Raman Spectroscopy for Instant Bladder Tumor Diagnosis: System Development and In Vivo Proof-Of-Principle Study in Accordance with the European Medical Device Regulation (MDR2017/745)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
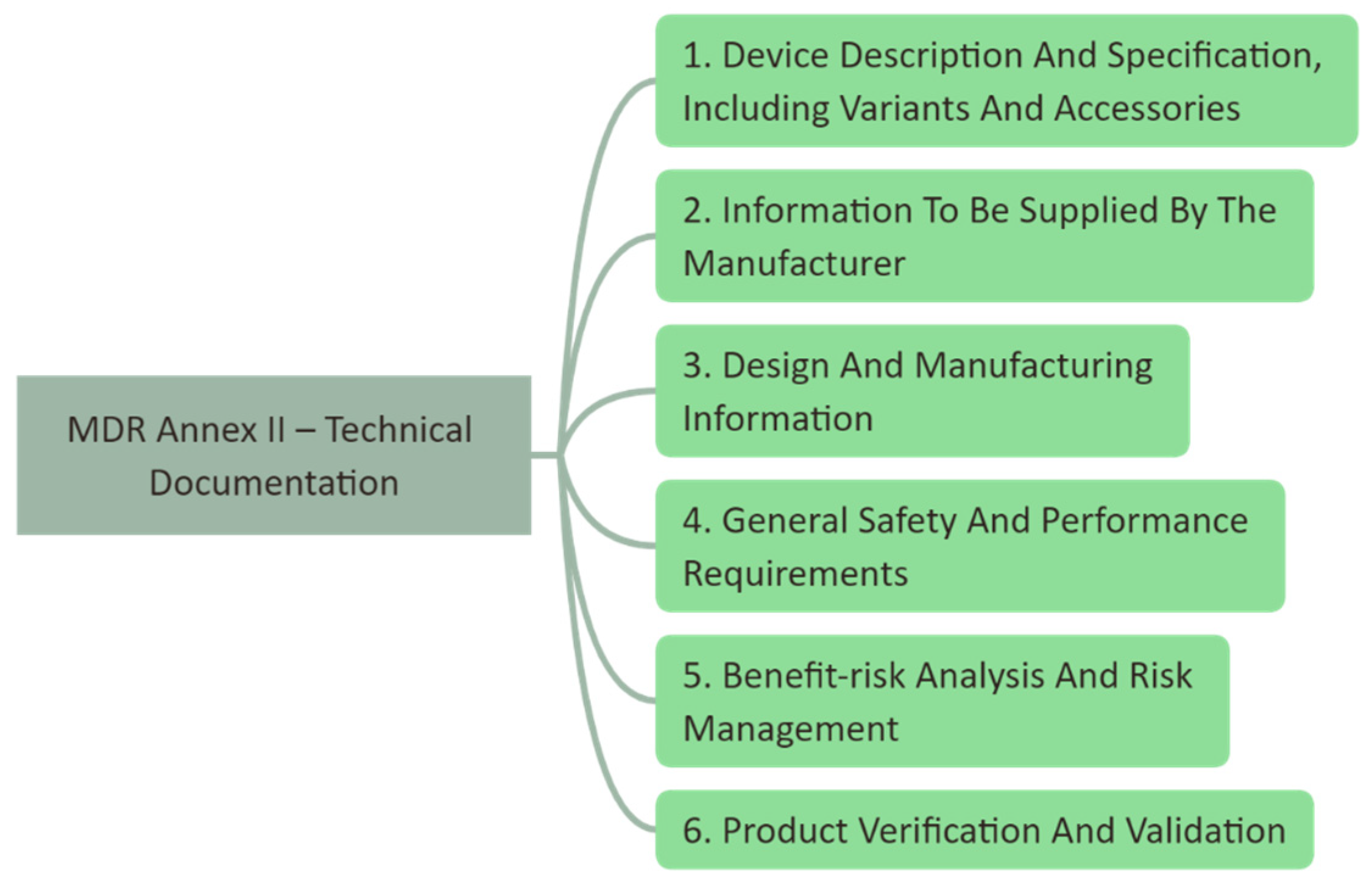
