Simple Summary
Prostate cancer (PCa) is among the most common cancers. While PCa is frequently diagnosed in elderly men as a slow-growing, low-risk disease, in about 10–15% of cases, it can become a life-threatening danger. Unfortunately, biomarkers able to discriminate indolent prostatic tumors from aggressive forms are lacking, and watchful waiting remains one of the best options. However, available data support the hypothesis that distinctive biological characteristics of PCa stroma can contribute to cancer progression in a clinically relevant way. According to the current view, tissue alterations induced by tumor growth affect both metabolism of resident normal cells and composition of the extracellular matrix and are able also to recruit cells from circulation. Here, we seek to respond to the challenge of identifying stroma-associated biomarkers that may be relevant to assist prognostic decisions in PCa patients.
Abstract
Prostate cancer (PCa), the most commonly diagnosed cancer in men worldwide, is particularly challenging for oncologists when a precise prognosis needs to be established. Indeed, the entire clinical management in PCa has important drawbacks, generating an intense debate concerning the possibility to individuate molecular biomarkers able to avoid overtreatment in patients with pathological indolent cancers. To date, the paradigmatic change in the view of cancer pathogenesis prompts to look for prognostic biomarkers not only in cancer epithelial cells but also in the tumor microenvironment. PCa ecology has been defined with increasing details in the last few years, and a number of promising key markers associated with the reactive stroma are now available. Here, we provide an updated description of the most biologically significant and cited prognosis-oriented microenvironment biomarkers derived from the main reactive processes during PCa pathogenesis: tissue adaptations, inflammatory response and metabolic reprogramming. Proposed biomarkers include factors involved in stromal cell differentiation, cancer-normal cell crosstalk, angiogenesis, extracellular matrix remodeling and energy metabolism.
1. Searching New Biomarkers in Prostate Cancer Stroma
Intense efforts have been performed by scientists in the field of prostate cancer (PCa) to identify new biomarkers able to discriminate PCa from benign prostatic conditions (diagnostic biomarker), between indolent and aggressive tumors (prognostic biomarker) and between responsive and unresponsive patients (predictive biomarker). The current diagnostic methods for PCa detection, including digital rectal exam, serum prostate-specific antigen (PSA) and derivatives (PSA density, PSA velocity, % between total and free PSAs) and transrectal ultrasound image-guided biopsy, lack the necessary specificity/sensitivity or are limited by inter-observer variability [1]. Indeed, it is estimated that the current diagnostic methods may miss up to 30% of clinically significant PCa [2]. In addition, the identification of new biomarkers that support physicians in their decision-making activity could help in identifying those patients who are suitable for active surveillance, avoiding unnecessary or invasive treatment (e.g., radical prostatectomy (RP), hormone therapy, chemotherapy) and ensuring a more precise clinical management [3,4].
Initially, carcinogenesis and cancer progression were considered a consequence of pathological alterations affecting only cancer cells. This consideration was abandoned when it was ascertained that the tumor microenvironment (TME) provides indispensable fertile soil for cancer growth. A more recent and radical suggestion envisions that the TME not only supports cancer growth but exerts a clinically relevant selective pressure for cancer progression. The assumption that cancer development is accompanied by a profound alteration in neighbor tissues is a new angle in oncology timeline [5]. The hypothesis of an active role of the TME in cancer growth is supported in PCa by at least two types of evidence: (1) the contribution of reactive stroma in PCa carcinogenesis and progression recapitulates the physiologic support of stroma to epithelial cell growth during normal development (embryonic reactivation hypothesis), and (2) the stromal response during PCa growth is similar to the physiological mechanisms associated with regenerative response to an emerging noxa (non-healing wound hypothesis).
According to the first pieces of evidence, it is well known that prostate formation requires paracrine interactions between mesenchymal and epithelial components. Mesenchyme–epithelial interactions play a central role in prostate organogenesis, and some studies demonstrated key similarities in molecular mechanisms involved in prostate development and cancer growth [6,7]. Indeed, tissue recombinant assays clearly demonstrated that urogenital sinus mesenchyme is necessary and sufficient to form prostate tissue in the presence of several different endodermal and ectodermal tissues [8]. Key regulators of branching morphogenesis include FGF, hedgehog, Bmp, TGF-β, Wnt pathways as well as matrix remodeling enzymes, and, importantly, the same factors are recognized to play important roles in carcinogenesis [9].
Secondly, tumor growth activates those pathways that are normally associated with inflammation and tissue healing, involving modification in extracellular matrix, production of de novo growth factors and altered phenotype in stromal cell. Evidence of tumor-induced blood vessel permeability was the basis for the well-known theory expressed in 1986 by H.D. Dvorak that compares tumors to wounds that do not heal [10]. Several chronic features associated with tumor growth can mimic characteristics that are typical of non-healing wounds, including necrosis, apoptosis, hypoxia, extracellular matrix damage and release of cytokines/growth factors. These aspects contribute to activate the wound-healing program triggering functional and phenotypic changes in stromal cells adjacent to tumor mass. A frequently reported response program associated with both wound healing and reactive stroma includes the activation of autophagy. Multiple metabolic stressors, such as hypoxia, nutrient deprivation and degradation of the extracellular matrix (ECM), can activate autophagy both in stromal and tumor cells. Autophagy is considered an inherently cytoprotective mechanism, supplying energy and compounds for cell survival and metabolism. However, autophagy has a paradoxical role during different stages of tumor development and in different cell types, rendering poorly feasible a future application of a specific autophagic signature in clinic prognostic procedures [11].
The challenging questions in this landscape are whether the success of tumor growth is strictly dependent on specific modifications in the surrounding tissue and whether this transformation has a diagnostic or prognostic value. A first answer was proposed by J. Levin following his study about the transplantability of cancers in mice [12]. Interestingly, Levin used the term “reactive stroma” in order to describe modifications in the mouse tissue that received the tumor xenograft, and, currently, the same term is adopted to define the altered characteristics of the TME closely adjacent to tumor mass. Characteristics of reactive stroma include inflammation, hyperplasia, angiogenesis and fibrosis. In addition, reactive stroma has been proposed as an early promotion event in prostate tumorigenesis, and, indeed, mouse model studies in PCa were among the first to show the tumor-promoting role of stroma from tumor-bearing prostate. In fact, it has been demonstrated that the inoculation of non-tumorigenic or low-tumorigenic prostate epithelial cells with mesenchymal cells significantly improved the tumorigenicity in vivo [13]. Ayala et al. developed a semi-quantitative grading scale of the reactive stroma in PCa, in which reactive stroma grade (RSG) 0 was associated with the reactive stroma comprising between 0 and 5% of the tumor area, RSG 1 with 5–15%, RSG 2 with 15–50% and RSG 3 with >50%. The authors showed that a higher level of reactive stromal response is connected to biochemical recurrence (BCR) and increased risk of death due to PCa [14,15]. Several studies have linked high reactive stroma content to a worse clinical outcome, including BCR, development of castration-resistant prostate cancer (CRPC) and prostate cancer-specific mortality [16,17,18,19,20,21].
Although adaptation processes seen in prostate reactive stroma could be involved in many diseases, and not specific for tumors, they offer the plausible biological bases for investigating gene expression signatures also in normal cells present in the TME. Of note, in the presence of tumor cells, prostate stromal cells show a significantly different gene signature with respect to normal tissue [22]. More than three thousand gene expression changes were observed between normal volunteer-derived prostate biopsy samples and stroma from near tumors. Among the most common and relevant differentially expressed genes, numerous were associated with expression in nerve and muscle. In addition, the evaluation of biochemical associations revealed upregulation in specific pathways, including TGF-β axis and Wnt pathways. The diagnostic application of the stroma-specific signature was also able to predict the tumor status of patients [23]. Another expression signature study found significant upregulation in specific processes, such as neurogenesis and axonogenesis, beside alterations that could be associated with wound-healing pathways, including DNA damage/repair and stemness. Different gene expression signatures were identified in stroma adjacent to high-grade and low-grade cancer, confirming the role of the microenvironment in guiding tumor progression. In addition, the prostate stroma gene expression profiling demonstrated a tumor-specific adaptation able to drive tissue-specific metastatic progression. In this context, bone remodeling pathways were upregulated in xenograft models and in high compared to low-Gleason grade cases, suggesting the importance of selective pressure by PCa stroma in conditioning tumor cell osteotropism [22,24]. Accordingly, profiling of stromal gene expression led to development of a stroma-derived metastasis signature that was validated as independently prognostic for the metastatic potential of PCa. A total of 124 differentially expressed stromal genes were identified in PCa patient-derived xenograft models and include genes involved in the modulation of cell–cell and cell–matrix interactions (such as galectin1 and 3, osteopontin and Adam12) [25]. Collectively, data accumulated from gene expression analyses suggest a profound modification of tumor stroma with respect to normal stroma, which supports the importance of cancer–stromal interactions during the process of PCa growth and metastasis. In the TME, mesenchymal cells play pivotal roles in PCa progression and metastasis [26,27,28]. Recently several studies demonstrated a complex spatial and functional heterogeneity of the TME-associated mesenchymal cells during PCa progression by using scRNA sequencing [29,30,31]. Stromal cell-mediated autocrine and paracrine mechanisms influence the structure and function of the TME during disease progression [32,33]. Congruently, it is recognized that stromal AR (androgen receptor) signaling affects the phenotype of prostate epithelial cells at different stages of PCa progression [26,34,35]. During PCa progression, stromal AR signaling avoids progression toward a more aggressive status (e.g., neuroendocrine status). Furthermore, Pakula et al. demonstrated that low AR expression is correlated with high expression of periostin, reduced overall survival, higher Gleason score and neuroendocrine status in PRN mouse model [36,37,38,39].
