Risk of Esophageal and Gastric Cancer in Patients with Type 2 Diabetes Receiving Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A National Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
3.2.1. Risk of Gastric Cancer
3.2.2. Risk of Esophageal Cancer
4. Discussion
4.1. GLP-1 Receptor Agonists: Indications, Benefits, and Concerns
4.2. Esophageal Cancer: Epidemiology, Incidence and Prevalence, Risk Factors, and Genetic Predisposition
4.3. Gastric Cancer: Epidemiology, Incidence and Prevalence, Risk Factors, and Genetic Predisposition
4.4. Our Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, W.; Kaelber, D.C.; Xu, R.; Berger, N.A. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients with Type 2 Diabetes, with and without Overweight/Obesity. JAMA Oncol. 2024, 10, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Chai, S.; Zhao, X.; Ji, L. Risk of Malignant Neoplasia with Glucagon-like Peptide-1 Receptor Agonist Treatment in Patients with Type 2 Diabetes: A Meta-Analysis. J. Diabetes Res. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gier, B.; Matveyenko, A.V.; Kirakossian, D.; Dawson, D.; Dry, S.M.; Butler, P.C. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the KrasG12D Mouse Model. Diabetes 2012, 61, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Stöckli, M.; Waser, B.; Reubi, J.C. GLP-1 Receptor Expression in Human Tumors and Human Normal Tissues: Potential for In Vivo Targeting. J. Nucl. Med. 2007, 48, 736–743. [Google Scholar] [CrossRef]
- Körner, M.; Christ, E.; Wild, D.; Reubi, J.C. Glucagon-like peptide-1 receptor overexpression in cancer and its impact on clinical applications. Front. Endocrinol. 2012, 3, 35115. [Google Scholar] [CrossRef]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Ito, S.; Ogawa, Y.; Kobayashi, M.; Hanazaki, K. Diagnosis and management of insulinoma. World J. Gastroenterol. WJG 2013, 19, 829–837. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Madsen, L.W.; Andersen, S.; Almholt, K.; De Boer, A.S.; Drucker, D.J.; Gotfredsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010, 151, 1473–1486. [Google Scholar] [CrossRef]
- Ayoub, M.; Tomanguillo, J.; Faris, C.; Anwar, N.; Chela, H.; Daglilar, E. SARS-CoV-2 Infection Is an Independent Risk Factor for Decompensation in Cirrhosis Patients. Diseases 2024, 12, 46. [Google Scholar] [CrossRef]
- Hunt, B.; Malkin, S.J.P.; Moes, R.G.J.; Huisman, E.L.; Vandebrouck, T.; Wolffenbuttel, B.H.R. Once-weekly semaglutide for patients with type 2 diabetes: A cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res. Care 2019, 7, e000705. [Google Scholar] [CrossRef]
- Burcelin, R.; Gourdy, P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes. Rev. 2017, 18, 86–98. [Google Scholar] [CrossRef]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Its Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S158–S178. [Google Scholar] [CrossRef]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S191–S202. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 101. [Google Scholar] [CrossRef]
- Zhang, F.; Tong, Y.; Su, N.; Li, Y.; Tang, L.; Huang, L.; Tong, N. Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes 2015, 7, 329–339. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Caroleo, P.; Giuliano, S.; Greco, E.; Luque, R.M.; Puccio, L.; Foti, D.P.; Aversa, A.; et al. Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin plus Insulin Secretagogues in Obesity and Type 2 Diabetes. J. Clin. Med. 2021, 10, 985. [Google Scholar] [CrossRef]
- Halawi, H.; Khemani, D.; Eckert, D.; O’Neill, J.; Kadouh, H.; Grothe, K.; Clark, M.M.; Burton, D.D.; Vella, A.; Acosta, A.; et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol. Hepatol. 2017, 2, 890–899. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Ellis, S.L. GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2015, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011, 34 (Suppl. 2), S279–S284. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2016, 18, 317–332. [Google Scholar] [CrossRef]
- Unger, J.R.; Parkin, C.G. Glucagon-like peptide-1 (GLP-1) receptor agonists: Differentiating the new medications. Diabetes Ther. 2011, 2, 29–39. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; De Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of Glucagon-like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 513–519. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Shlomai, G.; Neel, B.; LeRoith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef]