2.1. Regulatory Aspects
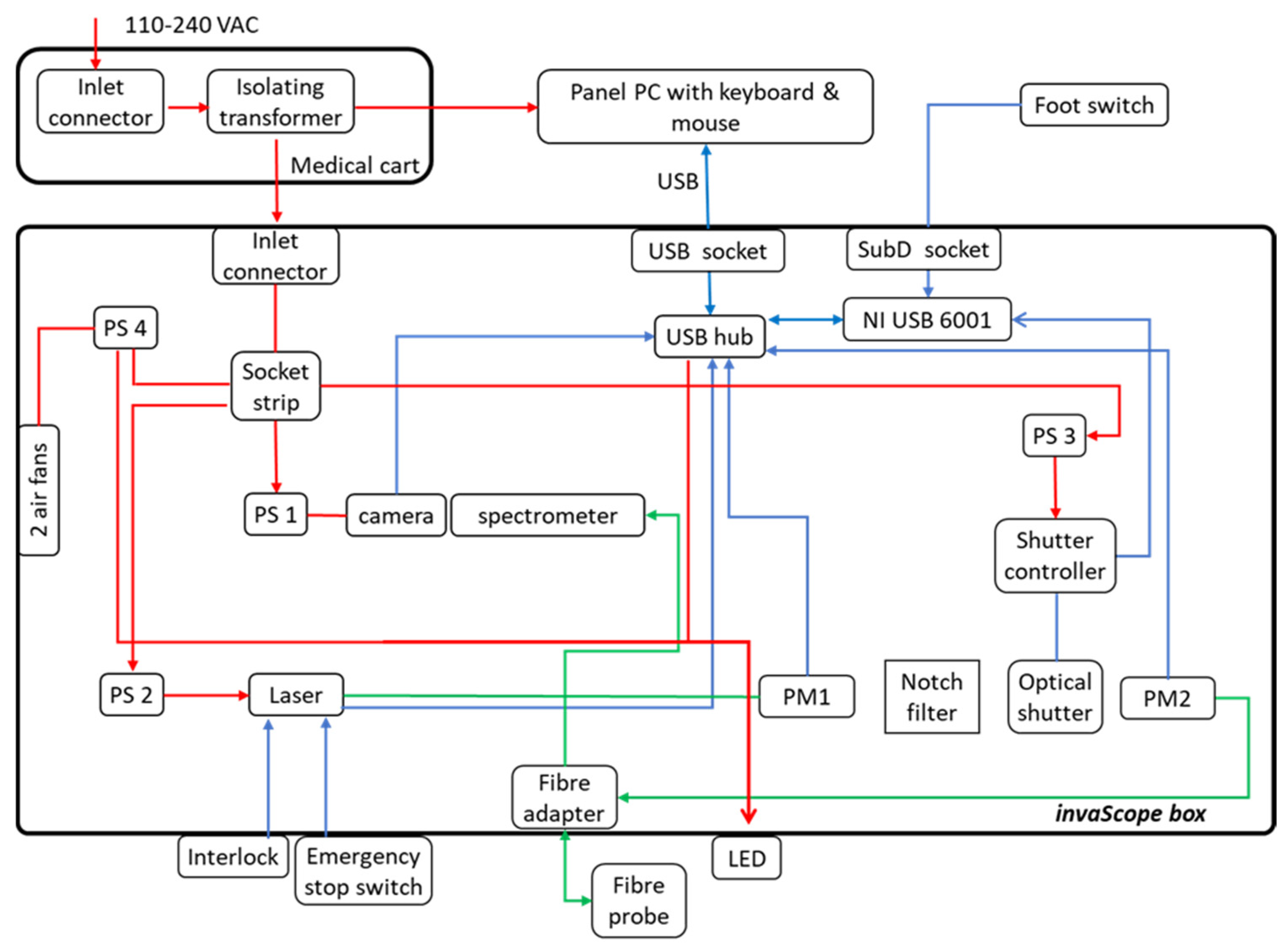
2.2. Description of Raman invaScope
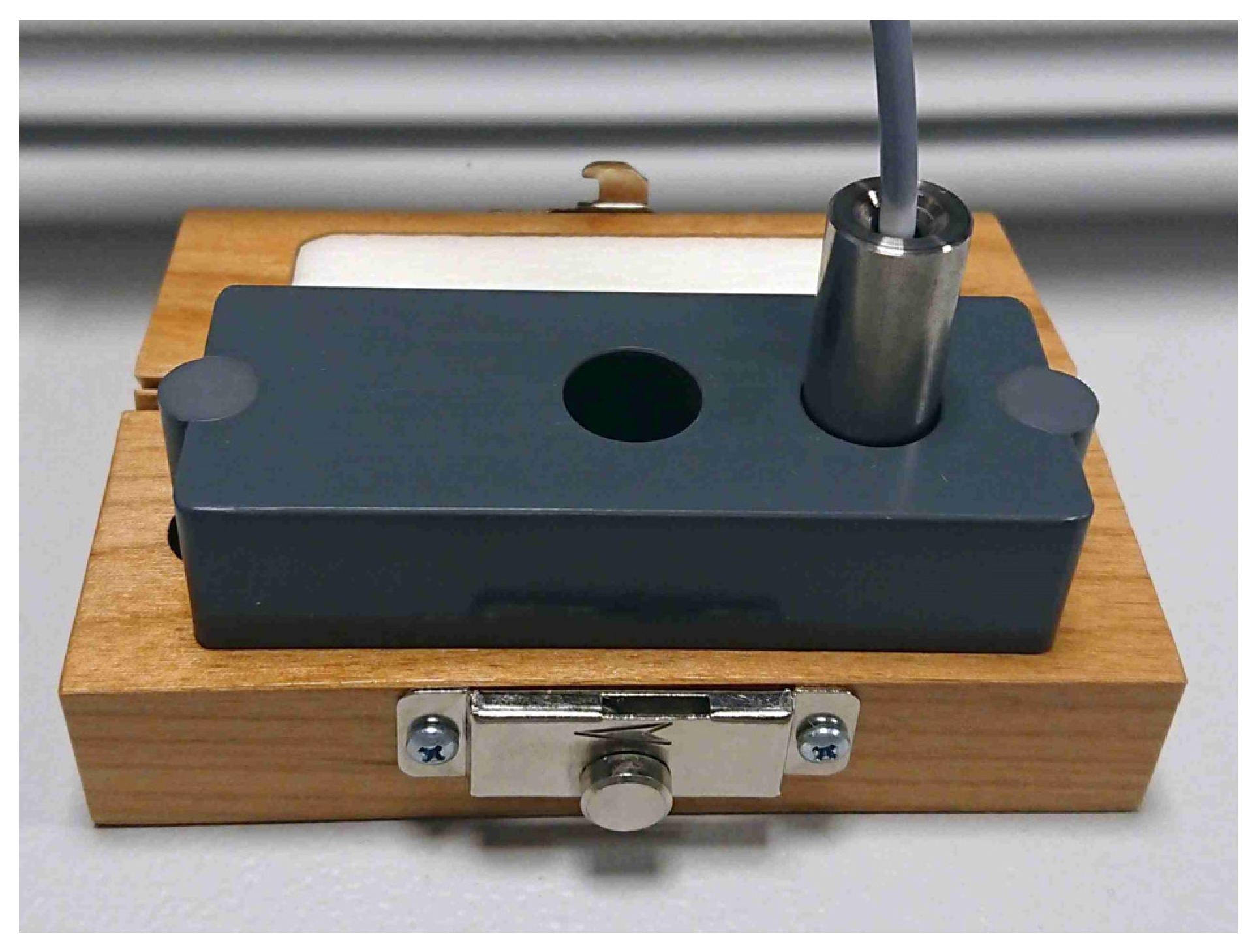
2.2.1. Raman Fiber Probe
- For easy and reliable use, the Raman fiber probe is designed as a contact probe.
