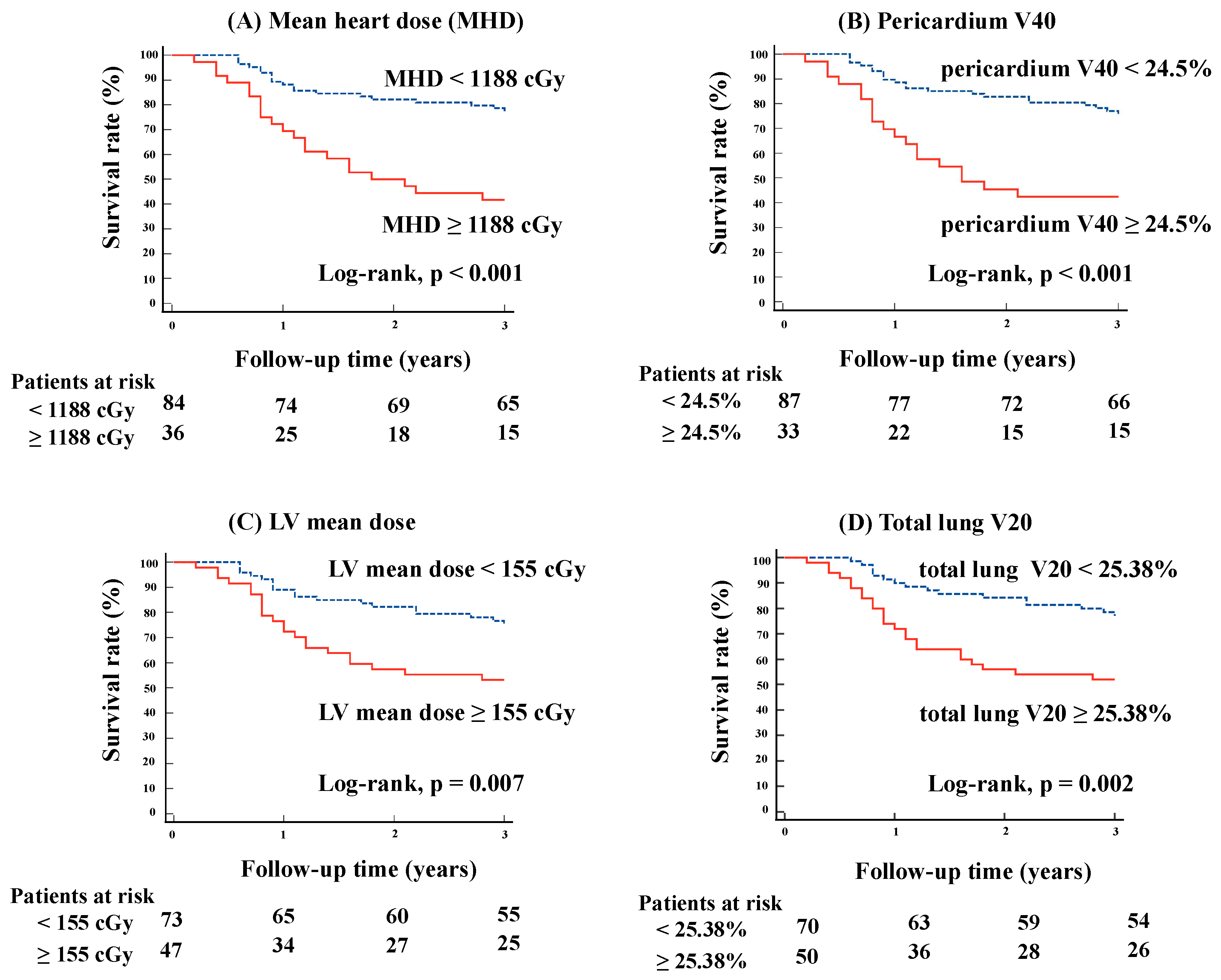
3.3. Kaplan–Meier Curve Analysis for Each Lung and Heart Dose and Their Combination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CRT | three-dimensional conformal radiation therapy |
| CCRT | concurrent chemoradiotherapy |
| CRT | chemoradiotherapy |
| CT | computed tomography |
| DVH | dose–volume histogram |
| HR | hazard ratio |
| IMRT | intensity-modulated radiation therapy |
| LA-NSCLC | locally advanced non-small-cell lung cancer |
| LV | left ventricular |
| MHD | mean heart dose |
| NCCN | National Comprehensive Cancer Network |
| NSCLC | non-small-cell lung cancer |
| OARs | organs at risk |
| OS | overall survival |
| ROC | receiver operating characteristic |
| RP | radiation pneumonitis |
| RT | radiation therapy |
| RTOG | Radiation Therapy Oncology Group |
| Vx | the percentage of the volume of the organ receiving at least xGy |
| VMAT | volumetric modulated arc therapy |
References
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar]
- Shen, L.; Liu, C.; Jin, J.; Han, C.; Zhou, Y.; Zheng, X.; Gong, C.; Chen, M.; Xie, C.; Jin, X. Association of lung and heart dose with survival in patients with non-small cell lung cancer underwent volumetric modulated arc therapy. Cancer Manag. Res. 2019, 11, 6091–6098. [Google Scholar] [CrossRef]
- Inoue, A.; Kunitoh, H.; Sekine, I.; Sumi, M.; Tokuuye, K.; Saijo, N. Radiation pneumonitis in lung cancer patients: A retrospective study of risk factors and the long-term prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; McGale, P.; Taylor, C.W.; Peto, R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005, 6, 557–565. [Google Scholar] [CrossRef]
- Aleman, B.M.; van den Belt-Dusebout, A.W.; De Bruin, M.L.; van’t Veer, M.B.; Baaijens, M.H.; Boer, J.P.D.; Hart, A.A.; Klokman, W.J.; Kuenen, M.A.; Ouwens, G.M.; et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007, 109, 1878–1886. [Google Scholar] [CrossRef]
- Tucker, S.L.; Liu, A.; Gomez, D.; Tang, L.L.; Allen, P.; Yang, J.; Liao, Z.; Grosshans, D. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother. Oncol. 2016, 119, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Molotievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradley, J.D.; Robinson, C.G. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef]