In the following chapters, we describe PCa stroma modifications utilizing three main categories: 1—tissue adaptation, including alterations in the ECM and in cells that regulate its homeostasis; 2—inflammatory response and immune cells activation; and 3—metabolic reprogramming, including alteration in energy metabolism ensuing from the synergy between normal and cancer cells (Figure 1). These processes deeply contribute to PCa initiation, progression and response to treatment, and the identification of process-specific biomarkers could potentially contribute to discover a new generation of precision biomarkers.
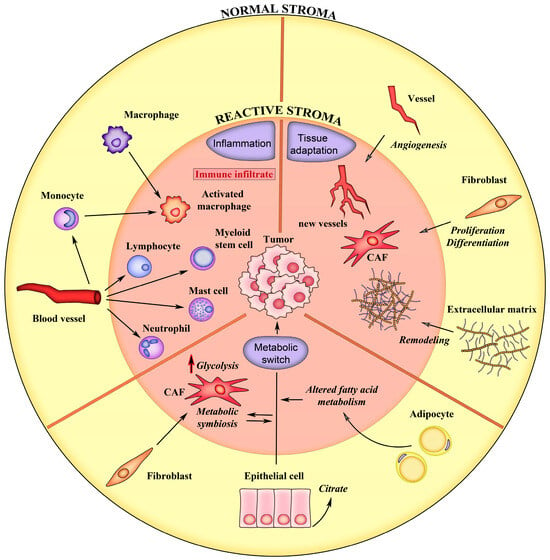
Figure 1.
Schematic representation of the main events associated with the formation of reactive stroma in prostate cancer. The normal tissue characteristics are depicted on yellow background. The processes of the reactive stroma are depicted on pink background. See text for details.
2. Tissue Adaptations
With the denomination of “tissue adaptation”, we collectively refer to phenotypic changes in stromal resident cells in consequence of adjacent tumor growth. The non-epithelial tissue of the prostate comprises primarily smooth muscle cells and less-abundant populations of fibroblasts, vascular cells, nerve cells and non-resident infiltrating immune cells. As previously described, during carcinogenesis and tumor progression, prostate stroma could undergo tissue adaptations typical of chronic wounds. These adaptations include formation of new blood vessels, differentiation of fibroblasts and ECM remodeling [40,41].
2.1. Angiogenesis
Blood capillary architecture, and in particular microvessel density (MVD), could offer important hints in the diagnostic procedure. MVD count does not vary significantly in physiological condition; on the contrary, angiogenesis is an early requirement for the maintenance of tumor growth. The increase in MVD offers important physiological advantages to tumor growth, including the necessary oxygen and nutrient supplies. However, endothelial cells could play a direct role in PCa progression, and, in particular, the tissue expression of biglycan, a factor specifically released by vessels, has demonstrated a significant relationship with progression to CRPC [42]. The level of new vessels in tumor and peri-tumor tissue was frequently analyzed by the evaluation of vessel density after staining of specific markers for endothelial cells. The immunohistochemical staining for vWF, CD31, CD34 and CD105 to measure MVD was suggested to be a reproducible method for characterizing the individual tumor [43,44,45,46]. Indeed, MVD is significantly higher in cancer tissue than in the adjacent benign and hyperplastic tissue and it increases toward the center of the tumor [47]. Over the past ten years, research has posited that MVD serves as a useful prognostic indicator in the context of PCa. In addition, it also provides insights into the extent of angiogenic activity within PCa tumors. Employing MVD scoring is recognized as a valuable, straightforward and practical histological technique for the routine evaluation of PCa. Furthermore, MVD has been identified as a reliable forecaster of BCR in PCa patients. In particular, cases of PCa in the earlier T stages with high MVD show a significant correlation with BCR [48].
High-grade prostatic intraepithelial neoplasia (HGPIN) and atypical small acinar proliferation (ASAP) are the most likely precursors of PCa, as demonstrated by the high percentage of new adenocarcinoma diagnoses following repeat biopsies in presence of these conditions [49]. In addition, Proliferative Inflammatory Atrophy (PIA) has been hypothesized to be a precursor lesion of PIN because it is preferentially observed in the peripheral zone of the prostate where PCa arises more frequently, and, furthermore, it shows a similar chromosomic instability to PIN and PCa [50]. PIN is a precancerous lesion in which some luminal cells of the prostate epithelium start to look and behave abnormally. They exhibit enlarged nuclei and nucleoli and increased abnormal proliferation [49,51]. Although PIN itself is usually asymptomatic, it is considered a precursor of PCa. It is often discovered in biopsies taken when PCa is suspected, and it harbors many of the genetic alterations classically present in PCa [52]. Once HGPIN is detected in a prostatic biopsy, the risk of finding PCa in a subsequent biopsy is 15 times greater than in biopsies without PIN [53,54]. The PIN lesion areas have been frequently reported to express an MVD similar to BPH and lower than carcinoma [55,56]. However, also in PIN, the stroma immediately adjacent to the epithelial basement membranes may contain a richer network of capillaries, in agreement with the evidence of strong immunoexpression of VEGF in HGPIN [55]. In addition, as demonstrated by Montironi et al., a deeper analysis of blood capillary permits to individuate early modification in MVD with capillaries that appeared located in the proximity of the basement membrane of ducts and acini [57]. During the progression from HGPIN to adenocarcinoma, an increase in shorter capillaries with open lumen and greater number of endothelial cells were also observed. This suggests that, beside MVD, the morphology of capillaries could represent an early marker of cancer progression. However, quantitative immunohistochemistry of PIN lesions demonstrated that they acquire a significant increase in MVD with respect to benign lesions only in the presence of a relatively widespread fragmentation of the basement membrane [58]. The constant transformation of vascular morphology toward aberrant network is certainly a confounding variable and a likely underlying reason for a lack of a statistically significant association between MVD and PCa stage, condition reported in some studies. This difficulty is augmented by the difference in pathological significance and prognostic roles of different markers of endothelial cells (Table 1) [59].
The activation of angiogenesis in tumor tissues can also be evaluated by analyzing the expression of vascular growth factors and their receptors. VEGFs are angiogenic growth factors that play an important role in PCa progression [60]. They are secreted by cancer cells, macrophages, fibroblasts and mast cells [61,62,63]. VEGFs produced by myofibroblasts promote the accumulation of macrophages at site of fibrosis, that in turn induces angiogenesis and invasion. The expression of VEGFs in PCa is associated with advanced clinical stage, Gleason score, pathologic tumor stage, progression, metastasis and poor survival [64,65,66]. The inhibition of VEGF-A blocked angiogenesis and tumor growth, supporting the role of VEGF-A in PCa progression and its therapeutic use for treating patients with advanced PCa [67,68,69]. Among all the VEGFs, the most predominant growth factor, overexpressed in PCa, is VEGF-A [64,70]. High expressions of VEGF-A and VEGFR-2 in the stroma were independently associated with higher incidence of biochemical failure [46]. Additionally, these two factors have been identified as markers indicative of PCa cases with elevated risk of cancer progression [71].
On the contrary, VEGF-D and VEGFR-3 expressions were significantly reduced in tumor stroma compared to benign tissue, suggesting a prognostic role for lymphangiogenesis [72]. However, differential expression of VEGF ligands and receptors has been associated only sporadically with metastatic spread.
Overall, it is widely accepted that histological biomarkers, able to predict angiogenesis, could be useful prognostic indicators [73,74].
2.2. Cancer-Associated Fibroblasts
In the prostatic stroma, fibroblasts are responsible for maintaining the integrity of the epithelial cells by secreting precursors (i.e., collagen type I and III, fibronectin) of the ECM and paracrine factors [75]. Several studies demonstrated that cancer-associated fibroblasts (CAFs) play an important role during prostatic neoplastic transformation creating the reactive stroma through the crosstalk with PCa cells [76,77,78]. CAFs are a heterogeneous cell population that controls the homeostasis of prostate ECM, and their contribution to PCa initiation and progression was demonstrated in several studies. Establishment of a reactive stroma is already observable in PIN, including through differentiation of normal fibroblasts and myofibroblasts surrounding the lesions [79]. The functions of CAFs also evolve along with cancer progression, and, for example, as demonstrated in an orthotopic spheroid culture xenograft model, patient-derived CAFs stimulated metastatic spreading [80]. Prostate fibroblasts’ transition toward CAFs generates a stable phenotype that is considered the result of epigenetic alterations, particularly enriched at regulatory regions of the genome [81]. A large set of differentially methylated regions are shared across CAFs from PCa at different stages, and a specific methylation signature can be utilized to discriminate aggressive PCa from moderate-risk patients. Interestingly, EDARADD gene that showed the greatest difference in methylation in high-Gleason grade cohort was associated with defective paracrine interactions between stroma and epithelium during aberrant development of ectodermal tissues [82].