- To improve usability and reduce the complexity of probe manufacturing, an unbranched design is used.
- The Raman fiber probe is designed for plug-and-play use, using a keyed snap-in linear connector with guide pins to ensure a precise alignment.
- The Raman probe parts that will come in direct contact with the patient have to be biocompatible.
- The fiber probe needs to be long enough, so that the clinician cannot touch the patient and the device at the same time.
- Laser engraved serial numbers for traceability of use are required.
2.2.2. Raman System
2.2.3. Software
2.2.4. Risk Analysis
- In the event of software failure, no measurements can be taken with the device. There is no harm to the user or the patient.
- The device is not intended to be used for diagnosis or monitoring, i.e., the clinician is not using any information to base a medical decision based on the information from the device.
- The device does not store critical patient care data or sensitive data.
- The invaScope has no emergency, critical, or life-sustaining functions; as such, the wellbeing of the patient does not depend on the device.
- There are no software-dependent risk control measures.
3. Results
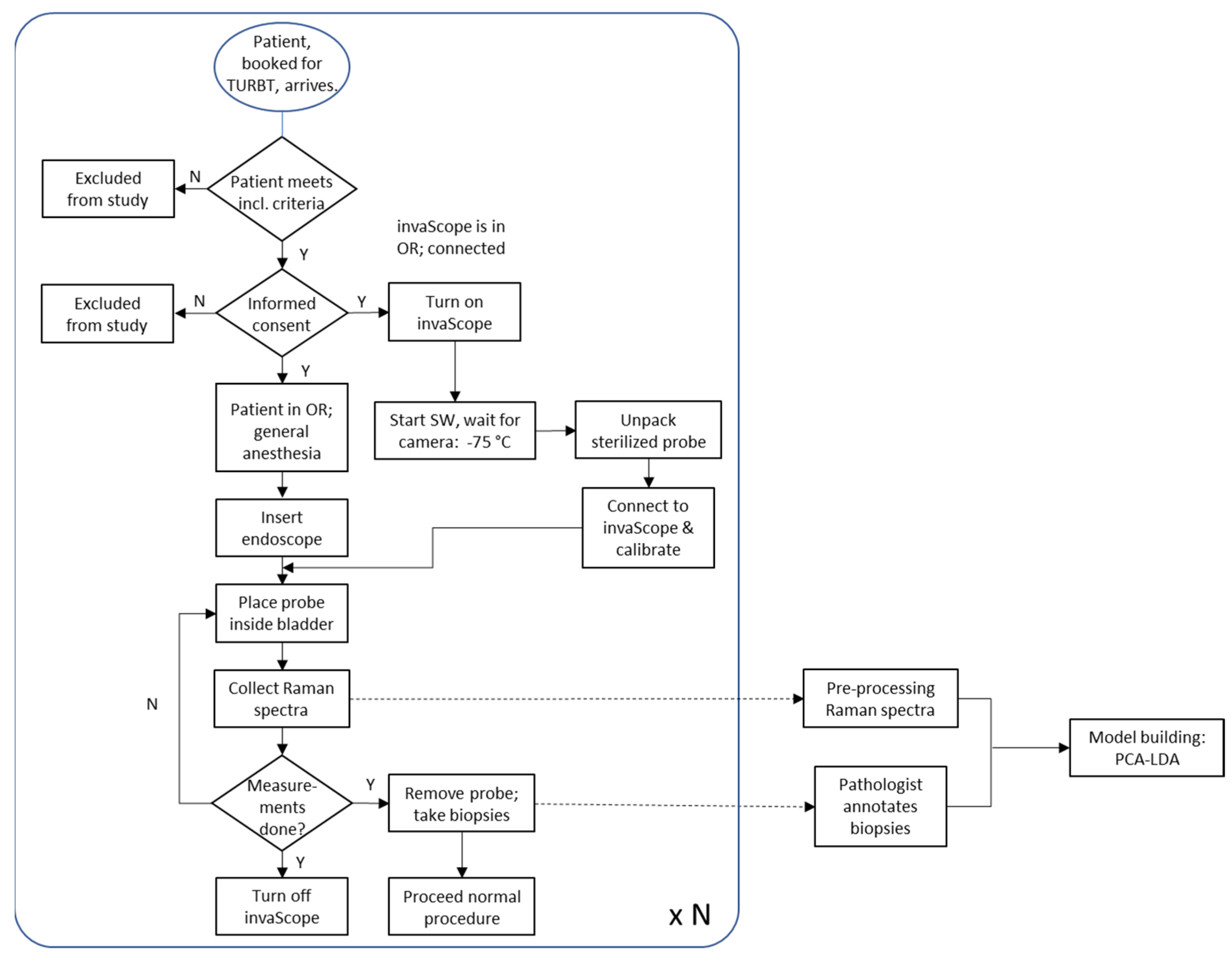
3.1. Clinical Phase I Study
3.2. Raman Measurement Protocol and Workflow
- The wavenumber calibration of the spectrometer was performed using a polystyrene sample.
