Immunological Tumor Microenvironment of Solitary Fibrous Tumors—Associating Immune Infiltrate with Variables of Prognostic Significance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
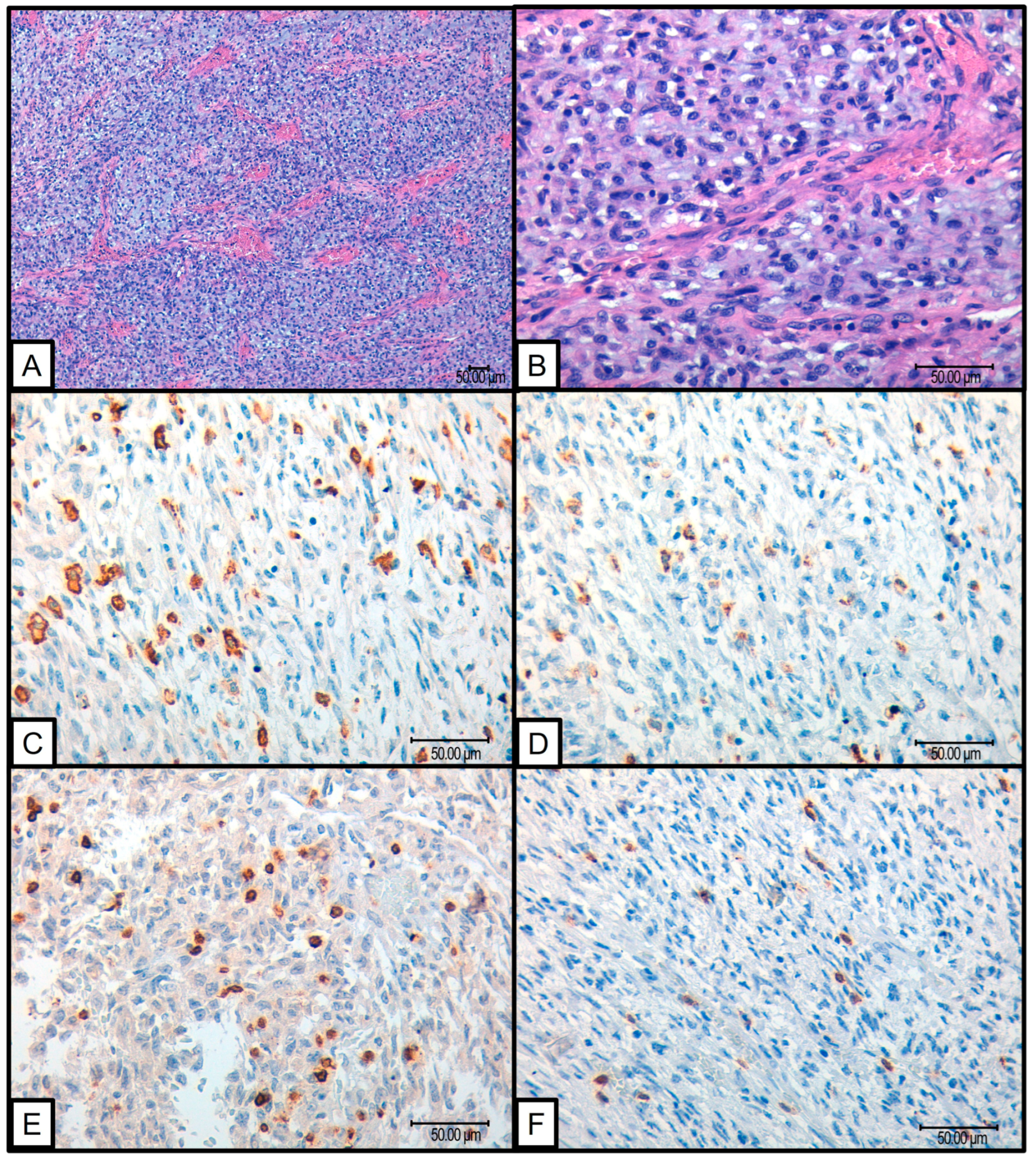
2.1. Immunohistochemistry
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinslow, C.J.; Wang, T.J.C. Incidence of Extrameningeal Solitary Fibrous Tumors. Cancer 2020, 126, 4067. [Google Scholar] [CrossRef] [PubMed]
- de Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.M.; et al. Nationwide Incidence of Sarcomas and Connective Tissue Tumors of Intermediate Malignancy over Four Years Using an Expert Pathology Review Network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef]
- Machado, I.; Nieto-Morales, G.; Cruz, J.; Navarro, S.; Giner, F.; Ferrandez, A.; María Victoria, L.-S.; Javier, L.; Llombart-Bosch, A. Controversial Issues in Soft Tissue Solitary Fibrous Tumors: A Pathological and Molecular Review. Pathol. Int. 2020, 70, 129–139. [Google Scholar] [CrossRef]
- Akaike, K.; Kurisaki-Arakawa, A.; Hara, K.; Suehara, Y.; Takagi, T.; Mitani, K.; Kaneko, K.; Yao, T.; Saito, T. Distinct Clinicopathological Features of NAB2-STAT6 Fusion Gene Variants in Solitary Fibrous Tumor with Emphasis on the Acquisition of Highly Malignant Potential. Hum. Pathol. 2015, 46, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Fritchie, K.J.; Han, A. Solitary Fibrous Tumor. In WHO Classification of Tumours of Soft Tissue and Bone; International Agency for Research on Cancer: Lyon, France, 2020; pp. 104–108. [Google Scholar]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary Fibrous Tumor: A Clinicopathological Study of 110 Cases and Proposed Risk Assessment Model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Wagner, M.J.; Maki, R.G.; Gupta, V.; Iofin, I.; Lazar, A.J.; Wang, W.L. Risk Assessment in Solitary Fibrous Tumors: Validation and Refinement of a Risk Stratification Model. Mod. Pathol. 2017, 30, 1433–1442. [Google Scholar] [CrossRef]
- Medina-Ceballos, E.; Machado, I.; Giner, F.; Bujeda, Á.B.; Navarro, S.; Ferrandez, A.; Lavernia, J.; Ruíz-Sauri, A.; Llombart-Bosch, A. Solitary Fibrous Tumor: Can the New Huang Risk Stratification System for Orbital Tumors Improve Prognostic Accuracy in Other Tumor Locations? Pathol. Res. Pract. 2024, 254, 155143. [Google Scholar] [CrossRef]
- Sugita, S.; Segawa, K.; Kikuchi, N.; Takenami, T.; Kido, T.; Emori, M.; Akiyama, Y.; Takada, K.; Hinotsu, S.; Hasegawa, T. Prognostic Usefulness of a Modified Risk Model for Solitary Fibrous Tumor That Includes the Ki-67 Labeling Index. World J. Surg. Oncol. 2022, 20, 29. [Google Scholar] [CrossRef]
- Georgiesh, T.; Boye, K.; Bjerkehagen, B. A Novel Risk Score to Predict Early and Late Recurrence in Solitary Fibrous Tumour. Histopathology 2020, 77, 123–132. [Google Scholar] [CrossRef]