- Stam, B.; van der Bijl, E.; van Diessen, J.; Rossi, M.M.; Tijhuis, A.; Belderbos, J.S.; Damen, E.; Sonke, J.J. Heart dose associated with overall survival in locally advanced NSCLC patients treated with hypofractionated chemoradiotherapy. Radiother. Oncol. 2017, 125, 62–65. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, A.; Kennedy, J.; Hodgson, C.; Osorio, E.V.; Faivre-Finn, C.; Van Herk, M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur. J. Cancer 2017, 85, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef]
- Dess, R.T.; Sun, Y.; Matuszak, M.M.; Sun, G.; Soni, P.D.; Bazzi, L.; Murthy, V.L.; Hearn, J.W.; Kong, F.M.; Kalemkerian, G.P.; et al. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2017, 35, 1395–1402. [Google Scholar] [CrossRef]
- Guberina, M.; Eberhardt, W.; Stuschke, M.; Gauler, T.; Heinzelmann, F.; Cheufou, D.; Kimmich, M.; Friedel, G.; Schmidberger, H.; Darwiche, K.; et al. Heart dose exposure as prognostic marker after radiotherapy for resectable stage IIIA/B non-small-cell lung cancer: Secondary analysis of a randomized trial. Ann. Oncol. 2017, 28, 1084–1089. [Google Scholar] [CrossRef]
- Beukema, J.C.; Kawaguchi, Y.; Sijtsema, N.M.; Zhai, T.T.; Langendijk, J.A.; van Dijk, L.V.; van Luijk, P.; Teshima, T.; Muijs, C.T. Can we safely reduce the radiation dose to the heart while compromising the dose to the lungs in oesophageal cancer patients? Radiother. Oncol. 2020, 149, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.M.; O’Connor, J.; Harbinson, M.; Duane, F.; McCann, C.; McKavanagh, P.; Johnston, D.I.; Giacometti, V.; McAleese, J.; Hounsell, A.R.; et al. The association of incidental radiation dose to the heart base with overall survival and cardiac events after curative-intent radiotherapy for non-small cell lung cancer: Results from the NI-HEART Study. Clin. Oncol. (R. Coll. Radiol.) 2024, 36, 119–127. [Google Scholar] [CrossRef]
- Yegya-Raman, N.; Lee, S.H.; Friedes, C.; Wang, X.; Iocolano, M.; Kegelman, T.P.; Duan, L.; Li, B.; Berlin, E.; Kim, K.N.; et al. Cardiac radiation dose is associated with inferior survival but not cardiac events in patients with locally advanced non-small cell lung cancer in the era of immune checkpoint inhibitor consolidation. Radiother. Oncol. 2024, 190, 110005. [Google Scholar] [CrossRef]
- Donovan, E.K.; Pond, G.R.; Seow, H.; Ellis, P.M.; Swaminath, A. Cardiac morbidity following chemoradiation in stage III non-small cell lung cancer patients: A population-based cohort study. Clin. Oncol. (R. Coll. Radiol.) 2023, 35, e182–e188. [Google Scholar] [CrossRef]