- The intensity calibration of the detector was carried out using the NIST-certified SRM-2241, specifically designed for the 785 nm laser wavelength.
- Saturation of the detector due to high auto-fluorescence of the tissue.
- Interfering spectral features from the white or blue light sources of the endoscope used to illuminate the inner volume of the bladder. These light sources can be set to a low light intensity but cannot be easily switched off completely (during the procedure). Some of these light sources interfere with the Raman measurement, i.e., they are visible in the collected Raman spectra.
- Some patients were injected with hexaminolevulinate (Hexvix, [4,5,45]) prior to surgery. However, the use of Hexvix in the bladder can interfere with Raman spectroscopy. The strong fluorescence and Raman signals of Hexvix may overlap and interfere with Raman signals from the bladder tissue. Therefore, it is recommended that the dye is not used simultaneously with Raman experiments to avoid signal interference and maintain accurate spectroscopic data collection.
3.3. Biopsy Annotations
3.4. Raman Spectral Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Hexvix Label | # Biopsies | # spc 1 Normal | # spc Low Grade (LG) | # spc High Grade (HG) | |
|---|---|---|---|---|---|
| Patient 1 | No | 1 | 14 | - | - |
| Patient 3 | No | 2 | 16 | 14 | - |
| Patient 4 | Yes | 2 | - | - | 31 |
| Patient 5 | Yes | 2 | 31 | - | - |
| Patient 6 | Yes | 1 | 16 | - | 16 |
| Patient 7 | Yes | 2 | 15 | 16 | - |
| Patient 12 | Yes | 2 | 15 | - | 30 |
| Patient 13 | No | 2 | 20 | - | 30 |
| Patient 14 | Yes | 1 | 12 | - | - |
| Patient 15 | Yes | 2 | - | - | 21 |
| Patient 17 | No | 1 | - | - | 31 |
| Patient 18 | Yes | 2 | 28 | 30 | - |
| Patient 19 | Yes | 2 | 15 | - | 15 |
| Total | - | 22 | 182 | 60 | 174 |
References
- Zynger, D.L. Staging-Bladder Carcinoma. Available online: https://www.pathologyoutlines.com/topic/bladderstaging.html (accessed on 9 May 2022).
- Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging (accessed on 12 February 2023).
- Babjuk, M.; Bohle, A.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; et al. EAU Guidelines on Nonmuscle-Invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, S.; Bazargani, S.T.; Bivalacqua, T.J.; Holzbeierlein, J.M.; Willard, B.; Taylor, J.M.; Liao, J.C.; Pohar, K.; Tierney, J.; Konety, B. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 361.e1–361.e6. [Google Scholar] [CrossRef] [PubMed]
- Ray, E.R.; Chatterton, K.; Khan, M.S.; Chandra, A.; Thomas, K.; Dasgupta, P.; O’brien, T.S. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guerin. BJU Int. 2010, 105, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.; Christensen, K.B.; Vrang, M.-L.; Hermann, G.G. Hermann Hospitalization for transurethral bladder resection reduces quality of life in Danish patients with non-muscle-invasive bladder tumour. Scand. J. Urol. 2016, 50, 170–174. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Mok, K.; Lemieux-Leduc, C.; Mercier, J.; St-Arnaud, K.; Urmey, K.; Guiot, M.-C.; Marple, E.; Petrecca, K.; et al. Characterization of a Raman Spectroscopy Probe System for Intraoperative Brain Tissue Classification. Biomed. Opt. Express 2015, 6, 2380. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.A.; Obaid, S.; Marple, E.T.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A New Method Using Raman Spectroscopy for in Vivo Targeted Brain Cancer Tissue Biopsy. Sci. Rep. 2018, 8, 1792. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Vargis, E.; Rudin, A.; Slaughter, J.C.; Thomas, G.; Newton, J.M.; Reese, J.; Bennett, K.A.; Mahadevan-Jansen, A. In Vivo Raman Spectroscopy for Biochemical Monitoring of the Human Cervix throughout Pregnancy. Am. J. Obstet. Gynecol. 2018, 218, 528.e1–528.e18. [Google Scholar] [CrossRef]