The heterogeneity renders difficult to precisely characterize CAF phenotype; thus, they are defined by a combination of morphology, tissue position, lack of lineage markers that are expressed in other cell types and fibroblast subtype markers. However, the majority of the studies revealed that the prevalent phenotypic transition in cancer-associated stroma is the presence of myofibroblasts, characterized by expression of smooth muscle α-actin (α-SMA). CAFs make up approximately 50% of reactive stroma in Gleason 3 focus and are progressively replaced by myofibroblasts in Gleason 4 to 5 foci [79]. Also, vimentin and platelet-derived growth factor receptor-α (PDGFR-α) expressions were suggested as predictive markers of myofibroblastic transition associated with PCa progression [83]. At the same time, a decrease in calponin in the TME could be indicative of a reduction in the presence of smooth muscle cells [79].
Significant examples of early reactive stroma derived from transgenic models for spontaneous PCa are present in the literature [84]. In fact, also considering the histologic differences resulting in a thinner fibromuscular stroma in mouse prostate with respect to human prostate in the TRAMP model, it is evident that a stromal response adjacent to moderately well-differentiated acini is detectable [84]. In mouse stroma, beside smooth muscle cells (CD34+/SMA+), another three types of fibroblasts were found: subepithelial cells, wrapping cells associated with smooth muscle cells and interstitial fibroblasts [85]. In probasin-large T antigen transgenic (LPB-Tag) mice, a model recapitulating tumorigenesis, reactive stromal proliferation was induced and continued to develop throughout the progression to high-grade dysplasia, carcinoma in situ and adenocarcinoma [86]. Immunohistochemical analyses in LPB-Tag indicated that most stromal cells stained positively for α-SMA, suggesting that hyperplastic stroma is determined by mesenchymal cells that had differentiated into smooth muscle cells. However, beside procedures based upon few reliable antibodies, to date, the accurate recording of CAF numbers and subtypes within clinical samples is challenging, and future diagnostic procedures involving CAF phenotyping will potentially benefit from standardization of multiplex technology [87].
The presence of TGF-β in vitro was sufficient to induce a stromal reaction characterized by differentiation of fibroblasts to myofibroblasts [88]. TGF-β mediates the transition of fibroblasts to CAFs and promotes angiogenesis and metastasis [89]. Phenotypic transformation associated with a new secretome was demonstrated in mouse prostate reconstitution (MPR) models where desmoplastic promotion activity was associated with the capacity of C57BL/6 mesenchyme to produce elevated TGF-β1 levels [90]. In addition, expression of TGF-β by PCa cells was fundamental in modulating the stromal microenvironment and accelerating the progression of the tumor [91]. TGF-β acts as promoter in early stages of a spontaneous prostate Pten KO tumorigenesis mouse model [92]. Indeed, genesis of the reactive stroma phenotype occurs also in precancerous PIN lesions and may be associated with elevated TGF-β expression by PIN epithelial cells [79]. Interestingly, a loss of TGF-β receptor type II was observed in epithelial but not in stromal cells during PCa development, and this could explain the direct antagonistic role of TGF-β against cancer cells during progression [93,94]. Although many studies reported decreased levels of TGF-β in PCa [95], others explained that PCa patients with higher Gleason scores showed elevated expressions of TGF-β, indicating that this factor could be a prognostic biomarker of PCa progression. In particular, CXCL13 production induced in myofibroblasts by TGF-β, in castration-resistant mouse models, was fundamental in the emergence of more aggressive cancer types [96]. Due to the complexity of TGF-β signaling in prostate TME, further studies are needed to better understand the role of TGF-β in prostate carcinogenesis and its potential use as a diagnostic target.
Beside upregulation of TGF-β expression, tumor-associated reactive stroma involve other signaling pathways normally associated with wound repair, including FGF and Wnt. PCa stroma secrete Wnt proteins that activate Wnt signaling in tumor cells and promote therapy resistance and disease progression. Wnt/β-catenin signaling is an essential mechanism underlying myofibroblast differentiation [97]. Wnt3a promotes the formation of a myofibroblast-like phenotype in cultured fibroblasts, in part, by upregulating TGF-β signaling, whereas studies in mice show that stromal Wnt3a activates canonical Wnt signaling in the epithelium, facilitating progression of PIN lesions to adenocarcinoma [98,99]. Moreover, Wnt is involved in neuroendocrine differentiation and CRPC cells as a consequence of the release of SFRP1 by endoglin-positive CAFs [96,100]. Increased mRNA expression of several secreted members of Wnt family, including Wnt2, Wnt4 and Wnt9a, have been associated with the development of multi-focal hormone-sensitive PIN lesions in an epigenetic modulated mouse model. In the same mouse model, overexpression of Wnt antagonists in the stroma strongly suppressed the development of precancerous lesions [101]. The knockdown of Wnt10B in prostate stromal cell lines resulted in decreased tumorigenesis in a xenograft model containing LNCaP cells. In addition, in stromal cells, the knockdown determined in stromal cell downmodulation of multiple cytokines such as VEGF-A, Bdnf and CSF2 [102].
Autochthonous transgenic mouse models demonstrated that Wnt signaling alone in the stroma is associated with a reactive stroma phenotype in synergy with FGFR1-induced proliferation and pre-malignant transformation [103]. FGF family members and FGF receptor variants represent key factors in the two-way stromal–epithelial cell dialogue in premalignant lesions. The FGF family contains 18 secreted members that are grouped into six subfamilies based on sequence homology. Different stromal subtypes express specific FGF patterns, and this correlates with potential heterogeneity in the stromal response and tumorigenesis. Prostate stroma secretes FGF-2, FGF7 and FGF10, and they exert a potent mitogenic activity on epithelial cells [104]. Stromal FGF-2 expression is detectable in a large proportion of advanced PCa where it is associated with adverse clinicopathological features and chromosomal instability in tumor cells [105]. Previously, it was demonstrated that stromal FGF-2 expression and release were critical in a xenograft model in sustaining TGF-β-induced PCa growth and MVD [106]. Importantly, FGF7 and FGF10 are expressed in stromal cells but absent in epithelial cells. They are involved in prostate organogenesis and bind the receptor FGFR2IIIb exclusively expressed by epithelial cells, where it has a suppressive role on proliferation [107]. Fibroblasts mainly expressed FGF10 and FGFR1, whereas FGFR3 was detected only in α-actin-positive stromal cells [108]. FGF1, that is expressed at only trace levels in normal adult prostates, was also upregulated in the stroma of malignant tumors that evolve from premalignant, but this evidence was observed only in a murine model of PCa [109]. Recently, Ruder and collaborators, using digital image analysis and a machine learning approach, developed a biomarker of the prostate stroma called quantitative reactive stroma (qRS) [110]. qRS is a measure of percentage of tumor area with a distinct reactive stromal architecture. This algorithm was coupled with Kaplan–Meier analysis to determine survival in a large retrospective cohort of radical prostatectomy samples, supporting the idea that qRS could be a potential biomarker able to add information on the standard predictive models.
2.3. Extracellular Matrix
ECM is a fundamental determinant of tissue homeostasis both in health and disease. Prostate ECM is composed of a mixture of collagens, glycoproteins laminins, fibronectins, tenascins and hyaluronan. ECM remodeling is vital for wound healing allowing the migration of cells and modulating cell phenotype. These mechanisms are possible through the ability of cells to bind ECM proteins, activating specific signal transduction pathways. Frequently, changes in the composition and architecture of ECM associated with different pathophysiologic events are determinant in guiding the adaptation of the tissue [111]. It is well known that tumors benefit by an inherently altered ECM with respect to normal tissue, and aberrant ECM is considered an important risk factor for tumor initiation and metastasis [112]. Significant ECM alteration was frequently reported in PCa from early stages to metastatic disease [113]. Dynamic changes in the ECM due to CAF activity could be mainly referred to as “desmoplasia”. In fact, ECM remodeling in PCa progression is associated with an increased collagen deposition and stiffer matrices, characteristics that have been associated with more aggressive cancer cell phenotypes and resistance to therapy [114]. Tumor tissue stiffening was proposed as an early event in PCa tumorigenesis, and increased type I collagen synthesis was observed in activated periacinar fibroblasts adjacent to PIN [79]. Interestingly, PCa cells implanted orthotopically demonstrated accelerated tumor growth in aged compared with young mice. Indeed, the immunohistochemistry analysis of tumors from aged mice revealed a substantial increase in collagen fibers compared with tumors in young mice [115]. A possible explanation for an increased fibrotic response to cancer cells in aged mice is a reduced ability by stromal cells to maintain the structure and composition of ECM [116]. Ultrasound shear wave elastography confirmed collagen remodeling around localized PCa and showed characteristic changes according to Gleason grade. In particular, when comparing the collagen orientation, it was evident that collagen fibers became more oriented as the PCa became more aggressive [117]. In murine models, it has been shown that radial alignment of collagen fibers relative to tumor mass facilitates invasion and thus cancer progression [118].