- Huang, A.; Su, M.; Jing, Y.; He, S.; He, X.; Ma, J.; Liu, H. Orbital Primary Solitary Fibrous Tumor: A Proposed Recurrence Risk Prediction Model Based on 92 Cases. Hum. Pathol. 2023, 137, 85–93. [Google Scholar] [CrossRef]
- Machado, I.; Blázquez Bujeda, Á.; Giner, F.; Nieto Morales, M.G.; Cruz, J.; Lavernia, J.; Navarro, S.; Ferrandez, A.; Ruiz-Sauri, A.; Llombart-Bosch, A. Evaluation of Alternative Risk Stratification Systems in a Large Series of Solitary Fibrous Tumors with Molecular Findings and Ki-67 Index Data: Do They Improve Risk Assessment? Int. J. Mol. Sci. 2022, 24, 439. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Morales, G.N.; Cruz, J.; Lavernia, J.; Giner, F.; Navarro, S.; Ferrandez, A.; Llombart-Bosch, A. Solitary Fibrous Tumor: A Case Series Identifying Pathological Adverse Factors—Implications for Risk Stratification and Classification. Virchows Arch. 2020, 476, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Kamamoto, D.; Ohara, K.; Kitamura, Y.; Yoshida, K.; Kawakami, Y.; Sasaki, H. Association between Programmed Cell Death Ligand-1 Expression and Extracranial Metastasis in Intracranial Solitary Fibrous Tumor/Hemangiopericytoma. J. Neurooncol. 2018, 139, 251–259. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung Cancer Immunotherapy: Progress, Pitfalls, and Promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From Inflammation to Cancer. Ir. J. Med. Sci. 2017, 186, 57. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Dvorak, H.F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the Tumor Stroma for Cancer Therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Z.; Jin, W.L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, M.; D’Angelo, E.; Bisogno, G.; Agostini, M.; Pozzobon, M. Carcinoma and Sarcoma Microenvironment at a Glance: Where We Are. Front. Oncol. 2020, 10, 76. [Google Scholar] [CrossRef]
- Issels, R.; Büclein, V.; Kampmann, E.; Knösel, T.; Nössner, E.; Subklewe, M.; Lindner, L. Dissecting the Role of Tumor-Infiltrating Lymphocytes (TIL) in Patients with High-Risk Soft-Tissue Sarcoma (STS) Receiving Neo-Adjuvant Chemotherapy (NAC) with Regional Hyperthermia (RHT). Ann. Oncol. 2016, 27, vi488. [Google Scholar] [CrossRef]
- Sorbye, S.W.; Kilvaer, T.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic Impact of Lymphocytes in Soft Tissue Sarcomas. PLoS ONE 2011, 6, 14611. [Google Scholar] [CrossRef]
- D’angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.-X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of Tumor-Infiltrating Lymphocytes and PD-L1 Expression in the Soft Tissue Sarcoma Microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef]
