Prognostic Implications of Maintaining the Target Thyroid-Stimulating Hormone Status Based on the 2015 American Thyroid Association Guidelines in Patients with Low-Risk Papillary Thyroid Carcinoma after Lobectomy: A 5-Year Landmark Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
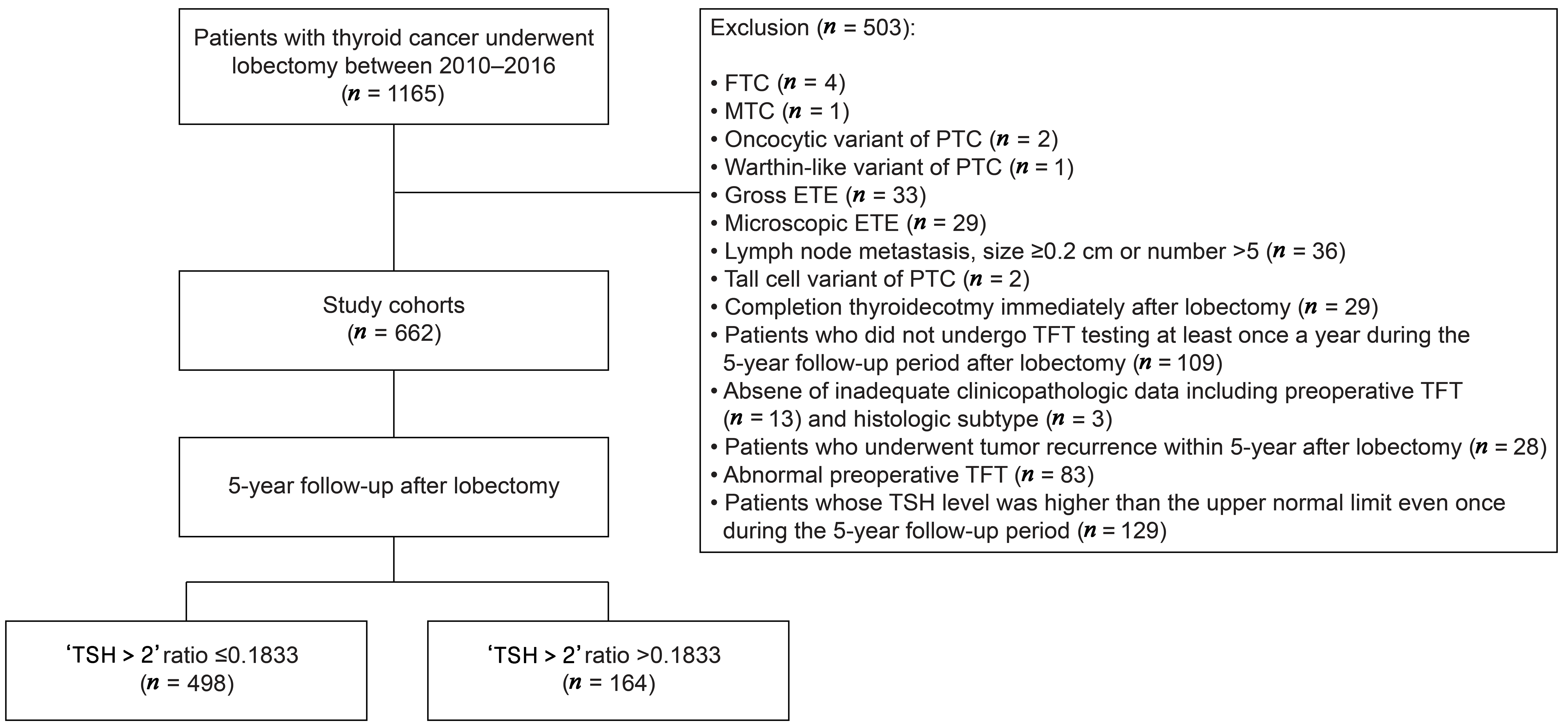
2.1. Study Cohorts and Patient Inclusion
2.2. Definition of Serum TSH Status during Follow-Up
2.3. Outcome
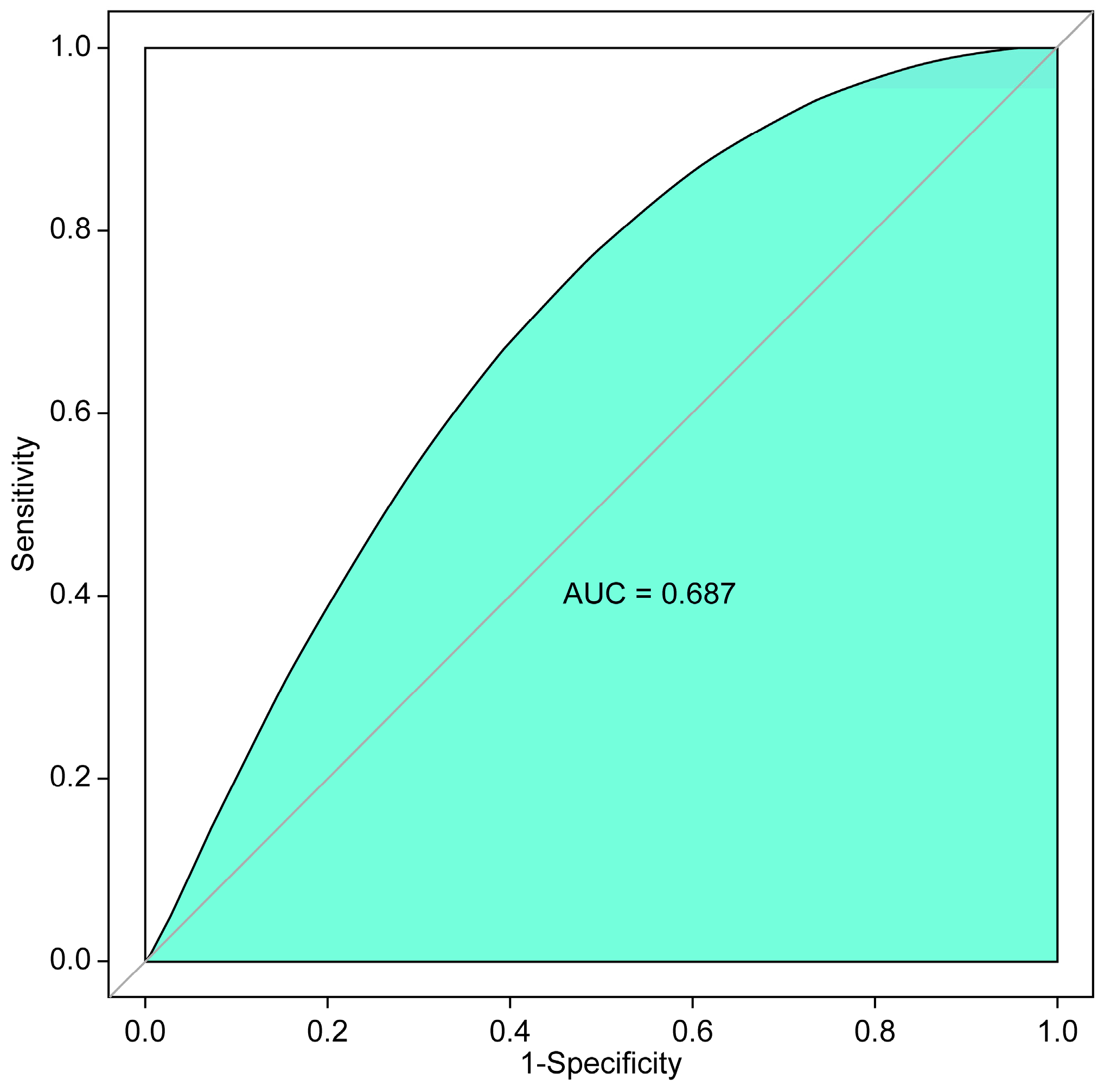
2.4. Statistical Analyses
3. Results
3.1. Patients and Tumor Characteristics
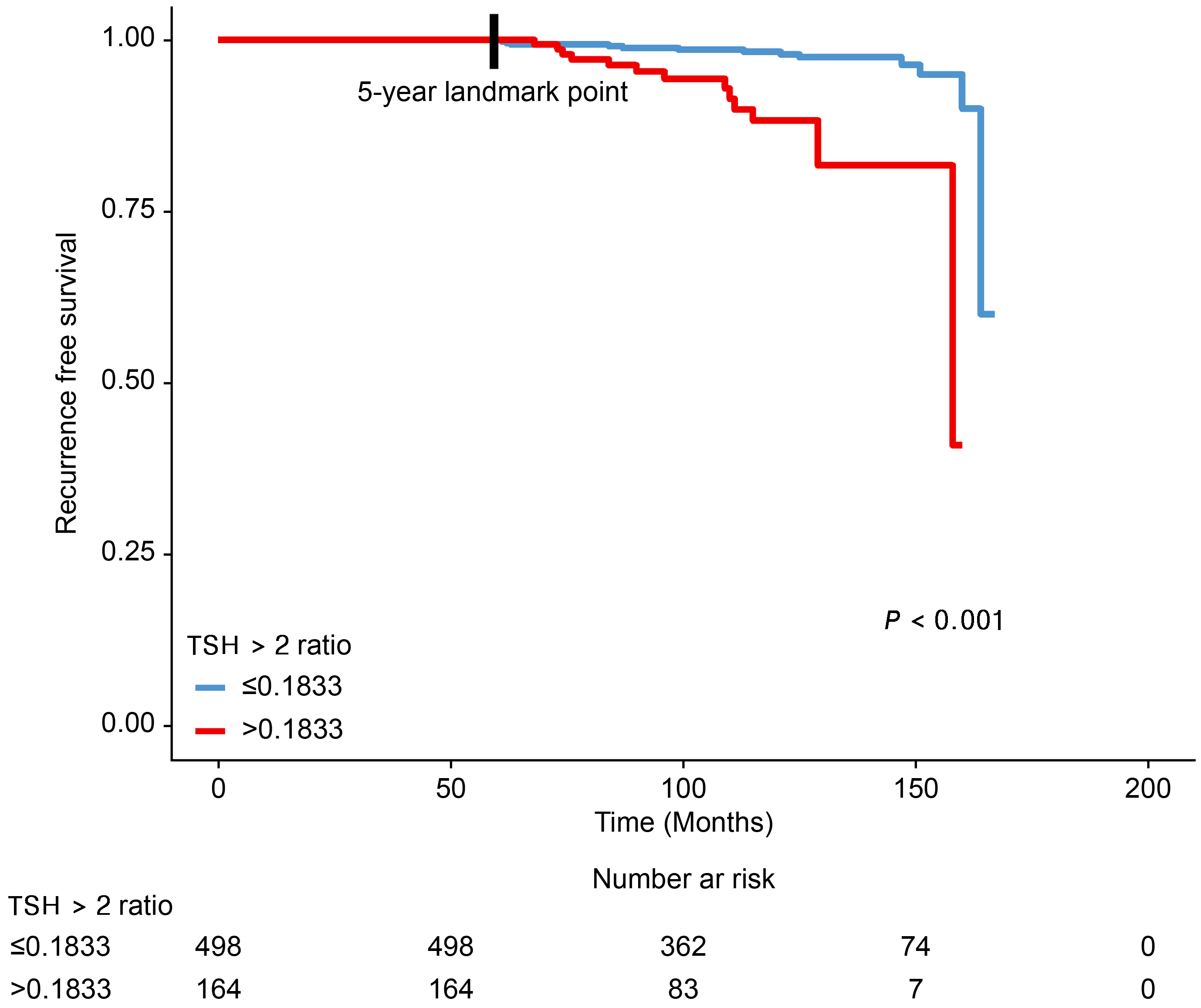
3.2. Association between the ‘TSH > 2 Ratio’ during the 5-Year Follow-Up Period after Thyroid Lobectomy and Tumor Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer ‘epidemic’—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Grani, G.; Arvat, E.; Nervo, A.; Zatelli, M.C.; Rossi, R.; Puxeddu, E.; Morelli, S.; Torlontano, M.; Massa, M.; et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: What to expect (ITCO#2). Endocr. Relat. Cancer 2018, 25, L7–L11. [Google Scholar]
- Lamartina, L.; Handkiewicz-Junak, D. Follow-up of low risk thyroid cancer patients: Can we stop follow-up after 5 years of complete remission? Eur. J. Endocrinol. 2020, 182, D1–D16. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What changed and why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Watt, T.; Christoffersen, T.; Brogaard, M.B.; Bjorner, J.B.; Bentzen, J.; Hahn, C.H.; Nygaard, B.; Feldt-Rasmussen, U. Quality of life in thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101732. [Google Scholar] [CrossRef]
- Pötter, E.; Horn, R.; Scheumann, G.F.; Dralle, H.; Costagliola, S.; Ludgate, M.; Vassart, G.; Dumont, J.E.; Brabant, G. Western blot analysis of thyrotropin receptor expression in human thyroid tumours and correlation with TSH-binding. Biochem. Biophys. Res. Commun. 1994, 205, 361–367. [Google Scholar] [CrossRef]
- Brabant, G. Thyrotropin suppressive therapy in thyroid carcinoma: What are the targets? J. Clin. Endocrinol. Metab. 2008, 93, 1167–1169. [Google Scholar] [CrossRef]
- Cooper, D.S.; Specker, B.; Ho, M.; Sperling, M.; Ladenson, P.W.; Ross, D.S.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Haugen, B.R.; et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National thyroid Cancer Treatment Cooperative Registry. Thyroid 1998, 8, 737–744. [Google Scholar] [CrossRef]
- Jonklaas, J.; Sarlis, N.J.; Litofsky, D.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Cooper, D.S.; Haugen, B.R.; Ladenson, P.W.; Magner, J.; et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006, 16, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Bosley, M.; Southerland, L.B.; Ahmadi, S.; Perkins, J.; Roman, S.; Sosa, J.A.; Carneiro-Pla, D. Lobectomy