Beside collagen deposition and remodeling, CAFs produce other potentially prognostic extracellular matrix glycoproteins, including fibronectin and tenascin-C (TNC). Fibronectin polymerization is a critical regulator of ECM organization and stability, and this implicates a potential role for fibronectin in mediating PCa progression. However, available data are contradictory; in fact, whereas fibronectin-rich ECM guides cancer cells to migrate directionally, in advanced PCa, the expression pattern of fibronectin was sporadic or reduced [119]. High expression of TNC has been associated more consistently with poor prognosis in a variety of malignant tumors [120]. TNC was among the most abundant stromal genes upregulated in patient-derived PCa xenografts and highly expressed in castration-resistant models [24]. In vivo studies demonstrated that PCa cells induced stromal reprogramming associated with TNC secretion and that TNC knockdown decreased metastasis to bone [121]. The evaluation of TNC expression via immunohistochemistry in a large cohort of PCa tissue samples confirmed its clinicopathological relevance and its correlation with poor prognosis in PCa patients [122].
Asporin (ASPN) is an extracellular secreted protein initially identified in human cartilage. Asporin was also detected in PCa stroma, but not in benign tissue, and is positively associated with reactive stroma and disease progression [123]. Interestingly, polymorphisms in the D-repeat-sequence variants (ASPN-D) were reported to favor PCa progression and metastatic recurrence, data that were confirmed also in orthotopic xenografts [124].
Periostin is an extracellular matrix glycoprotein frequently detected in several tissues during tissue repair and regeneration. Whereas periostin expression was weak or absent in normal prostates, stronger staining was found in peritumoral stroma. A positive correlation between total periostin staining and Gleason score was observed in different studies [125]. In addition, high stromal periostin was significantly associated with shortened PSA relapse free survival times. Interestingly, circulating periostin was also proposed as a useful marker in improving predictive values of clinicopathological variables [126].
Stromal cells significantly contribute to remodeling TME also by releasing extracellular matrix proteases, such as metalloproteases (MMPs). MMPs are a multigene family of zinc-dependent endopeptidases that share similar structures and the ability to virtually degrade all components of the ECM, playing a central role in morphogenesis, wound healing, tissue repair and remodeling to deal with injuries [127]. Some studies have also shown that MMPs are correlated with Gleason score, disease-free survival, tumor recurrence and other factors related to PCa [128,129,130]. MMP2 and MMP9 stand out because their biomarker potential has been frequently studied and documented in the literature [131].
Indeed, MMP2 is expressed by stromal cells in more than 70% of PCa tissues analyzed [132], and Murray et al. suggested that its expression in bone marrow micrometastasis is associated with worse prognosis in PCa patients after radical prostatectomy. While similar findings sustain a potential role of MMP9 as biomarkers in PCa, current data are still ambiguous and do not support the use of metalloproteases as PCa biomarkers [132,133,134,135].
Tissue inhibitors of matrix metalloproteinases (TIMPs) control MMP activity [136]. The unbalance between TIMPs and MMPs was observed during PCa progression and metastasis [136,137]. In addition, the treatment and prognosis of PCa are strictly dependent from the balance between these two molecules. A recent study demonstrated that TIMP-1 can be used as a biomarker to decide the best treatment for patients with metastatic prostate cancer [138]. These findings suggest that MMPs and TIMPs could be used as potential prognostic biomarkers for patients with PCa, but further investigations are needed [136,139].
Recently, Pu and collaborators demonstrated that elevated monoamine oxidase B (MAOB) in stromal fibroblasts contributes to PCa progression by promoting a reactive stroma, through enhanced ROS production and increased levels of stromal markers (i.e., TGF-B1, VIM and FAP), and favoring the interaction between stromal and epithelial cells via CXCL12/CXCR4 signaling activation [140]. In fact, pharmacological inhibition of stromal MAOB reduces tumor growth in vivo. Additionally, high expression of MAOB in tumor stroma is associated with poor clinical outcomes in patients with PCa. Given the important role of stromal-derived MOAB in PCa tumor progression and invasion, it could be used as a new biomarker to improve the prognosis of PCa patients. The inhibition of MAOB could be a potential new strategy to counteract PCa tumor progression and ameliorate the clinical outcome of PCa patients [140].
3. Prostate Cancer Metabolic Reprogramming: The Contribution of TME
Prostate gland shows a unique metabolism based on the elevated production and secretion of citrate, a major component of the prostatic fluid and a key factor for sperm viability [141]. To support citrate production, healthy prostate cells have a truncated tricarboxylic acid (TCA) cycle and do not oxidase this metabolite that instead represents the final product of the Krebs cycle. To this end, high levels of zinc are shuttled into the mitochondria from the extracellular space via SLC39 transporter, whose expression is regulated by androgen receptor (AR) signaling. Accumulated zinc inhibits (m)-aconitase (ACO-2), the enzyme responsible for citrate oxidation. Therefore, prostatic epithelial cells are characterized by reduced levels of oxidative phosphorylation (OXPHOS), with a 65% reduction in the production of ATP via TCA compared to citrate-oxidizing cells [141].
During oncogenic transformation, PCa cells undergo profound changes in their metabolism. Primary PCa shows enhanced OXPHOS [142,143,144,145,146]. In addition, a lipogenic phenotype, characterized by increased de novo lipogenesis (DNL), has been observed at early stages of PCa and further enhanced during cancer progression [142,143,144,145,146,147,148,149]. Differently from the majority of solid cancers, primary PCa is less glycolytic, as confirmed by the low uptake of 18-fluorodeoxyglucose (FDG) [150]. On the contrary, glycolytic metabolism becomes fundamental in advanced CRPC, along with amino acid metabolism, mainly in the form of glutamine anaplerosis into the TCA cycle [151,152,153]. Glutamine is taken up by PCa cells and activates mTOR signaling, favoring their neuroendocrine differentiation [154].
AR signaling has been recognized as the major molecular driver of PCa progression and many pieces of evidence showed how this hormone-sensitive transcription factor is crucial to rewire PCa metabolism toward OXPHOS and DNL during oncogenic transformation by regulating several downstream effectors, including mitochondrial pyruvate carrier (MPC), estrogen-related receptor ERRγ, μTOR and fatty acid synthase (FASN) [145,146,148,155,156]. The reactivated AR signaling is also crucial in CRPC setting, where elevated AR-dependent lipogenic gene signatures (FASN, long-chain acyl-CoA Synthetase 3 (ACSL3), Membrane-bound O-acyltransferase domain containing protein 2 (MBOAT2) and fatty acid elongases 5 and 7 (ELOVL5/7)) and increased expressions of the glucose transporter 1 (GLUT1) are associated with poor patient outcome [151,152,157,158]. However, the metabolic rewiring of PCa cells is not exclusively dependent on cell-autonomous mechanisms but is also strongly influenced by surrounding TME.
High reactive stroma content in PCa tissues, characterized by high amounts of CAFs and proinflammatory immune cells, was associated with a specific metabolic profile linked to increased inflammation and ECM remodeling, including low levels of citrate and spermine and high levels of leucine (Table 1) [21]. It is well recognized that CAFs establish a metabolic symbiotic cooperation with PCa cells through lactate shuttle that fuels tumor progression [159]. Human prostate fibroblasts undergo Warburg effect upon exposure to PCa-conditioned media, thus becoming PCa-activated fibroblasts, characterized by increased expression of glucose transporter 1 (GLUT1), elevated glucose uptake and extrusion of lactate. In turn, the CAF-secreted lactate is uploaded by PCa cells to fuel both OXPHOS and anabolic pathways supporting cell proliferation and tumor growth in glucose-free milieu. This metabolic coupling between CAFs and PCa cells involves the overexpression of (i) lactate monocarboxylate transporter 4 (MCT4) in CAFs, responsible for lactate extrusion in the TME and (ii) MCT1 in PCa cells, which is the main transporter for lactate uptake [159]. Notably, both MCT4 and MCT1 levels have a prognostic relevance in PCa. Higher MCT4 expression in the stroma of human PCa has been associated with advanced tumor stages and poor clinical outcomes [160,161]. Likewise, MCT1 expression and lactate uptake have been observed in p53-null PCa, with elevated levels of MCT1 being found in patients with worse prognoses [162]. Moreover, when the expression of MCT1 and MCT4 in PCa cells and surrounding stroma was explored in a large cohort of patients, the elevated co-expression of MCT1 in tumor and MCT4 in stroma was found to be independently associated with a worse biochemical failure-free survival, and the strength of this association was as strong as having a Gleason score [163].
Another key modulator of the lactate-dependent metabolism displayed by PCa cells upon CAF exposure in highly infiltrated cancers is the pyruvate kinase PKM2 isoform, the rate-limiting enzyme of the last step of glycolysis. In vitro studies showed that the metabolic activity of PKM2 in PCa cells is inhibited by CAF conditioning through the induction of PKM2 oxidation and phosphorylation. In this way, PCa cells are forced to shut down glycolysis and switch to CAF-derived lactate-driven OXPHOS. In addition, this metabolically inactive form of PMK2 is able to translocate to the nucleus, where, in association with hypoxia-inducible factor (HIF-1) and the transcriptional repressor Differentially Expressed in Condrocytes 1 (DEC1), it drives the epithelial–mesenchymal transition (EMT) program by repressing the expression of miR-205 [164]. Accordingly, targeting PKM2 nuclear translocation by DASA-58 destroyed the CAF–PCa cell metabolic coupling by restoring glucose dependency and impaired metastatic spread. The clinical relevance of this CAF-dependent PMK2/OXPHOS/EMT axis was confirmed by finding nuclear localization of PMK2 in tumor specimens with higher Gleason scores (8–10) and cancer aggressiveness.