- McKenzie, E.; Zhang, S.; Zakariaee, R.; Guthier, C.V.; Hakimian, B.; Mirhadi, A.; Kamrava, M.; Padda, S.K.; Lewis, J.H.; Nikolova, A.; et al. Left anterior descending coronary artery radiation dose association with all-cause mortality in NRG oncology trial RTOG 0617. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1138–1143. [Google Scholar] [CrossRef]
- Olloni, A.; Brink, C.; Lorenzen, E.L.; Jeppesen, S.S.; Hofmann, L.; Kristiansen, C.; Knap, M.M.; Møller, D.S.; Nygård, L.; Persson, G.F.; et al. Heart and Lung Dose as Predictors of Overall Survival in Patients with Locally Advanced Lung Cancer. A National Multicenter Study. JTO Clin. Res. Rep. 2024, 5, 100663. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, M.D.; Gore, E.M.; Ad, V.B.; Robinson, C.G.; Bradley, J.D. Defining a novel cardiac contouring atlas for NSCLC using cadaveric anatomy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S658. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Fournel, P.; Robinet, G.; Thomas, P.; Souquet, P.J.; Léna, H.; Vergnenégre, A.; Delhoume, J.Y.; Le Treut, J.; Silvani, J.A.; Dansin, E.; et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J. Clin. Oncol. 2005, 23, 5910–5917. [Google Scholar] [CrossRef]
- Pan, L.; Lei, D.; Wang, W.; Luo, Y.; Wang, D. Heart dose linked with cardiac events and overall survival in lung cancer radiotherapy: A meta-analysis. Medicine 2020, 99, e21964. [Google Scholar] [CrossRef]
- Tsujino, K.; Hashimoto, T.; Shimada, T.; Yoden, E.; Fujii, O.; Ota, Y.; Satouchi, M.; Negoro, S.; Adachi, S.; Soejima, T. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 983–990. [Google Scholar] [CrossRef]
- Heo, J.; Noh, O.K.; Kim, H.I.; Chun, M.; Cho, O.; Park, R.W.; Yoon, D.; Oh, Y.T. Lung dose and the potential risk of death in postoperative radiation therapy for non-small cell lung cancer: A study using the method of stratified grouping. Radiother. Oncol. 2018, 129, 61–67. [Google Scholar] [CrossRef]
- Xu, C.; Guo, L.; Liao, Z.; Wang, Y.; Liu, X.; Zhao, S.; Wang, J.; Yuan, Z.; Wang, P.; Lin, S.H. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin. Transl. Radiat. Oncol. 2019, 17, 17–23. [Google Scholar] [CrossRef]
- Lee, C.C.; Chua, G.W.Y.; Zheng, H.; Soon, Y.Y.; Foo, L.L.; Thiagarajan, A.; Yap, S.P.; Siow, T.R.; Ng, W.L.; Chua, K.L.M.; et al. Are heart doses associated with survival in patients with non-small cell lung cancer who received post-operative thoracic radiotherapy?: A national population-based study. Medicine 2019, 98, e17020. [Google Scholar] [CrossRef]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Finn, C.F. Cardiac toxicity of thoracic radiotherapy: Existing evidence and future directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No. of Patients (%) (n = 120) |