- Anderson, S.L.; Trujillo, J.M. Association of pancreatitis with glucagon-like Peptide-1 agonist use. Ann. Pharmacother. 2010, 44, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Piccoli, G.F.; Mesquita, L.A.; Stein, C.; Aziz, M.; Zoldan, M.; Degobi, N.A.H.; Spiazzi, B.F.; Lopes Junior, G.L.; Colpani, V.; Gerchman, F. Do GLP-1 Receptor Agonists Increase the Risk of Breast Cancer? A Systematic Review and Meta-analysis. J Clin Endocrinol Metab 2021, 106, 912–921. [Google Scholar] [CrossRef]
- Alves, C.; Batel-Marques, F.; Macedo, A.F. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res. Clin. Pract. 2012, 98, 271–284. [Google Scholar] [CrossRef]
- Wenjing, H.; Shao, Y.; Yu, Y.; Huang, W.; Feng, G.; Li, J. Exendin-4 enhances the sensitivity of prostate cancer to enzalutamide by targeting Akt activation. Prostate 2020, 80, 367–375. [Google Scholar] [CrossRef]
- Nomiyama, T.; Kawanami, T.; Irie, S.; Hamaguchi, Y.; Terawaki, Y.; Murase, K.; Tsutsumi, Y.; Nagaishi, R.; Tanabe, M.; Morinaga, H.; et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63, 3891–3905. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.-Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Lin, Y.; Totsuka, Y.; He, Y.; Kikuchi, S.; Qiao, Y.; Ueda, J.; Wei, W.; Inoue, M.; Tanaka, H. Epidemiology of Esophageal Cancer in Japan and China. J. Epidemiol. 2013, 23, 233–242. [Google Scholar] [CrossRef]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Nasrollahzadeh, D.; Safiri, S.; Sepanlou, S.G.; Fitzmaurice, C.; Ikuta, K.S.; Bisignano, C.; Islami, F.; Roshandel, G.; Lim, S.S.; et al. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Yousef, F.; Cardwell, C.; Cantwell, M.M.; Galway, K.; Johnston, B.T.; Murray, L. The incidence of esophageal cancer and high-grade dysplasia in barrett’s esophagus: A systematic review and meta-analysis. Am. J. Epidemiol. 2008, 168, 237–249. [Google Scholar] [CrossRef]
- Di Pardo, B.J.; Bronson, N.W.; Diggs, B.S.; Thomas, C.R.; Hunter, J.G.; Dolan, J.P. The Global Burden of Esophageal Cancer: A Disability-Adjusted Life-Year Approach. World J. Surg. 2016, 40, 395–401. [Google Scholar] [CrossRef]
- Kubo, A.; Levin, T.R.; Block, G.; Rumore, G.J.; Quesenberry, C.P.; Buffler, P.; Corley, D.A. Dietary patterns and the risk of barrett’s esophagus. Am. J. Epidemiol. 2008, 167, 839–846. [Google Scholar] [CrossRef]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016, 27 (Suppl. 5), v38–v49. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713. [Google Scholar] [CrossRef]
- Chiang, T.H.; Chang, W.J.; Chen, S.L.S.; Yen, A.M.F.; Fann, J.C.Y.; Chiu, S.Y.H.; Chen, Y.R.; Chuang, S.L.; Shieh, C.F.; Liu, C.Y.; et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut 2021, 70, 243–250. [Google Scholar]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.-L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 June 2024).
- Loomans-Kropp, H.A.; Umar, A. Analysis of Body Mass Index in Early and Middle Adulthood and Estimated Risk of Gastrointestinal Cancer. JAMA Netw. Open 2023, 6, e2310002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Wang, X.; Wang, J.; Yan, Z.; Cheng, J.; Gong, G.; Li, G. Body mass index and risk of gastric cancer: Ameta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. Familial gastric cancer: Genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015, 16, e60–e70. [Google Scholar] [CrossRef]
- Taja-Chayeb, L.; Vidal-Millán, S.; Trejo-Becerril, C.; Pérez-Cárdenas, E.; Chávez-Blanco, A.; Domínguez-Gómez, G.; González-Fierro, A.; Romo-Pérez, A.; Dueñas-González, A. Hereditary diffuse gastric cancer (HDGC). An overview. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101820. [Google Scholar] [CrossRef]
- Rudloff, U. Gastric adenocarcinoma and proximal polyposis of the stomach: Diagnosis and clinical perspectives. Clin. Exp. Gastroenterol. 2018, 11, 447–459. [Google Scholar] [CrossRef]
- Genetics of Gastric Cancer (PDQ®)—NCI. Available online: https://www.cancer.gov/types/stomach/hp/gastric-genetics-pdq (accessed on 4 September 2024).