Recently, Ippolito et al. identified the axis sirtuin 1 (SIRT1)/peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1α) as a crucial player in the CAF-dependent mitochondrial re-education of PCa cells and a marker of PCa invasiveness [165]. Upon CAF-derived lactate uptake, PCa cells experience an unbalanced NAD+/NADH ratio, which in turn caused the SIRT1-dependent activation of PGC-1α, a key regulator of mitochondrial biogenesis and OXPHOS. Accordingly, mitochondrial activity and mitochondrial mass were both increased in CAF-exposed PCa cells. Moreover, CAF-reprogrammed PCa cells showed an increased oxygen consumption rate (OCR). This elevated exploitation of mitochondria also resulted in (i) TCA deregulation, (ii) accumulation of oncometabolites like succinate and fumarat and (iii) increased production of mitochondrial reactive oxygen species, all events that drove CAF-induced EMT signature in PCa cells. Beside this lactate-dependent mitochondria reshape, highly glycolytic CAFs were also proven to donate their dispensable mitochondria to PCa cells through the formation of cellular bridges, thus further enhancing OXPHOS addiction of cancer cells and ultimately their malignancy [165].
A glutamine-mediated paracrine reprogramming of PCa cells has been also reported by Mishra et al. [154]. By performing a DNA methylome analysis, the authors found the epigenetic repression of the Ras inhibitor RASAL3 in prostatic CAFs and showed how this epigenetic alteration initiates a cascade of stromal–epithelial interactions. Indeed, RASAL3 epigenetic silencing promoted the Ras-dependent macropinocytosis for the uptake of albumin, which was in turn degraded to generate glutamine. Stromal glutamine was then shuttled into PCa cells via the increased expression of the glutamine transporter SLC1A5 where it was converted to glutamate by glutaminase (GLS) to replenish the TCA cycle and support the energetic need of proliferating cancer cells. Moreover, CAF-derived glutamine was necessary and sufficient to promote PCa neuroendocrine differentiation via activation of the nutrient sensor mTOR. Accordingly, genetic or pharmacologic blockade of either SLC1A5 or GLS impaired ATP production, cell proliferation and neuroendocrine differentiation observed in CAF-activated PCa cells. The authors found that epigenetic modifications in prostatic CAFs induced stromal–epithelial interactions that, in turn, promoted PCa progression and resistance to androgen-deprivation therapy (ADT). Notably, ADT further promoted RASAL3 epigenetic silencing and enhanced glutamine secretion by CAFs. Accordingly, elevated glutamine blood levels were found in the cohort of patients showing resistance to ADT compared to the ADT responder group, suggesting that glutamine plasma levels can be used as prognostic biomarkers for monitoring ADT response and development of resistance [154].
Reduced expression of sequestrosome 1/p62 in the stromal component of PCa is also associated with progression to the most aggressive stages [166]. Indeed, while p62 normally acts as a suppressor of inflammation, its loss promotes the instauration of the CAF proinflammatory phenotype, associated with increased expressions of IL-6 and TGF-β, which in turn drives tumor progression. Valencia et al. further showed that loss of p62 was associated with lower levels of reduced glutathione (GSH) and the consequent accumulation of ROS, which in turn were required for IL-6 overexpression. Accordingly, p62-depleted CAFs showed decreased NADPH/NADP ratio, as consequence of a lower glycolytic rate that fails in maintaining NADPH via pentose phosphate pathway. p62 loss was also associated with reduced glutamine consumption and the downregulation of glutamine and cysteine transporters, including SLC7A11, responsible for cysteine uptake, a rate-limiting step in glutathione synthesis. This metabolic/inflammatory reprogramming in p62-lacking stromal cells was due to the inhibition mTORC1/c-Myc axis, which is instead activated by p62 in normal fibroblasts to suppress the establishment of the proinflammatory phenotype [166].
Emerging evidence suggested that white adipose tissue-derived cells contributed to CAF population in PCa, as obesity in PCa patients is associated with cancer progression and increased content of CAFs, and the intake of saturated fatty acids has been recently shown to promote PCa progression [167]. Zhang et al. reported that palmitate induces PCa lineage plasticity via paracrine secretion of the Wnt ligand 5 (Wnt5) from CAFs [168]. In this study, the authors showed how in CAFs the palmitate-induced secretion of Wnt5, previously associated with PCa aggressiveness, induction of PCa bone metastasis and poor prognosis [169], was able to activate Sonic hedgehog (HH) signaling in PCa cells. In turn, palmitate/Wnt5 paracrinal-induced HH pathway promoted in prostate epithelial cells the expression of SOX2, well known to be a regulator of cell trans-differentiation to AR-independent basal-like phenotype, which portends to poor prognosis in PCa patients [100,170,171]. Accordingly, inhibition of fatty acid intake by using anti-CD36 antibodies resulted in the reduction in Wnt5 and SOX2 expressions by CAFs and PCa cells, respectively, decreased proliferation and elevated apoptosis in mice fed with high-fat diet and treated with enzalutamide, suggesting a role for saturated fatty acid signaling in mediating cancer progression and therapy resistance [172].
Based on these findings, we can envisage that the deeper understanding of the metabolic interactions between PCa and TME that fuel PCa progression may be crucial in the future to realize the full diagnostic, prognostic and therapeutic potentials embodied in this crosstalk.
4. Inflammation
The link between inflammation and cancer is well characterized, and it is widely accepted that most malignant tumors are associated with chronic inflammation [173]. Inflammation is considered an early event associated with carcinogenesis. PIA is closely associated with chronic inflammation and describes a frequently observed lesion in prostate biopsies characterized by atrophic glandular structures and chronic inflammatory cell infiltrate, including mast cells and macrophages. The affected epithelial luminal cells exhibit enlarged nuclei, increased proliferation and a reduced apoptotic rate [174].
Further, it has been suggested that tumors can be divided into different prognostic subtypes based on their immune phenotype, i.e., ‘immune inflamed’, ‘immune excluded’ and ‘immune desert’ [175]. Importantly, these immune phenotypes are characterized by different immune cell infiltration patterns in stroma (peritumoral) and epithelium (intratumoral), indicating that the spatial location of infiltrating immune cells is important for tumor biology and patient outcome. While earlier studies of PCa highlighted infiltrating mast cells, T lymphocytes, natural killer cells and macrophages as possible predictors of outcome, conflicting reports exist [176,177,178,179,180,181].
TME frequently comprises immune cells such as macrophages, natural killer (NK) cells, mast cells and lymphocytes that secrete multiple biomarkers (i.e., cytokines, chemokines and growth factors) able to fuel carcinogenesis [182,183]. In the TME, macrophages represent the principal type of immune cells and are involved in tumor promotion and progression [184]. In addition, the expression of tumor-associated macrophages (TAMs) is also associated with resistance to therapy [185,186]. Macrophages are highly plastic cells that can elicit pro- or anti-tumorigenic effects on cancer cells depending on contextual cues from their environment.
When recruited into the primary cancer site, macrophages can be polarized into M1 or M2 TAMs in response to cytokine concentrations in the TME. Classically activated macrophages (M1) exert anti-tumoral activities by secreting IL-1β, IL-12 and TNF-α, while alternatively activated macrophages (M2) fuel tumor growth through immune suppression and promotion of angiogenesis and metastasis [187]. Given the presence of M2 TAMs in the prostate tumor milieu, and their correlation with poor clinical outcomes, they could be used as potential prognostic predictors of disease. It is well known that higher presence of M2-like macrophages is correlated with poor clinical outcomes in several malignant diseases including PCa [187,188]. Recently, several genes (i.e., ACSL1, DLGAP5, KIF23 and NCAPG) highly expressed in tumor tissue and correlated with M2 TAMs have been identified as prognostic biomarkers in PCa due to their correlation with poor overall survival [189]. However, additional studies are needed in order to elucidate the role of these genes as potential biomarkers in PCa.
CAFs cooperate to regulate immune response not only with tumor cells but also with immune cells within the TME by secreting several cytokines, chemokines and growth factors [190]. CAF proliferation has been shown to lead to the development of a fibrous stroma, which induces localized vasculature remodeling and a state of hypoxia and chronic inflammation [96].
Several studies demonstrated that crosstalk between CAFs and TAMs during carcinogenesis and tumor progression leads to the generation of a pro-tumoral microenvironment [191]. CAFs produce several factors (i.e., IL-6, CXCL8, TGF monocyte chemotactic protein-1 (MCP-1) and stromal-derived growth factor-1 (SDF-1)) that regulate the macrophage polarization toward the M2-like phenotype and, consequently, their functions [192,193,194,195]. In turn, M2 TAMs influence phenotypic transition of fibroblasts, leading to their transformation into CAFs [196]. It has been shown that CAFs can orchestrate tumor-promoting inflammation via NF-κB activation [197]. In particular, CAFs recruit monocytes via SDF-1, monocyte chemotactic protein-1 (MCP-1), prostaglandin E2 (PGE2), CXC motif chemokine ligand 14 (CXCL14) and CXC motif chemokine receptor 4 (CXCR4) secretion and promote their differentiation toward a M2 pro-tumoral phenotype both in vitro and in vivo [195,198]. Recently, different subtypes of CAFs have been identified based on differential chemokine expressions (i.e., CCL2 and CXCL12) and with their capability to recruit immune cells within the TME [199]. In addition to acting directly on macrophages, CAFs can affect M2 polarization also indirectly by inducing mast cell to secrete interleukin-13 (IL-13) [200].