for treatment of differentiated thyroid cancer: Can patients avoid postoperative thyroid hormone supplementation and be compliant with the American Thyroid Association guidelines? Surgery 2018, 163, 75–80. [Google Scholar] [CrossRef]
- Bae, M.R.; Nam, S.H.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma. Endocrine 2022, 75, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, W.G.; Han, M.; Jeon, M.J.; Kwon, H.; Kim, M.; Sung, T.Y.; Kim, T.Y.; Kim, W.B.; Hong, S.J.; et al. Thyrotropin suppressive therapy for low-risk small thyroid cancer: A propensity score-matched cohort study. Thyroid 2017, 27, 1164–1170. [Google Scholar] [CrossRef]
- Lee, M.C.; Kim, M.J.; Choi, H.S.; Cho, S.W.; Lee, G.H.; Park, Y.J.; Park, D.J. Postoperative thyroid-stimulating hormone levels did not affect recurrence after thyroid lobectomy in patients with papillary thyroid cancer. Endocrinol. Metab. 2019, 34, 150–157. [Google Scholar] [CrossRef]
- Won, H.R.; Jeon, E.; Chang, J.W.; Kang, Y.E.; Song, K.; Kim, S.W.; Lim, D.M.; Ha, T.K.; Chung, K.W.; Kim, H.J.; et al. Is maintaining thyroid-stimulating hormone effective in patients undergoing thyroid lobectomy for low-risk differentiated thyroid cancer? A systematic review and meta-analysis. Cancers 2022, 14, 1470. [Google Scholar] [CrossRef]