- Carvalho, J.; Oliveira, P.; Senz, J.; São José, C.; Hansford, S.; Teles, S.P.; Ferreira, M.; Corso, G.; Pinheiro, H.; Lemos, D.; et al. Redefinition of familial intestinal gastric cancer: Clinical and genetic perspectives. J. Med Genet. 2021, 58, 1–11. [Google Scholar] [CrossRef]
- Setia, N.; Clark, J.W.; Duda, D.G.; Hong, T.S.; Kwak, E.L.; Mullen, J.T.; Lauwers, G.Y. Familial Gastric Cancers. Oncologist 2015, 20, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, R.; Kaelber, D.C.; Berger, N.A. Glucagon-Like Peptide 1 Receptor Agonists and 13 Obesity-Associated Cancers in Patients with Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2421305. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Faris, C.; Juranovic, T.; Chela, H.; Daglilar, E. The Use of Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus Does Not Increase the Risk of Pancreatic Cancer: A U.S.-Based Cohort Study. Cancers 2024, 16, 1625. [Google Scholar] [CrossRef] [PubMed]

| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Characteristic | GLP-1 RA (n = 167,077) | No GLP-1 RA (n = 2,581,354) | p-Value | GLP-1 RA (n = 146,277) | No GLP-1 RA (n = 146,277) | p-Value |
| Demographics | ||||||
| Age (Mean ± SD) | 58.2 ± 13.5 | 61.6 ± 16.4 | <0.001 | 58.3 ± 13.5 | 58.3 ± 14.4 | 0.995 |
| White | 64.1% | 55.5% | <0.001 | 63.3% | 63.9% | 0.293 |
| Black or African American | 15.7% | 15.2% | <0.001 | 15.8% | 15.7% | 0.385 |
| Asian | 4.5% | 6.1% | <0.001 | 4.5% | 4.3% | 0.482 |
| Female | 51.1% | 47.1% | <0.001 | 51.2% | 51.4% | 0.207 |
| Medications | ||||||
| Rabeprazole | 0.09% | 0.04% | <0.001 | 0.1% | 0.1% | 0.699 |
| Lansoprazole | 1% | 0.4% | <0.001 | 1% | 0.8% | 0.083 |
| Esomeprazole | 1.8% | 0.7% | <0.001 | 1.8% | 1.6% | 0.144 |
| Pantoprazole | 8.4% | 2.9% | <0.001 | 8.3% | 7.7% | 0.174 |
| Omeprazole | 7.2% | 2.2% | <0.001 | 7% | 6.8% | 0.430 |