Yoshihara and collaborators described the crosstalk between stromal and immune cells by using “Estimation of STromal and Immune cells in Malignant Tumor tissues using Expression data” (ESTIMATE) algorithm, which utilizes gene expression data for inferring the proportions of stromal and immune cells in a tumor sample [201]. Furthermore, the functional enrichment of single-sample genes can be predicted using GO and KEGG pathway analyses [202,203]. The authors predicted the levels of infiltrating stromal and immune cells by calculating immune and stromal scores, which formed the basis for calculating the ESTIMATE score of tumor purity for tumor tissues. Zhao and colleagues identified a set of TME-related genes which are predictive of poor prognosis in these patients by using TCGA-PRAD data. The identified list of genes provide a better understanding of this disease and clarify the relationship between prognosis and TME in patients with PRAD [204].
As for TAMs, myeloid-derived suppressor cells (MDSCs), a heterogeneous population of immature myeloid cells, contribute to create an immunosuppressive TME [205]. It has been demonstrated that a subpopulation of CAFs expressing high levels of fibroblast activation protein (FAP) recruits MDSCs via STAT3-C-C motif chemokine ligand 2 (CCL2) signaling, thus supporting a pro-tumorigenic milieu [206]. Furthermore, Wen and collaborators suggested that MDSCs are more susceptible to infiltrate the PCa stromal area rather than the epithelial compartment supporting neoangiogenesis [207]. Additionally, CAFs are able to recruit neutrophils in the TME via IL-6/JAK/STAT3 in various cancer types [208,209].
During tumor progression, the proliferation and invasion of tumor cells as well as the EMT process are controlled by TME-mediated secretion of several growth factors and cytokines including TGF-β, IL-6, IL-8, IL-10 and VEGF [78,210,211,212]. The heterogeneity of secreted molecules is due to the presence of different cell subpopulations within the TME. The transcription factor NF-κB is the principal factor that controls several processes during tumorigenesis by regulating the expression of numerous genes involved in survival, proliferation, angiogenesis and inflammation [213]. It has been demonstrated that constitutive activation of NF-κB is a hallmark of several cancers, including PCa [213,214]. NF-κB is overexpressed in the PCa TME and regulates the expression of numerous tumor-promoting genes including cyclin-D1, IL-6, TNFα, VEGF, IL-8 and IL-1β [215,216,217]. The activation of NF-κB in PCa sustains cancer cell survival via activation of anti-apoptotic pathways, angiogenesis and metastasis, indicating that this factor is associated with advanced PCa. Accordingly, NF-κB overactivation also promotes EMT of PCa cells [218], suggesting that NF-κB could be a potential prognostic biomarker in human PCa. Indeed, serum markers of inflammation have been proposed as useful tools for estimating PCa risk and evaluating prognosis [219]. Interestingly, accumulated evidence suggests that PCa inflammatory biomarkers could be detected in urine via non-invasive procedures [219,220].
In PCa, the most important mediator of chronic inflammation is IL-6, a multifunctional cytokine that sustains prostate cancer cell proliferation, inhibits cell death and promotes T cell infiltration to the TME, EMT and metastatic processes both in vitro and in vivo [221,222]. In addition, IL-6 influences the sensibility to androgens via different signaling pathways [223]. IL-6 is secreted by different types of cells including inflammatory and immune cells [224]. IL-6 stimulates PCa cells to secrete VEGF via the activation of STAT3 and MAPK signaling pathways under ADT [210]. Recently, it has been demonstrated that IL-6 promotes the progression of PCa to CRPC via AR activation both in vitro and in vivo [225]. Additionally, elevated IL-6 signaling in PCa samples is associated with advanced stages of diseases and with worse prognoses (Table 1) [226]. IL-6 activates in vitro TGF-β-SMAD2 axis and promotes neuroendocrine differentiation (NED) of PCa cells under androgen depletion conditions [220,227]. Given its important role in tumor progression, as well as the established correlation between high levels of serum IL-6 and shorter survival [228], IL-6 could be a prognostic biomarker for PCa patients. In this context, IL-6 and IL-8 mediate PCa NED through MAPK and STAT3 signaling, supporting their role as potential biomarkers for advanced PCa [227,228,229]. Although no biomarkers able to predict risk of NED are available to date, strong efforts have been made by several groups to investigate the role of secreted protein acidic and rich in cysteine (SPARC) as a potential biomarker. Controversial results are reported due to its expression in the tumor cells and the stromal compartment, but Enriquez et al. unveiled that castration triggers a tumor–stroma crosstalk leading to stromal Sparc downregulation that could be a crucial step for Neuroendocrine prostate cancer (NEPC), supporting its role as a new potential biomarker. Furthermore, although SPARC can limit IL-6 production by a mechanism that is still elusive and needs a deeper investigation, an upregulation of the NF-κB pathway, which in turn promotes IL-6 production, has been observed in Sparc−/− fibroblasts [230,231,232,233].
IL-18 is produced by different immune cells such as B and T cells, NK cells, monocytes and macrophages and controls the immune response [234]. Congruently, IL-18 promotes angiogenesis and metastasis, leading to tumor progression and contributing to immune escape [90,235]. Recently, it has been documented that IL-18 is overexpressed in several cancers including PCa and it is associated with advanced tumor stage and metastasis [236]. Accordingly, high expression of IL-18 correlates with poor prognosis in PCa patients [236]. These findings suggest that IL-18 could be used as a novel prognostic biomarker to identify high-risk patients and as a new potential therapeutic target. Since this cytokine is also produced by other cells, a deeper understanding of the role of IL-18 in PCa will help to better evaluate its prognostic role.
Another important proinflammatory cytokine secreted by inflammatory cells and implicated in PCa progression and castration resistance is IL-8 [237]. IL-8 expression is mediated by NF-κB [238]. IL-8 is also regulated by TGF-β and induces angiogenesis and tumor metastasis [239]. In addition, IL-8 promotes the EMT and the recruitment of lymphocytes to the TME [222]. Also, IL-8 induces prostate cancer proliferation in vitro and stimulates TAMs to secrete growth factors that in turn sustain the proliferation of tumor cells. Accordingly, the inhibition of IL-8 signaling reduces cell proliferation and invasiveness both in vitro and in vivo [240,241,242]. It has been demonstrated that IL-8 is overexpressed in serum of metastatic PCa patients and correlates with poor overall survival [237,243]. Recent studies reported a controversial role of IL-8 expression in association with the expression of androgens [244]; therefore, further investigations are necessary in order to clarify the precise relationship between IL-8 and androgen expression and the potential utility of IL-8 as a prognostic and predictive biomarker in aggressive PCa.
The anti-inflammatory, immune-suppressive cytokine IL-10, secreted by both tumor cells and immune cells, promotes cancer progression by inhibiting the anti-tumor response via different signaling pathways [245]. As for several other cytokines, elevated serum levels of IL-10 are associated with poor prognosis and high Gleason scores in patients with PCa as well as with drug resistance [246]. A growing number of studies are working on the identification of stromal-specific genomic signatures able to predict the progression risk. Among these, Rasmussen et al. identified a specific stromal subtype (using clinicopathological characteristics such as Gleason grade, gene set enrichment analysis and stromal and immune cell infiltration patterns) in primary tumor samples of localized prostate cancer (LPC) and validated their characteristics in two independent cohorts. A specific stromal subtype has been demonstrated to be more aggressive in LPC, showing stromal dysfunction at both the cellular and molecular levels with increased M2-polarized macrophages and CD8+ T cell infiltration [247].
5. Conclusions
Data from early studies investigating the role of TME in PCa carcinogenesis and progression had a pioneering value in the field of ecology of cancer. Indeed, the hypothesis supporting the role of CAFs in tumorigenesis has been formulated mainly using data obtained in PCa models. However, the clinical translation of this aspect in useful diagnostic or prognostic hints is far from being available. A first problem resides in the high number of modifications evaluable in reactive stroma and in our capacity to discriminate stromal alterations able to support tumor growth from those that are neutral for cancer progression. Indeed, although the cellular and molecular processes involved in the formation of reactive stroma are well defined, cancer-promoting pathways are frequently intertwined with the physiologic defense mechanisms. In addition, it is possible, as seen for cancer cell phenotypes, that a unique promoting pattern of reactive stroma does not exist and that different combinations of molecular characteristics in TME could equally result in a positive effect on tumorigenesis. An example is the metabolic symbiosis between stroma and cancer cells observed only in a subpopulation of PCa patients. Another important weakness is of technical relevance. To date, the gold method for investigating stromal biomarkers is immunohistochemistry, which, however, suffers from different limitations. Among others, we can cite the use of non-standardized antibodies and inter-operator variability. Thus, the future of TME biomarkers will certainly benefit from new high-throughput methodologies that, together with an increased capacity to investigate precisely TME excluding the contribution from cancer cells, will also guarantee a higher reproducibility of the results.
In conclusion, current evidence strongly supports a modern prognostic approach that takes into consideration reference markers associated with the stroma modification and, in particular, with changes in stromal cell phenotype, in ecological metabolisms and in patterns of secreted inflammatory cytokines.

Table 1.
Potential prognostic biomarkers detectable in reactive stroma. Main biological effects associated with their expression and experimental models used for their validation are also shown.
Table 1.