|---|---|
| Age | |
| <65 | 46 (38.3) |
| ≥65 | 74 (61.7) |
| Sex | |
| Male | 92 (76.7) |
| Female | 28 (23.3) |
| ECOG performance status | |
| 0 | 64 (53.3) |
| 1 | 54 (45.0) |
| 2 | 1 (0.8) |
| 3 | 1 (0.8) |
| T stage * | |
| 1 | 29 (24.2) |
| 2 | 28 (23.3) |
| 3 | 27 (22.5) |
| 4 | 36 (30.0) |
| N stage * | |
| 0 | 5 (4.2) |
| 1 | 11 (9.2) |
| 2 | 66 (55.0) |
| 3 | 38 (31.7) |
| Clinical stage * | |
| IIB | 9 (7.5) |
| IIIA | 34 (28.3) |
| IIIB | 65 (54.2) |
| IIIC | 12 (10.0) |
| Histology | |
| Adenocarcinoma | 62 (51.7) |
| Squamous cell | 40 (33.3) |
| Other | 18 (15.0) |
| Chemotherapy | |
| Yes | 113 (94.2) |
| No | 7 (5.8) |
| Durvalumab after CRT | |
| Yes | 31 (25.8) |
| No | 89 (74.2) |
| Radiation technique | |
| 3D-CRT | 105 (87.5) |
| IMRT | 15 (12.5) |
| Prescribed dose/fractionation | |
| 60 Gy/30 fr | 111 (92.5) |
| 64 Gy/32 fr | 3 (2.5) |
| 66 Gy/33 fr | 3 (2.5) |
| 58 Gy/29 fr | 2 (1.7) |
| 56 Gy/28 fr | 1 (0.8) |
| Dose Parameters | Median (Interquartile Range) |
|---|---|
| Heart Doses | |
| Heart mean dose (cGy) | 741 (381–1500) |
| Heart V50 (%) | 4.0 (0.1–12.9) |
| Heart V40 (%) | 6.9 (1.0–16.6) |
| Heart V30 (%) | 10.9 (3.0–20.5) |
| Pericardium mean dose (cGy) | 1362 (1004–2021) |
| Pericardium V50 (%) | 13.8 (7.0–20.4) |
| Pericardium V40 (%) | 17.4 (11.0–26.2) |
| Pericardium V30 (%) | 21.5 (16.0–31.0) |
| LV mean dose (cGy) | 114 (53–284) |
| Lung Doses | |
| Total lung mean dose (cGy) | 1277 (922–1527) |
| Total lung V20 (%) | 23.2 (17.1–28.0) |
| Total lung V5 (%) | 38.2 (29.3–44.0) |
| Ipsilateral lung mean dose (cGy) | 2106 (1585–2557) |
| Ipsilateral lung V20 (%) | 40.1 (30.9–48.0) |
| Ipsilateral lung V5 (%) | 57.9 (48.2–68.0) |
| Contralateral lung mean dose (cGy) | 384 (203–586) |
| Contralateral lung V20 (%) | 5.5 (2.3–9.2) |
| Contralateral lung V5 (%) | 16.6 (7.7–25.3) |
| Variables | p-Value | HR (95% CI) |
|---|---|---|
| Patient Data | ||
| Age ≥ 65 | 0.041 | 2.1 (1.0–4.3) |
| Male | 0.024 | 3.3 (1.2–9.3) |
| ECOG PS (0, 1, 2, 3) | 0.034 | 1.9 (1.0–3.4) |
| TNM stage (IIB, IIIA, IIIB, IIIC) | 0.003 | 2.0 (1.3–3.1) |
| Adenocarcinoma (vs. SCC) | 0.449 | 0.8 (0.4–1.5) |
| Concurrent chemotherapy | 0.026 | 0.3 (0.1–0.9) |
| Durvalumab after CRT | 0.024 | 0.3 (0.1–0.9) |
| Dose Data | ||
| Heart Doses | ||
| Heart mean dose (≥1188 cGy) | <0.001 | 3.4 (1.8–6.3) |
| Heart V50 (≥10.64%) | 0.002 | 2.7 (1.4–5.0) |