- Pence, I.J.; Beaulieu, D.B.; Horst, S.N.; Bi, X.; Herline, A.J.; Schwartz, D.A.; Mahadevan-Jansen, A. Clinical Characterization of In Vivo Inflammatory Bowel Disease with Raman Spectroscopy. Biomed. Opt. Express 2017, 8, 524. [Google Scholar] [CrossRef]
- Cordero Bautista, E.; Latka, I.; Matthäus, C.; Schie, I.W.; Popp, J. In-Vivo Raman Spectroscopy: From Basics to Applications. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Kerr, L.T.; Domijan, K.; Cullen, I.; Hennelly, B.M. Applications of Raman Spectroscopy to the Urinary Bladder for Cancer Diagnostics. Photonics Lasers Med. 2014, 3, 193–224. [Google Scholar] [CrossRef]
- Jin, H.; Lin, T.; Han, P.; Yao, Y.; Zheng, D.; Hao, J.; Hu, Y.; Zeng, R. Efficacy of Raman Spectroscopy in the Diagnosis of Bladder Cancer. Medicine 2019, 98, e18066. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Broderick, N.; Liu, Y.; Zhou, Y.; Han, J.; Xu, W. Identification and Characterization of Bladder Cancer by Low-Resolution Fiber-Optic Raman Spectroscopy. J. Biophotonics 2018, 11, e201800016. [Google Scholar] [CrossRef] [PubMed]
- Crow, P.; Molckovsky, A.; Stone, N.; Uff, J.; Wilson, B.C.; Wongkeesong, L.M. Assessment of Fiberoptic Near-Infrared Raman Spectroscopy for Diagnosis of Bladder and Prostate Cancer. Urology 2005, 65, 1126–1130. [Google Scholar] [CrossRef]
- Bovenkamp, D.; Sentosa, R.; Rank, E.; Erkkilä, M.T.; Placzek, F.; Püls, J.; Drexler, W.; Leitgeb, R.A.; Garstka, N.; Shariat, S.F.; et al. Combination of High-Resolution Optical Coherence Tomography and Raman Spectroscopy for Improved Staging and Grading in Bladder Cancer. Appl. Sci. 2018, 8, 2371. [Google Scholar] [CrossRef]
- Cordero Bautista, E.; Rüger, J.; Marti, D.; Mondol, A.S.; Hasselager, T.; Mogensen, K.; Hermann, G.G.; Popp, J.; Schie, I.W. Bladder Tissue Characterization Using Probe-Based Raman Spectroscopy: Evaluation of Tissue Heterogeneity and Influence on the Model Prediction. J. Biophotonics 2020, 13, e201960025. [Google Scholar] [CrossRef]
- Placzek, F.; Cordero Bautista, E.; Kretschmer, S.; Wurster, L.M.; Knorr, F.; González-Cerdas, G.; Erkkilä, M.T.; Stein, P.; Ataman, Ç.; Hermann, G.G.; et al. Morpho-Molecular Ex Vivo Detection and Grading of Non-Muscle-Invasive Bladder Cancer Using Forward Imaging Probe Based Multimodal Optical Coherence Tomography and Raman Spectroscopy. Analyst 2020, 145, 1445–1456. [Google Scholar] [CrossRef]
- Schie, I.W.; Placzek, F.; Knorr, F.; Cordero Bautista, E.; Wurster, L.M.; Hermann, G.G.; Mogensen, K.; Hasselager, T.; Drexler, W.; Popp, J.; et al. Morpho-Molecular Signal Correlation between Optical Coherence Tomography and Raman Spectroscopy for Superior Image Interpretation and Clinical Diagnosis. Sci. Rep. 2021, 11, 9951. [Google Scholar] [CrossRef]
- Osterberg, E.C.; Laudano, M.A.; Li, P.S. Clinical and Investigative Applications of Raman Spectroscopy in Urology and Andrology. Transl. Androl. Urol. 2014, 3, 84–88. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, F.; Yang, C.; Jiang, H. Use of in Vivo Raman Spectroscopy and Cryoablation for Diagnosis and Treatment of Bladder Cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123707. [Google Scholar] [CrossRef]
- Stomp-Agenant, M.; van Dijk, T.; Onur, A.R.; Grimbergen, M.; van Melick, H.; Jonges, T.; Bosch, R.; van Swol, C. In Vivo Raman Spectroscopy for Bladder Cancer Detection Using a Superficial Raman Probe Compared to a Nonsuperficial Raman Probe. J. Biophotonics 2022, 15, e202100354. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Paidi, S.K.; Prasad, R.; Pandey, R.; Barman, I. Advancing Raman Spectroscopy from Research to Clinic: Translational Potential and Challenges. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119957. [Google Scholar] [CrossRef] [PubMed]