Potential prognostic biomarkers detectable in reactive stroma. Main biological effects associated with their expression and experimental models used for their validation are also shown.
| TME Biomarker | Biological Effect | Experimental Model | References * |
|---|---|---|---|
| Tissue Adaptation | |||
| α-SMA, vimentin, PDGFR-α | myofibroblast differentiation | in vivo, ex vivo | [79,86] |
| Asporin | desmoplasia | in vivo, ex vivo | [123] |
| Collagen | desmoplasia | imaging in patients | [113,117] |
| Fibroblast methylation signature | CAF differentiation | ex vivo | [82] |
| FGF-2 | PCa growth, increased microvessel density | in vitro, in vivo, ex vivo | [105,106] |
| Periostin | desmoplasia | ex vivo | [125] |
| Tenascin-C | desmoplasia | in vivo, ex vivo | [94,95] |
| TGF-β | CAF differentiation | ex vivo | [79] |
| VEGF-A, VEGFR-2 | angiogenesis | ex vivo | [70] |
| vWF, CD31, CD34, CD105 | increased microvessel density, aberrant vessel morphology | in vivo, ex vivo | [56,57] |
| Wnt | CAF differentiation | in vitro, in vivo | [98,99,101] |
| MAOB | CAF differentiation, ECM remodeling | in vitro, in vivo | [140] |
| Metabolic Switch | |||
| Lactate | metabolic reprogramming of PCa cells toward OXPHOS and anabolic pathways | in vitro, in vivo, ex vivo | [159,160,165] |
| Leucine | increased inflammation and ECM remodeling | ex vivo | [21,163] |
| GLUT1 | Warburg effect | in vitro | [159] |
| Glutamine | metabolic reprogramming of PCa cells in the form of anaplerosis into TCA cycle and energy production | in vitro, ex vivo | [154] |
| MCT4 | increased lactate extrusion | in vitro, ex vivo | [160,161] |
| Inflammation | |||
| IL-6 | cell proliferation, T cell infiltration into the TME, EMT, metastasis | in vitro, in vivo, ex vivo | [226,228] |
| IL-8 | angiogenesis and metastasis, EMT, T cell recruitment into the TME and castration resistance | in vitro, in vivo, ex vivo | [237] |
| IL-10 | immune escape | in vitro, ex vivo | [246] |
| IL-18 | angiogenesis, metastasis and immune escape | ex vivo | [236] |
* Only selected references are indicated. See the text for further pertinent references.
Author Contributions
D.V. (Davide Vecchiotti) and D.V. (Daniela Verzella) wrote the “inflammation” section; D.C. wrote the “metabolic reprogramming” section; L.C. and E.C. performed bibliographic search and formatting; M.D.V.N. prepared figures; and A.A. and F.Z. wrote the “tissue adaptation” section. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by intramural “DISCAB GRANT 2022 code 07_DG_2022_02” to D.C. and “DISCAB GRANT 2024 code 07_DG_2024_23” to D.Ver. awarded by the Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-Specific Antigen and Prostate Cancer: Prediction, Detection and Monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Bullock, T.L.; Belani, J.S.; Traxel, E.; Yan, Y.; Bostwick, D.G.; Humphrey, P.A. Is There a Better Way to Biopsy the Prostate? Prospects for a Novel Transrectal Systematic Biopsy Approach. Urology 2007, 70, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Couñago, F.; López-Campos, F.; Díaz-Gavela, A.A.; Almagro, E.; Fenández-Pascual, E.; Henríquez, I.; Lozano, R.; Espinós, E.L.; Gómez-Iturriaga, A.; de Velasco, G.; et al. Clinical Applications of Molecular Biomarkers in Prostate Cancer. Cancers 2020, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, V.; Cooperberg, M.R.; Dall’Era, M.; Lin, D.W.; Montorsi, F.; Schalken, J.A.; Evans, C.P. Genomic Markers in Prostate Cancer Decision Making. Eur. Urol. 2018, 73, 572–582. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Pritchard, C.; Mecham, B.; Dumpit, R.; Coleman, I.; Bhattacharjee, M.; Chen, Q.; Sikes, R.A.; Nelson, P.S. Conserved Gene Expression Programs Integrate Mammalian Prostate Development and Tumorigenesis. Cancer Res. 2009, 69, 1739–1747. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Marchionni, L.; Huang, Z.; Simons, B.; Blackman, A.; Yu, W.; Parmigiani, G.; Berman, D.M. Androgen-Induced Programs for Prostate Epithelial Growth and Invasion Arise in Embryogenesis and Are Reactivated in Cancer. Oncogene 2008, 27, 7180–7191. [Google Scholar] [CrossRef]
- Taylor, R.A.; Wang, H.; Wilkinson, S.E.; Richards, M.G.; Britt, K.L.; Vaillant, F.; Lindeman, G.J.; Visvader, J.E.; Cunha, G.R.; St John, J.; et al. Lineage Enforcement by Inductive Mesenchyme on Adult Epithelial Stem Cells across Developmental Germ Layers. Stem Cells 2009, 27, 3032–3042. [Google Scholar] [CrossRef]
- Toivanen, R.; Shen, M.M. Prostate Organogenesis: Tissue Induction, Hormonal Regulation and Cell Type Specification. Development 2017, 144, 1382–1398. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal. Similarities between Tumor Stroma Generation and Wound Healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Loizzo, D.; Pandolfo, S.D.; Rogers, D.; Cerrato, C.; di Meo, N.A.; Autorino, R.; Mirone, V.; Ferro, M.; Porta, C.; Stella, A.; et al. Novel Insights into Autophagy and Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 3826. [Google Scholar] [CrossRef] [PubMed]
- Levin, I. The Relation of the Reactive Stroma Formation to the Transplantability of the Cancers of the White Rat. J. Exp. Med. 1911, 13, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.W. Fibroblasts Are Critical Determinants in Prostatic Cancer Growth and Dissemination. Cancer Metastasis Rev. 1991, 10, 263–274. [Google Scholar] [CrossRef]
- Ayala, G.; Tuxhorn, J.A.; Wheeler, T.M.; Frolov, A.; Scardino, P.T.; Ohori, M.; Wheeler, M.; Spitler, J.; Rowley, D.R. Reactive Stroma as a Predictor of Biochemical-Free Recurrence in Prostate Cancer. Clin. Cancer Res. 2003, 9, 4792–4801. [Google Scholar] [PubMed]
- Ayala, G.E.; Muezzinoglu, B.; Hammerich, K.H.; Frolov, A.; Liu, H.; Scardino, P.T.; Li, R.; Sayeeduddin, M.; Ittmann, M.M.; Kadmon, D.; et al. Determining Prostate Cancer-Specific Death through Quantification of Stromogenic Carcinoma Area in Prostatectomy Specimens. Am. J. Pathol. 2011, 178, 79–87. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Wei, W.; Hawley, S.; Auman, H.; Newcomb, L.F.; Boyer, H.D.; Fazli, L.; Simko, J.; Hurtado-Coll, A.; Troyer, D.A.; et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am. J. Surg. Pathol. 2016, 40, 1439–1456. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Li, R.; Rowley, D.; Liu, H.; Kadmon, D.; Miles, B.J.; Wheeler, T.M.; Ayala, G.E. Stromogenic Prostatic Carcinoma Pattern (Carcinomas with Reactive Stromal Grade 3) in Needle Biopsies Predicts Biochemical Recurrence-Free Survival in Patients after Radical Prostatectomy. Hum. Pathol. 2007, 38, 1611–1620. [Google Scholar] [CrossRef]
- Billis, A.; Meirelles, L.; Freitas, L.L.L.; Polidoro, A.S.; Fernandes, H.A.; Padilha, M.M.; Magna, L.A.; Reis, L.O.; Ferreira, U. Adenocarcinoma on Needle Prostatic Biopsies: Does Reactive Stroma Predicts Biochemical Recurrence in Patients Following Radical Prostatectomy? Int. Braz. J. Urol. 2013, 39, 320–327. [Google Scholar] [CrossRef][Green Version]
- Tomas, D.; Spajić, B.; Milosević, M.; Demirović, A.; Marusić, Z.; Kruslin, B. Intensity of Stromal Changes Predicts Biochemical Recurrence-Free Survival in Prostatic Carcinoma. Scand. J. Urol. Nephrol. 2010, 44, 284–290. [Google Scholar] [CrossRef]
- Wu, J.-P.; Huang, W.-B.; Zhou, H.; Xu, L.-W.; Zhao, J.-H.; Zhu, J.-G.; Su, J.-H.; Sun, H.-B. Intensity of Stromal Changes Is Associated with Tumor Relapse in Clinically Advanced Prostate Cancer after Castration Therapy. Asian J. Androl. 2014, 16, 710–714. [Google Scholar] [CrossRef]