- Papaleontiou, M.; Chen, D.W.; Banerjee, M.; Reyes-Gastelum, D.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Thyrotropin suppression for papillary thyroid cancer: A physician survey study. Thyroid 2021, 31, 1383–1390. [Google Scholar] [CrossRef]
- Díaz-Soto, G.; Fernández-Velasco, P.; Torres Torres, B.; López Gómez, J.J.; García Calvo, S.; de Luis Román, D. Evolution of suppressing TSH therapy at diagnosis and in the long term follow-up in a cohort of differentiated thyroid cancer. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022, 69, 844–851. [Google Scholar] [CrossRef]
- Lee, E.K.; Kang, Y.E.; Park, Y.J.; Koo, B.S.; Chung, K.W.; Ku, E.J.; Won, H.R.; Yoo, W.S.; Jeon, E.; Paek, S.H.; et al. A multicenter, randomized, controlled trial for assessing the usefulness of suppressing thyroid stimulating hormone target levels after thyroid lobectomy in low to intermediate risk thyroid cancer patients (MASTER): A study protocol. Endocrinol. Metab. 2021, 36, 574–581. [Google Scholar] [CrossRef]
- Thyroid cancer: Assessment and management [N] Evidence review for duration of thyroid stimulating hormone suppression. In NICE Guideline NG230; National Institute for Health and Care Excellence (NICE): London, UK, 2022; Available online: https://www.nice.org.uk/guidance/ng230/evidence/n-duration-of-thyroid-stimulating-hormone-suppression-pdf-407307065370 (accessed on 25 March 2024).