- Schleusener, J.; Gluszczynska, P.; Reble, C.; Gersonde, I.; Helfmann, J.; Cappius, H.J.; Fluhr, J.W.; Meinke, M.C. Perturbation Factors in the Clinical Handling of a Fiber-Coupled Raman Probe for Cutaneous in Vivo Diagnostic Raman Spectroscopy. Appl. Spectrosc. 2015, 69, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Grimbergen, M.C.M.; van Swol, C.F.P.; van Moorselaar, R.J.A.; Uff, J.; Mahadevan-Jansen, A.; Stone, N. Raman Spectroscopy of Bladder Tissue in the Presence of 5-Aminolevulinic Acid. J. Photochem. Photobiol. B Biol. 2009, 95, 170–176. [Google Scholar] [CrossRef]
- Grimbergen, M.C.M.; van Swol, C.F.P.; Draga, R.O.P.; van Diest, P.; Verdaasdonk, R.M.; Stone, N.; Bosch, J.H.L.R. Bladder Cancer Diagnosis during Cystoscopy Using Raman Spectroscopy. Photonic Ther. Diagn. V 2009, 7161, 716114. [Google Scholar] [CrossRef]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman Spectroscopy for Medical Diagnostics—From in-Vitro Biofluid Assays to In-Vivo Cancer Detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef]
- Barik, A.K.; Sanoop Pavithran, M.; Lukose, J.; Upadhya, R.; Pai, M.V.; Kartha, V.B.; Chidangil, S. In Vivo Spectroscopy: Optical Fiber Probes for Clinical Applications. Expert Rev. Med. Devices 2022, 19, 657–675. [Google Scholar] [CrossRef]
- Cameron, J.M.; Rinaldi, C.; Rutherford, S.H.; Sala, A.; Theakstone, A.G.; Baker, M.J. Clinical Spectroscopy: Lost in Translation? Appl. Spectrosc. 2021, 76, 393–415. [Google Scholar] [CrossRef]
- Stevens, O.; Petterson, I.E.I.; Day, J.C.C.; Stone, N. Developing Fibre Optic Raman Probes for Applications in Clinical Spectroscopy. Chem. Soc. Rev. 2016, 45, 1919–1934. [Google Scholar] [CrossRef]
- The European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. OJL 2017, 117, 1–175. [Google Scholar]
- Shim, M.G.; Wilson, B.C.; Marple, E.T.; Wach, M. Study of Fiber-Optic Probes for in Vivo Medical Raman Spectroscopy. Appl. Spectrosc. 1999, 53, 619–627. [Google Scholar] [CrossRef]
- ASTM E1840-96; Standard Guide for Raman Shift Standards for Spectrometer Calibration. ASTM: West Conshohocken, PA, USA, 2014.
- Choquette, S.J.; Etz, E.S.; Hurst, W.S.; Blackburn, D.H.; Leigh, S.D. Relative Intensity Correction of Raman Spectrometers: NIST SRMs 2241 through 2243 for 785 Nm, 532 Nm, and 488 Nm/514.5 Nm Excitation. Appl. Spectrosc. 2007, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-1-2:2014+A1:2020; Medical Electrical Equipment—Part 1–2: General Requirements for Basic Safety and Essential Performance—Collateral Standard: Electromagnetic Disturbances—Requirements and Tests. IEC: Geneva, Switzerland, 2020.
- CISPR 11:2015; Industrial, Scientific and Medical Equipment—Radio-Frequency Disturbance Characteristics—Limits and Methods of Measurement. IEC: Geneva, Switzerland, 2015.
- DIN EN 60601-1; Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. IEC: Geneva, Switzerland, 1996.
- IEC 60601-2-22:2019; Medical Electrical Equipment—Part 2–22: Particular Requirements for Basic Safety and Essential Performance of Surgical, Cosmetic, Therapeutic and Diagnostic Laser Equipment. IEC: Geneva, Switzerland, 2013.
- IEC 60825; Safety of Laser Products—Part 1: Equipment Classification and Requirements. IEC: Geneva, Switzerland, 2014.
- EN ISO 17664; Processing of Health Care Products—Information to Be Provided by the Medical Device Manufacturer for the Processing of Medical Devices. ISO: Geneva, Switzerland, 2017.
- EN 62366-1; Medical Devices—Part 1: Application of Usability Engineering to Medical Devices. IEC: Geneva, Switzerland, 2015.
- IEC TR 62366-2; Medical Devices—Part 2: Guidance on the Application of Usability Engineering to Medical Devices. IEC: Geneva, Switzerland, 2016.
- EN 62304 (2006+A1:2015); Medical Device Software—Software Life Cycle Processes. ISO: Geneva, Switzerland, 2006.
- Available online: https://www.hexvix.com/safety-information (accessed on 2 February 2023).