| Heart V40 (≥14.08%) | 0.001 | 2.8 (1.5–5.2) |
| Heart V30 (≥17.66%) | <0.001 | 3.1 (1.7–5.8) |
| Pericardium mean dose (≥1493.5 cGy) | 0.002 | 2.8 (1.5–5.3) |
| Pericardium V50 (≥17%) | 0.001 | 2.8 (1.5–5.3) |
| Pericardium V40 (≥24.5%) | <0.001 | 3.2 (1.7–6.0) |
| Pericardium V30 (≥23%) | 0.001 | 2.9 (1.5–5.7) |
| LV mean dose (≥155 cGy) | 0.009 | 2.3 (1.2–4.3) |
| Lung Doses | ||
| Total lung mean dose (≥1192.6 cGy) | 0.009 | 2.6 (1.3–5.4) |
| Total lung V20 (≥25.38%) | 0.003 | 2.6 (1.4–5.0) |
| Total lung V5 (≥38.12%) | 0.010 | 2.4 (1.2–4.6) |
| Ipsilateral lung mean dose (≥2191 cGy) | 0.048 | 1.9 (1.0–3.5) |
| Ipsilateral lung V20 (≥43.89%) | 0.021 | 2.1 (1.1–3.9) |
| Ipsilateral lung V5 (≥57%) | 0.090 | 1.8 (0.9–3.4) |
| Contralateral lung mean dose (≥565.4 cGy) | 0.165 | 1.6 (0.8–3.0) |
| Contralateral lung V20 (≥9%) | 0.101 | 1.7 (0.9–3.2) |
| Contralateral lung V5 (≥11.7%) | 0.141 | 0.6 (0.3–1.2) |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| MHD | 0.002 | 2.8 (1.4–5.3) | ||||
| Pericardium V40 | 0.005 | 2.6 (1.3–5.0) | ||||
| LV mean dose | 0.108 | 1.7 (0.9–3.4) | ||||
| Total lung V20 | 0.042 | 2.0 (1.0–3.9) | 0.041 | 2.0 (1.0–3.9) | 0.028 | 2.1 (1.1–4.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, Y.; Koide, Y.; Shimizu, H.; Aoyama, T.; Shindo, Y.; Hashimoto, S.; Tachibana, H.; Kodaira, T. Risk Stratification by Combination of Heart and Lung Dose in Locally Advanced Non-Small-Cell Lung Cancer after Radiotherapy. Cancers 2024, 16, 3255. https://doi.org/10.3390/cancers16193255
Watanabe Y, Koide Y, Shimizu H, Aoyama T, Shindo Y, Hashimoto S, Tachibana H, Kodaira T. Risk Stratification by Combination of Heart and Lung Dose in Locally Advanced Non-Small-Cell Lung Cancer after Radiotherapy. Cancers. 2024; 16(19):3255. https://doi.org/10.3390/cancers16193255
Chicago/Turabian StyleWatanabe, Yui, Yutaro Koide, Hidetoshi Shimizu, Takahiro Aoyama, Yurika Shindo, Shingo Hashimoto, Hiroyuki Tachibana, and Takeshi Kodaira. 2024. "Risk Stratification by Combination of Heart and Lung Dose in Locally Advanced Non-Small-Cell Lung Cancer after Radiotherapy" Cancers 16, no. 19: 3255. https://doi.org/10.3390/cancers16193255
APA StyleWatanabe, Y., Koide, Y., Shimizu, H., Aoyama, T., Shindo, Y., Hashimoto, S., Tachibana, H., & Kodaira, T. (2024). Risk Stratification by Combination of Heart and Lung Dose in Locally Advanced Non-Small-Cell Lung Cancer after Radiotherapy. Cancers, 16(19), 3255. https://doi.org/10.3390/cancers16193255