- Sousa, L.M.; Almeida, J.S.; Fortes-Andrade, T.; Santos-Rosa, M.; Freitas-Tavares, P.; Casanova, J.M.; Rodrigues-Santos, P. Tumor and Peripheral Immune Status in Soft Tissue Sarcoma: Implications for Immunotherapy. Cancers 2021, 13, 3885. [Google Scholar] [CrossRef]
- Lotze, M.T.; Zeh, H.J.; Rubartelli, A.; Sparvero, L.J.; Amoscato, A.A.; Washburn, N.R.; DeVera, M.E.; Liang, X.; Tör, M.; Billiar, T. The Grateful Dead: Damage-Associated Molecular Pattern Molecules and Reduction/Oxidation Regulate Immunity. Immunol. Rev. 2007, 220, 60–81. [Google Scholar] [CrossRef]
- Patidar, A.; Selvaraj, S.; Sarode, A.; Chauhan, P.; Chattopadhyay, D.; Saha, B. DAMP-TLR-Cytokine Axis Dictates the Fate of Tumor. Cytokine 2018, 104, 114–123. [Google Scholar] [CrossRef]
- Chiaruttini, G.; Mele, S.; Opzoomer, J.; Crescioli, S.; Ilieva, K.M.; Lacy, K.E.; Karagiannis, S.N. B Cells and the Humoral Response in Melanoma: The Overlooked Players of the Tumor Microenvironment. Oncoimmunology 2017, 6, e1294296. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.L.; Bryant, V.L. B Cells in Lung Cancer—Not Just a Bystander Cell: A Literature Review. Transl. Lung Cancer Res. 2021, 10, 2830. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. Human Cancer Biology CD20 þ Tumor-Infiltrating Lymphocytes Have an Atypical CD27 À Memory Phenotype and Together with CD8 þ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin. Cancer. Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Saudi, A.; Banday, V.; Zirakzadeh, A.A.; Selinger, M.; Forsberg, J.; Holmbom, M.; Henriksson, J.; Waldén, M.; Alamdari, F.; Aljabery, F.; et al. Immune-Activated B Cells Are Dominant in Prostate Cancer. Cancers 2023, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.K.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic Impact of Peritumoral Lymphocyte Infiltration in Soft Tissue Sarcomas. BMC Clin. Pathol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Hu, C.; Huang, M.; Cen, W.; Ling, D.; Long, Y.; Yang, X.H.; Xu, B.; Peng, J.; et al. Comprehensive Analysis Reveals Potential Therapeutic Targets and an Integrated Risk Stratification Model for Solitary Fibrous Tumors. Nat. Commun. 2023, 14, 7479. [Google Scholar] [CrossRef]
- Smolle, M.A.; Herbsthofer, L.; Granegger, B.; Goda, M.; Brcic, I.; Bergovec, M.; Scheipl, S.; Prietl, B.; Pichler, M.; Gerger, A.; et al. T-Regulatory Cells Predict Clinical Outcome in Soft Tissue Sarcoma Patients: A Clinico-Pathological Study. Br. J. Cancer 2021, 125, 717–724. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Cell Metabolism Review Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Whitworth, P.W.; Pak, C.C.; Esgro, J.; Kleinerman, E.S.; Fidler, I.J. Macrophages and Cancer. Cancer Metastasis Rev. 1990, 8, 319–351. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Loke, P.; Allison, J.P. PD-L1 and PD-L2 Are Differentially Regulated by Th1 and Th2 Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5336–5341. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Amaya, C.N.; Wians, F.H.; Bryan, B.A. PD-1 and PD-L1 Expression in Bone and Soft Tissue Sarcomas. Pathology 2017, 49, 506–513. [Google Scholar] [CrossRef]
- Kösemehmetoğlu, K.; Özogul, E.; Babaoğlu, B.; Tezel, G.G.; Gedikoğlu, G. Programmed Death Ligand 1 (PD-L1) Expression in Malignant Mesenchymal Tumors. Turk. Patoloji. Derg. 2017, 1, 192–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Papakonstantinou, A.; Tsagkozis, P.; Linder-Stragliotto, C.; Haglund, F. Evaluation of PD-L1 Expression in Undifferentiated Pleomorphic Sarcomas, Liposarcomas and Chondrosarcomas. Biomolecules 2022, 12, 292. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, M.; Sung, M.; Lee, S.E.; Kim, Y.J.; Choi, Y.L. Status of Programmed Death-Ligand 1 Expression in Sarcomas. J. Transl. Med. 2018, 16, 303. [Google Scholar] [CrossRef]
- Wang, F.; Yu, T.; Ma, C.; Yuan, H.; Zhang, H.; Zhang, Z. Prognostic Value of Programmed Cell Death 1 Ligand-1 in Patients with Bone and Soft Tissue Sarcomas: A Systemic and Comprehensive Meta-Analysis Based on 3680 Patients. Front. Oncol. 2020, 10, 749. [Google Scholar] [CrossRef]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Ceballos, E.; Machado, I.; Giner, F.; Blázquez-Bujeda, Á.; Espino, M.; Navarro, S.; Llombart-Bosch, A. Immunological Tumor Microenvironment of Solitary Fibrous Tumors—Associating Immune Infiltrate with Variables of Prognostic Significance. Cancers 2024, 16, 3222. https://doi.org/10.3390/cancers16183222
Medina-Ceballos E, Machado I, Giner F, Blázquez-Bujeda Á, Espino M, Navarro S, Llombart-Bosch A. Immunological Tumor Microenvironment of Solitary Fibrous Tumors—Associating Immune Infiltrate with Variables of Prognostic Significance. Cancers. 2024; 16(18):3222. https://doi.org/10.3390/cancers16183222
Chicago/Turabian StyleMedina-Ceballos, Emilio, Isidro Machado, Francisco Giner, Álvaro Blázquez-Bujeda, Mónica Espino, Samuel Navarro, and Antonio Llombart-Bosch. 2024. "Immunological Tumor Microenvironment of Solitary Fibrous Tumors—Associating Immune Infiltrate with Variables of Prognostic Significance" Cancers 16, no. 18: 3222. https://doi.org/10.3390/cancers16183222
APA StyleMedina-Ceballos, E., Machado, I., Giner, F., Blázquez-Bujeda, Á., Espino, M., Navarro, S., & Llombart-Bosch, A. (2024). Immunological Tumor Microenvironment of Solitary Fibrous Tumors—Associating Immune Infiltrate with Variables of Prognostic Significance. Cancers, 16(18), 3222. https://doi.org/10.3390/cancers16183222