- Afseth, N.K.; Kohler, A. Extended Multiplicative Signal Correction in Vibrational Spectroscopy, a Tutorial. Chemom. Intell. Lab. Syst. 2012, 117, 92–99. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of Multidimensional Data Processing Approaches for Raman and Infrared Spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef]
- Mogensen, K.; Glenthøj, A.; Toft, B.G.; Scheike, T.; Hermann, G.G. Outpatient photodynamic-guided diagnosis of carcinoma in situ with flexible cystoscopy: An alternative to conventional inpatient photodynamic-guided bladder biopsies in the operating theatre? Scand. J. Urol. 2017, 51, 376–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nardelli, C.; Aveta, A.; Pandolfo, S.D.; Tripodi, L.; Russo, F.; Imbimbo, C.; Castaldo, G.; Pastore, L. Microbiome Profiling in Bladder Cancer Patients Using the First-Morning Urine Sample. Eur. Urol. Open Sci. 2024, 59, 18–26. [Google Scholar] [CrossRef]




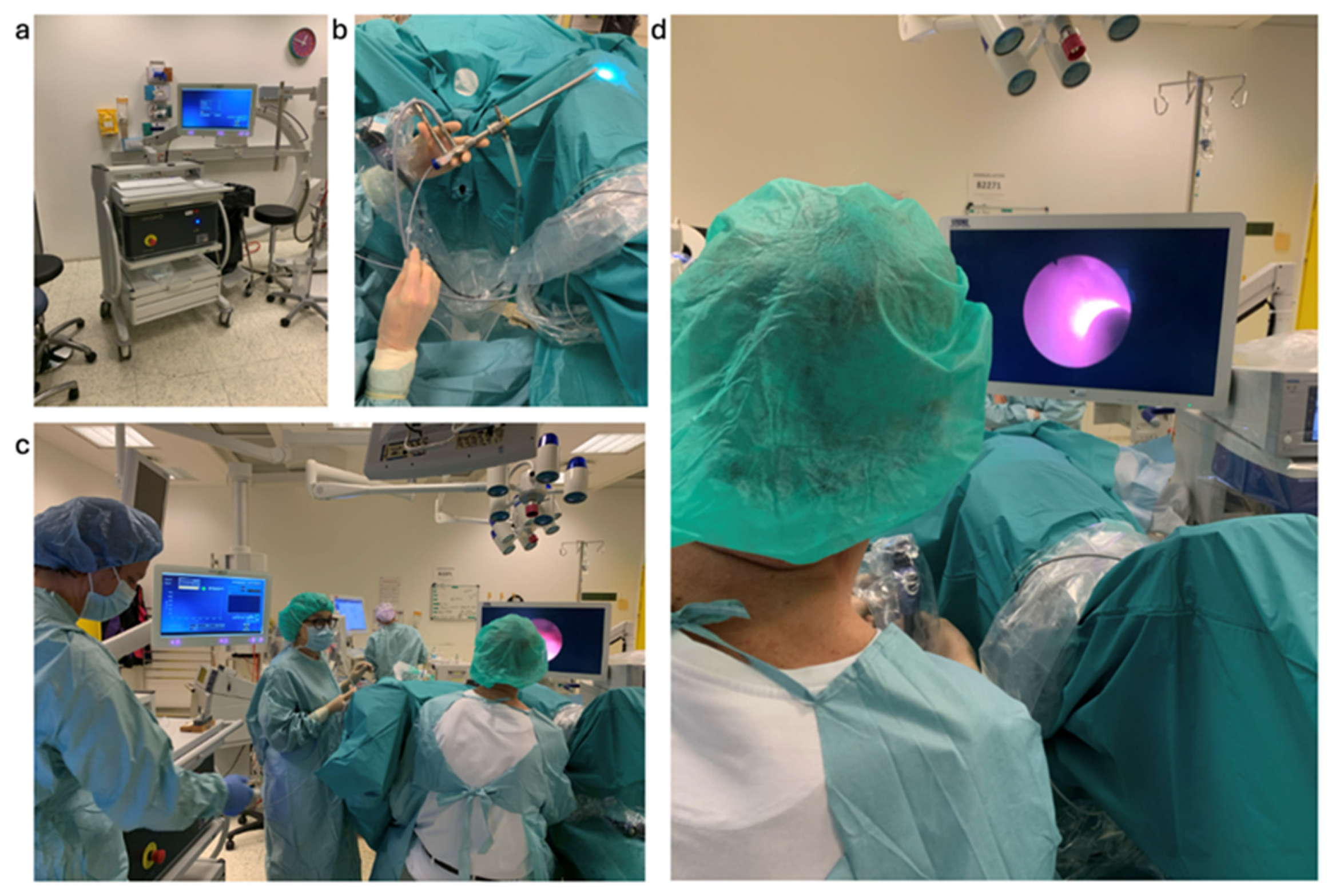

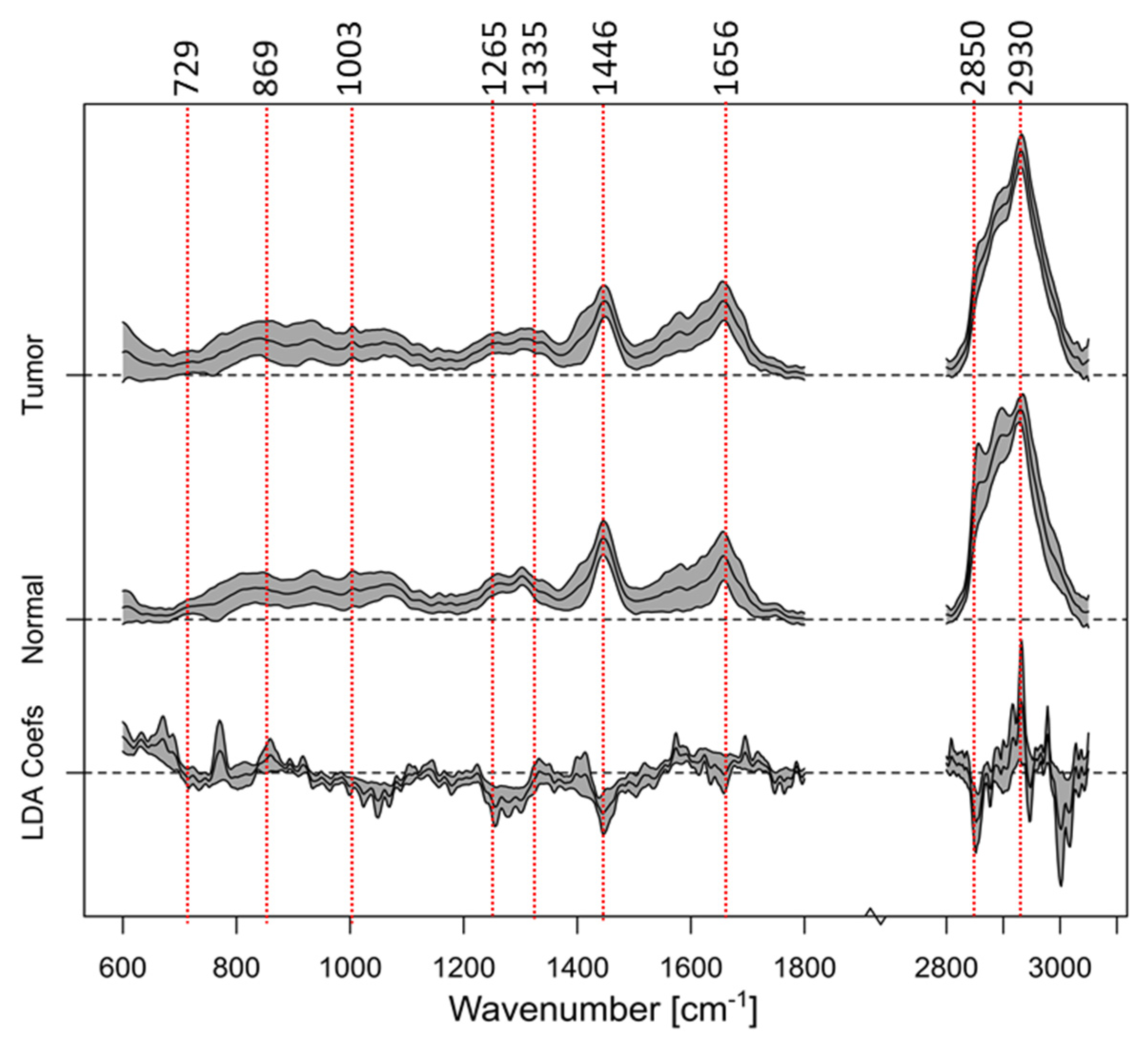

| Category | Description | Number of Biopsies |
|---|---|---|
| C1 | pT2, muscle invasive cancer | 1 |
| C2 | pTa (superficial tumor) with low-grade cells | 7 |
| C3 | CIS (carcinoma in situ) | 4 |
| C4 | Normal tissue | 25 |
| C5 | pT1 (tumor in the soft mucosa) with high-grade cells | 3 |
| C6 | pTa (superficial tumor) high-grade cells | 4 |
| Normal | Tumor | |
|---|---|---|
| Normal | 67 | 22 |
| Tumor | 12 | 104 |
| Sensitivity | 0.83 | |
| Specificity | 0.75 | |
| Wavenumber [cm−1] | Bond Assignment |
|---|---|
| 729 | C-C stretching, proline |
| 869 | C-C stretching, choline group |
| 1003 | Phenylalanine, C-C skeletal, phosphate group |
| 1265 | Amide III of collagen, v(CN), d(NH) amide III |
| 1335 | CH3CH2 wagging |
| 1446 | CH2 bending mode of proteins and lipids, CH2 deformation |