| Dexlansoprazole | 0.3% | 0.1% | <0.001 | 0.3% | 0.2% | 0.811 |
| Ranitidine | 2.2% | 0.8% | <0.001 | 2.2% | 1.7% | 0.278 |
| Cimetidine | 0.1% | 0.03% | <0.001 | 0.1% | 0.1% | 0.082 |
| Nizatidine | 0.0004% | 0.006% | <0.001 | 0.01% | 0.01% | 0.746 |
| Famotidine | 6.9% | 2.1% | <0.001 | 6.6% | 6.2% | 0.743 |
| Bismuth subcitrate | 0.02% | 0.003% | <0.001 | 0.01% | 0.01% | 0.771 |
| Biguanides | 56.8% | 8.6% | <0.001 | 51.7% | 50.2% | 0.092 |
| Sulfonylureas | 25.8% | 3.4% | <0.001 | 21.2% | 20.2% | 0.154 |
| Alpha glucosidase inhibitors | 0.4% | 0.01% | <0.001 | 0.3% | 0.2% | 0.761 |
| Thiazolidinediones | 6.7% | 0.7% | <0.001 | 5.1% | 5.3% | 0.078 |
| SGLT-2 inhibitors | 18.1% | 0.9% | <0.001 | 9.7% | 11.6% | 0.091 |
| Labs and Genetics * | ||||||
| HbA1C (Mean ± SD) | 8.3 ± 2.2 | 7.2 ± 2 | <0.001 | 8.2 ± 2.2 | 7.5 ± 2.2 | 0.084 |
| PMS2 Variants | 0.007% | 0.004% | <0.001 | 0.007% | 0.007% | 0.097 |
| MLH1 | 0.009% | 0.007% | <0.001 | 0.009% | 0.009% | 0.102 |
| MSH6 Variants | 0.007% | 0.003% | <0.001 | 0.007% | 0.007% | 0.405 |
| MSH2 Variants | 0.005% | 0.002% | <0.001 | 0.005% | 0.002% | 0.405 |
| STK11 Variants | 0.004% | 0.002% | <0.001 | 0.004% | 0.004% | 0.239 |
| TP53 Variants | 0.01% | 0.005% | <0.001 | 0.01% | 0.01% | 0.125 |
| EPCAM | 0.007% | 0.003% | <0.001 | 0.007% | 0.007% | 0.204 |
| PMS2 | 0.007% | 0.004% | <0.001 | 0.007% | 0.007% | 0.113 |
| CDH1 Variants | 0.002% | 0.001% | 0.009 | 0.002% | 0.002% | 0.502 |
| BMPR1A | 0.002% | 0.001% | 0.003 | 0.002% | 0.002% | 0.317 |
| SMAD4 | 0.01% | 0.005% | 0.009 | 0.01% | 0.01% | 0.103 |
| APC | 0.017% | 0.007% | <0.001 | 0.017% | 0.017% | 0.091 |
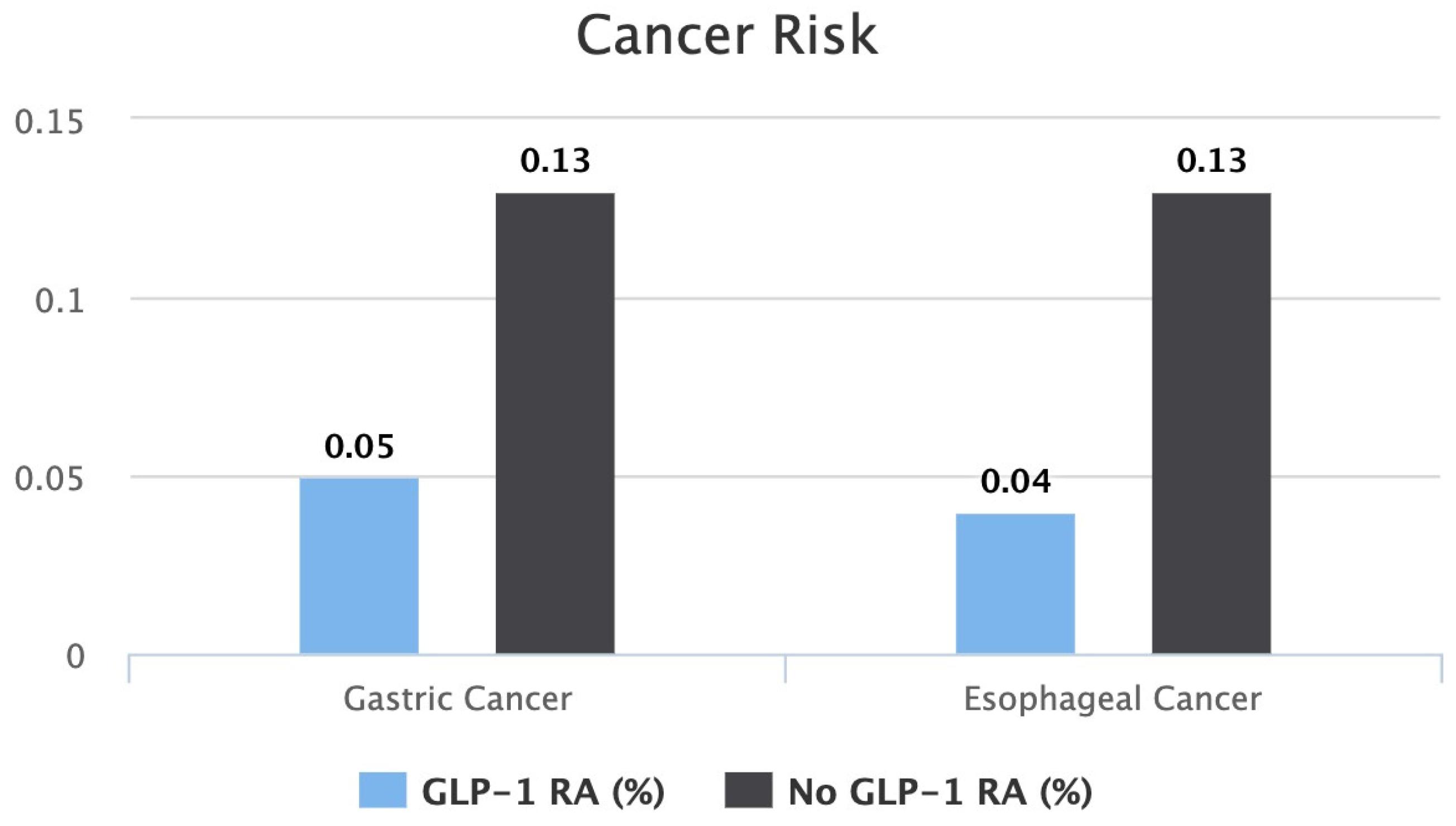
| Outcome Gastric Cancer |
GLP-1 RA (n = 146,277) |
NO GLP-1 RA (n = 146,277) | p-value | ||||
| 0.05% (n = 79) | 0.13% (n = 197) | <0.0001 | |||||
| Log-Rank Test | X2 | df | p | ||||