- Lee, Y.M.; Jeon, M.J.; Kim, W.W.; Sung, T.Y.; Chung, K.W.; Shong, Y.K.; Hong, S.J. Optimal thyrotropin suppression therapy in low-risk thyroid cancer patients after lobectomy. J. Clin. Med. 2019, 8, 1279. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, S.P.J.; Coerts, H.I.; Loncar, I.; Verhoef, C.; Kruijff, S.; Engelsman, A.F.; Peeters, R.P.; van Ginhoven, T.M. Deescalating follow-up after hemithyroidectomy for patients with low-risk papillary thyroid microcarcinoma. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Viola, D.; Torregrossa, L.; Giannini, R.; Romei, C.; Ugolini, C.; Molinaro, E.; Agate, L.; Biagini, A.; Lupi, C.; et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: Single-institution results from a large cohort study. J. Clin. Endocrinol. Metab. 2012, 97, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, M.R.; Dobrinja, C.; Casal Ide, E.; Zane, M.; Lora, O.; Toniato, A.; Mian, C.; Barollo, S.; Izuzquiza, M.; Guerrini, J.; et al. The role of BRAF(V600E) mutation as poor prognostic factor for the outcome of patients with intrathyroid papillary thyroid carcinoma. Biomed. Pharmacother. 2014, 68, 413–417. [Google Scholar] [CrossRef]
| All Patients | ‘TSH > 2 Ratio’ ≤ 0.1833 (n = 498) | ‘TSH > 2 Ratio’ > 0.1833 (n = 164) | p-Value | |
|---|---|---|---|---|
| Age (years) | 48.29 ± 16.09 | 48.99 ± 17.48 | 46.15 ± 10.61 | 0.03 |
| ≤55 | 513 (77.5%) | 378 (75.9%) | 135 (82.3%) | 0.088 |
| >55 | 149 (22.5%) | 120 (24.1%) | 29 (17.7%) | |
| Sex | ||||
| Female | 567 (85.6%) | 427 (85.7%) | 140 (85.4%) | 0.905 |
| Male | 95 (14.4%) | 71 (14.3%) | 24 (14.6%) | |
| Tumor size (cm) | 0.64 ± 0.35 | 0.63 ± 0.31 | 0.67 ± 0.44 | 0.694 |
| ≤1 cm | 609 (92.0%) | 466 (93.6%) | 143 (87.2%) | 0.009 |
| >1 cm | 53 (8%) | 32 (6.4%) | 21 (12.8%) | |
| Tumor location | ||||
| Right | 337 (50.9%) | 252 (50.6%) | 85 (51.8%) | 0.657 |
| Left | 317 (47.9%) | 241 (48.4%) | 76 (46.3%) | |
| Isthmus | 8 (1.2%) | 5 (1.0%) | 3 (1.8%) | |
| Multifocality | ||||
| No | 566 (85.5%) | 431 (86.5%) | 135 (82.3%) | 0.182 |
| Yes | 96 (14.5%) | 67 (13.5%) | 29 (17.7%) | |
| Histologic subtype | ||||
| Classic | 623 (94.1%) | 469 (94.2%) | 154 (93.9%) | 0.897 |
| Follicular variant | 39 (5.9%) | 29 (5.8%) | 10 (6.1%) | |
| Lymphatic invasion | ||||
| No | 650 (98.2%) | 494 (99.2%) | 156 (95.1%) | 0.002 |
| Yes | 12 (1.8%) | 4 (0.8%) | 8 (4.9%) | |
| Central lymph node dissection | ||||
| No | 439 (66.3%) | 311 (62.4%) | 128 (78.0%) | <0.001 |
| Yes | 223 (33.7%) | 187 (37.6%) | 36 (22.0%) | |
| Number of TSH tests during the 5 years after lobectomy | 9.23 ± 1.25 | 9.17 ± 1.27 | 9.4 ± 1.16 | 0.01 |
| FU duration (months) | 113.43 ± 30.19 | 117.28 ± 30.50 | 101.75 ± 26.02 | <0.001 |
| ‘TSH > 2 ratio’ during the 5 years after lobectomy | 0.1053 ± 0.1500 | 0.0324 ± 0.0510 | 0.3265 ± 0.1334 | <0.001 |
| Recurrence after the 5-year landmark point | ||||
| No | 635 (95.9%) | 485 (97.4%) | 150 (91.5%) | 0.001 |
| Yes | 27 (4.1%) | 13 (2.6%) | 14 (8.5%) | |