| 1656 | C-C lipids, amide I (proteins) |
| 2850 | υsCH2, lipids, fatty acids CH2 symmetric |
| 2930 | CH2 sym. stretching, chain-end CH3 sym. stretching |
| High Grade | Low Grade | |
|---|---|---|
| High grade | 79 | 5 |
| Low grade | 23 | 7 |
| Sensitivity | 0.77 | |
| Specificity | 0.53 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latka, I.; Mogensen, K.; Knorr, F.; Kuzucu, C.; Windirsch, F.; Sandic, D.; Popp, J.; Hermann, G.G.; Schie, I.W. Raman Spectroscopy for Instant Bladder Tumor Diagnosis: System Development and In Vivo Proof-Of-Principle Study in Accordance with the European Medical Device Regulation (MDR2017/745). Cancers 2024, 16, 3238. https://doi.org/10.3390/cancers16183238
Latka I, Mogensen K, Knorr F, Kuzucu C, Windirsch F, Sandic D, Popp J, Hermann GG, Schie IW. Raman Spectroscopy for Instant Bladder Tumor Diagnosis: System Development and In Vivo Proof-Of-Principle Study in Accordance with the European Medical Device Regulation (MDR2017/745). Cancers. 2024; 16(18):3238. https://doi.org/10.3390/cancers16183238
Chicago/Turabian StyleLatka, Ines, Karin Mogensen, Florian Knorr, Cansu Kuzucu, Florian Windirsch, Dragan Sandic, Jürgen Popp, Gregers G. Hermann, and Iwan W. Schie. 2024. "Raman Spectroscopy for Instant Bladder Tumor Diagnosis: System Development and In Vivo Proof-Of-Principle Study in Accordance with the European Medical Device Regulation (MDR2017/745)" Cancers 16, no. 18: 3238. https://doi.org/10.3390/cancers16183238
APA StyleLatka, I., Mogensen, K., Knorr, F., Kuzucu, C., Windirsch, F., Sandic, D., Popp, J., Hermann, G. G., & Schie, I. W. (2024). Raman Spectroscopy for Instant Bladder Tumor Diagnosis: System Development and In Vivo Proof-Of-Principle Study in Accordance with the European Medical Device Regulation (MDR2017/745). Cancers, 16(18), 3238. https://doi.org/10.3390/cancers16183238