| 45.626 | 1 | 0.000 | |||||
| Hazard Ratio and Proportionality | HR | 95% CI | X2 | df | p | ||
| 0.417 | (0.321, 0.542) | 7.370 | 1 | 0.001 | |||
| Outcome Esophageal Cancer |
GLP-1 RA (n = 146,277) |
NO GLP-1 RA (n = 146,277) | p -value | ||||
| 0.04% (n = 63) | 0.13% (n = 190) | <0.0001 | |||||
| Log-Rank Test | X2 | df | p | ||||
| 59.747 | 1 | 0.000 | |||||
| Hazard Ratio and Proportionality | HR | 95% CI | X2 | df | p | ||
| 0.341 | (0.257, 0.454) | 1.409 | 0.341 | 0.002 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, M.; Aibani, R.; Dodd, T.; Ceesay, M.; Bhinder, M.; Faris, C.; Amin, N.; Daglilar, E. Risk of Esophageal and Gastric Cancer in Patients with Type 2 Diabetes Receiving Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A National Analysis. Cancers 2024, 16, 3224. https://doi.org/10.3390/cancers16183224
Ayoub M, Aibani R, Dodd T, Ceesay M, Bhinder M, Faris C, Amin N, Daglilar E. Risk of Esophageal and Gastric Cancer in Patients with Type 2 Diabetes Receiving Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A National Analysis. Cancers. 2024; 16(18):3224. https://doi.org/10.3390/cancers16183224
Chicago/Turabian StyleAyoub, Mark, Rafi Aibani, Tiana Dodd, Muhammed Ceesay, Muhammad Bhinder, Carol Faris, Nisar Amin, and Ebubekir Daglilar. 2024. "Risk of Esophageal and Gastric Cancer in Patients with Type 2 Diabetes Receiving Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A National Analysis" Cancers 16, no. 18: 3224. https://doi.org/10.3390/cancers16183224
APA StyleAyoub, M., Aibani, R., Dodd, T., Ceesay, M., Bhinder, M., Faris, C., Amin, N., & Daglilar, E. (2024). Risk of Esophageal and Gastric Cancer in Patients with Type 2 Diabetes Receiving Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A National Analysis. Cancers, 16(18), 3224. https://doi.org/10.3390/cancers16183224