| All Patients | ‘TSH > 2 Ratio’ ≤ 0.1833 (n = 498) | ‘TSH > 2 Ratio’ > 0.1833 (n = 164) | |
|---|---|---|---|
| No recurrence | 635 (95.9%) | 485 (97.4%) | 150 (91.5%) |
| Contralateral lobe | 18 (2.7%) | 8 (1.6%) | 10 (6.1%) |
| Regional lymph nodes | 9 (1.4%) | 5 (1.0%) | 4 (2.4%) |
| Distant metastases | 0 (0%) | 0 (0%) | 0 (0%) |
| Variable | Univariable | Multivariable 1 | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age, years | ≤55 | 1 (Reference) | – | – | – |
| >55 | 1.498 (0.594–3.780) | 0.392 | – | – | |
| Sex | Female | 1 (Reference) | – | – | – |
| Male | 1.155 (0.437–3.052) | 0.772 | – | – | |
| Tumor size | ≤1 cm | 1 (Reference) | – | 1 (Reference) | – |
| >1 cm | 2.448 (0.835–7.173) | 0.103 | 3.080 (1.138–8.334) | 0.027 | |
| Tumor location | Rt. | 1 (Reference) | – | – | – |
| Lt. | 0.505 (0.211–1.206) | 0.124 | – | – | |
| Isthmus | 0.999 (0.107–9.331) | 0.999 | – | – | |
| Histologic subtype | Classic | 1 (Reference) | – | – | – |
| Follicular variant | 2.415 (0.671–8.689) | 0.177 | – | – | |
| Lymphatic invasion | No | 1 (Reference) | – | 1 (Reference) | – |
| Yes | 8.118 (2.072–31.803) | 0.003 | 7.542 (2.080–27.349) | 0.002 | |
| Central lymph node dissection | No | 1 (Reference) | – | – | – |
| Yes | 0.873 (0.373–2.042) | 0.754 | – | – | |
| Multifocality | No | 1 (Reference) | – | 1 (Reference) | – |
| Yes | 3.439 (1.477–8.011) | 0.004 | 3.103 (1.354–7.113) | 0.007 | |
| TSH > 2 ratio | ≤0.1833 | 1 (Reference) | – | 1 (Reference) | – |
| >0.1833 | 4.758 (2.033–11.135) | <0.001 | 4.795 (2.102–10.937) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.W.; Suh, Y.J.; Lim, S.T. Prognostic Implications of Maintaining the Target Thyroid-Stimulating Hormone Status Based on the 2015 American Thyroid Association Guidelines in Patients with Low-Risk Papillary Thyroid Carcinoma after Lobectomy: A 5-Year Landmark Analysis. Cancers 2024, 16, 3253. https://doi.org/10.3390/cancers16193253
Jeon YW, Suh YJ, Lim ST. Prognostic Implications of Maintaining the Target Thyroid-Stimulating Hormone Status Based on the 2015 American Thyroid Association Guidelines in Patients with Low-Risk Papillary Thyroid Carcinoma after Lobectomy: A 5-Year Landmark Analysis. Cancers. 2024; 16(19):3253. https://doi.org/10.3390/cancers16193253
Chicago/Turabian StyleJeon, Ye Won, Young Jin Suh, and Seung Taek Lim. 2024. "Prognostic Implications of Maintaining the Target Thyroid-Stimulating Hormone Status Based on the 2015 American Thyroid Association Guidelines in Patients with Low-Risk Papillary Thyroid Carcinoma after Lobectomy: A 5-Year Landmark Analysis" Cancers 16, no. 19: 3253. https://doi.org/10.3390/cancers16193253
APA StyleJeon, Y. W., Suh, Y. J., & Lim, S. T. (2024). Prognostic Implications of Maintaining the Target Thyroid-Stimulating Hormone Status Based on the 2015 American Thyroid Association Guidelines in Patients with Low-Risk Papillary Thyroid Carcinoma after Lobectomy: A 5-Year Landmark Analysis. Cancers, 16(19), 3253. https://doi.org/10.3390/cancers16193253