- Sæter, T.; Vlatkovic, L.; Waaler, G.; Servoll, E.; Nesland, J.M.; Axcrona, K.; Axcrona, U. The Prognostic Value of Reactive Stroma on Prostate Needle Biopsy: A Population-Based Study. Prostate 2015, 75, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tyekucheva, S.; Bowden, M.; Bango, C.; Giunchi, F.; Huang, Y.; Zhou, C.; Bondi, A.; Lis, R.; Van Hemelrijck, M.; Andrén, O.; et al. Stromal and Epithelial Transcriptional Map of Initiation Progression and Metastatic Potential of Human Prostate Cancer. Nat. Commun. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, Y.; Sawyers, A.; Yao, H.; Rahmatpanah, F.; Xia, X.-Q.; Xu, Q.; Pio, R.; Turan, T.; Koziol, J.A.; et al. Diagnosis of Prostate Cancer Using Differentially Expressed Genes in Stroma. Cancer Res. 2011, 71, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Karkampouna, S.; De Filippo, M.R.; Ng, C.K.Y.; Klima, I.; Zoni, E.; Spahn, M.; Stein, F.; Haberkant, P.; Thalmann, G.N.; Kruithof-de Julio, M. Stroma Transcriptomic and Proteomic Profile of Prostate Cancer Metastasis Xenograft Models Reveals Prognostic Value of Stroma Signatures. Cancers 2020, 12, 3786. [Google Scholar] [CrossRef]
- Mo, F.; Lin, D.; Takhar, M.; Ramnarine, V.R.; Dong, X.; Bell, R.H.; Volik, S.V.; Wang, K.; Xue, H.; Wang, Y.; et al. Stromal Gene Expression Is Predictive for Metastatic Primary Prostate Cancer. Eur. Urol. 2018, 73, 524–532. [Google Scholar] [CrossRef]
- Cunha, G.R.; Hayward, S.W.; Wang, Y.Z.; Ricke, W.A. Role of the Stromal Microenvironment in Carcinogenesis of the Prostate. Int. J. Cancer 2003, 107, 212–235. [Google Scholar] [CrossRef]
- Dakhova, O.; Ozen, M.; Creighton, C.J.; Li, R.; Ayala, G.; Rowley, D.; Ittmann, M. Global Gene Expression Analysis of Reactive Stroma in Prostate Cancer. Clin. Cancer Res. 2009, 15, 3979–3989. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive Stroma in Prostate Cancer Progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Hofree, M.; Choi, D.; Linton, E.L.; Turkekul, M.; Bejnood, A.; Carver, B.; Gopalan, A.; Abida, W.; Laudone, V.; et al. Regenerative Potential of Prostate Luminal Cells Revealed by Single-Cell Analysis. Science 2020, 368, 497–505. [Google Scholar] [CrossRef]
- Crowley, L.; Cambuli, F.; Aparicio, L.; Shibata, M.; Robinson, B.D.; Xuan, S.; Li, W.; Hibshoosh, H.; Loda, M.; Rabadan, R.; et al. A Single-Cell Atlas of the Mouse and Human Prostate Reveals Heterogeneity and Conservation of Epithelial Progenitors. Elife 2020, 9, e59465. [Google Scholar] [CrossRef]
- Pederzoli, F.; Raffo, M.; Pakula, H.; Ravera, F.; Nuzzo, P.V.; Loda, M. Stromal Cells in Prostate Cancer Pathobiology: Friends or Foes? Br. J. Cancer 2023, 128, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Need, E.F.; Toivanen, R.; Trotta, A.P.; Palethorpe, H.M.; Tamblyn, D.J.; Kopsaftis, T.; England, G.M.; Smith, E.; Drew, P.A.; et al. Stromal Androgen Receptor Regulates the Composition of the Microenvironment to Influence Prostate Cancer Outcome. Oncotarget 2015, 6, 16135–16150. [Google Scholar] [CrossRef] [PubMed]
- Cioni, B.; Nevedomskaya, E.; Melis, M.H.M.; van Burgsteden, J.; Stelloo, S.; Hodel, E.; Spinozzi, D.; de Jong, J.; van der Poel, H.; de Boer, J.P.; et al. Loss of Androgen Receptor Signaling in Prostate Cancer-Associated Fibroblasts (CAFs) Promotes CCL2- and CXCL8-Mediated Cancer Cell Migration. Mol. Oncol. 2018, 12, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Alarid, E.T.; Turner, T.; Donjacour, A.A.; Boutin, E.L.; Foster, B.A. Normal and Abnormal Development of the Male Urogenital Tract. Role of Androgens, Mesenchymal-Epithelial Interactions, and Growth Factors. J. Androl. 1992, 13, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Horton, C.; Yu, C.; Knudsen, B.; Stefanson, J.; Hu, K.; Stefanson, O.; Green, J.; Guo, C.; et al. Stromal AR Inhibits Prostate Tumor Progression by Restraining Secretory Luminal Epithelial Cells. Cell Rep. 2022, 39, 110848. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Rubagotti, A.; Zinoli, L.; Ricci, F.; Salvi, S.; Boccardo, S.; Boccardo, F. Prognostic Value of Stromal and Epithelial Periostin Expression in Human Prostate Cancer: Correlation with Clinical Pathological Features and the Risk of Biochemical Relapse or Death. BMC Cancer 2012, 12, 625. [Google Scholar] [CrossRef]
- Sasaki, H.; Lo, K.M.; Chen, L.B.; Auclair, D.; Nakashima, Y.; Moriyama, S.; Fukai, I.; Tam, C.; Loda, M.; Fujii, Y. Expression of Periostin, Homologous with an Insect Cell Adhesion Molecule, as a Prognostic Marker in Non-Small Cell Lung Cancers. Jpn. J. Cancer Res. 2001, 92, 869–873. [Google Scholar] [CrossRef]
- Tian, Y.; Choi, C.H.; Li, Q.K.; Rahmatpanah, F.B.; Chen, X.; Kim, S.R.; Veltri, R.; Chia, D.; Zhang, Z.; Mercola, D.; et al. Overexpression of Periostin in Stroma Positively Associated with Aggressive Prostate Cancer. PLoS ONE 2015, 10, e0121502. [Google Scholar] [CrossRef]
- Pakula, H.; Omar, M.; Carelli, R.; Pederzoli, F.; Fanelli, G.N.; Pannellini, T.; Socciarelli, F.; Van Emmenis, L.; Rodrigues, S.; Fidalgo-Ribeiro, C.; et al. Distinct Mesenchymal Cell States Mediate Prostate Cancer Progression. Nat. Commun. 2024, 15, 363. [Google Scholar] [CrossRef]
- Corn, P.G. The Tumor Microenvironment in Prostate Cancer: Elucidating Molecular Pathways for Therapy Development. Cancer Manag. Res. 2012, 4, 183–193. [Google Scholar] [CrossRef]
- Cunha, G.R.; Hayward, S.W.; Dahiya, R.; Foster, B.A. Smooth Muscle-Epithelial Interactions in Normal and Neoplastic Prostatic Development. Acta Anat. 1996, 155, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Furumido, J.; Maishi, N.; Yanagawa-Matsuda, A.; Kikuchi, H.; Matsumoto, R.; Osawa, T.; Abe, T.; Matsuno, Y.; Shinohara, N.; Hida, Y.; et al. Stroma Biglycan Expression Can Be a Prognostic Factor in Prostate Cancers. Int. J. Urol. 2023, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Offersen, B.V.; Borre, M.; Overgaard, J. Immunohistochemical Determination of Tumor Angiogenesis Measured by the Maximal Microvessel Density in Human Prostate Cancer. APMIS 1998, 106, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bono, A.V.; Celato, N.; Cova, V.; Salvadore, M.; Chinetti, S.; Novario, R. Microvessel Density in Prostate Carcinoma. Prostate Cancer Prostatic Dis. 2002, 5, 123–127. [Google Scholar] [CrossRef]
- Steiner, I.; Jung, K.; Miller, K.; Stephan, C.; Erbersdobler, A. Expression of Endothelial Factors in Prostate Cancer: A Possible Role of Caveolin-1 for Tumour Progression. Oncol. Rep. 2012, 27, 389–395. [Google Scholar] [CrossRef]
- Grivas, N.; Goussia, A.; Stefanou, D.; Giannakis, D. Microvascular Density and Immunohistochemical Expression of VEGF, VEGFR-1 and VEGFR-2 in Benign Prostatic Hyperplasia, High-Grade Prostate Intraepithelial Neoplasia and Prostate Cancer. Cent. Eur. J. Urol. 2016, 69, 63–71. [Google Scholar] [CrossRef]
- Siegal, J.A.; Yu, E.; Brawer, M.K. Topography of Neovascularity in Human Prostate Carcinoma. Cancer 1995, 75, 2545–2551. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Xiong, X.; Zhang, S.; Tan, D.; Yang, L.; Wei, Q. Different Predictive Values of Microvessel Density for Biochemical Recurrence among Different PCa Populations: A Systematic Review and Meta-Analysis. Cancer Med. 2023, 12, 2166–2178. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Lopez-Beltran, A. Molecular Biology of Prostatic Intraepithelial Neoplasia. Prostate 1996, 29, 117–134. [Google Scholar] [CrossRef]
- Nelson, W.G.; De Marzo, A.M.; Isaacs, W.B. Prostate Cancer. N. Engl. J. Med. 2003, 349, 366–381. [Google Scholar] [CrossRef]
- Montironi, R.; Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M. Mechanisms of Disease: High-Grade Prostatic Intraepithelial Neoplasia and Other Proposed Preneoplastic Lesions in the Prostate. Nat. Clin. Pract. Urol. 2007, 4, 321–332. [Google Scholar] [CrossRef] [PubMed]
- DeMarzo, A.M.; Nelson, W.G.; Isaacs, W.B.; Epstein, J.I. Pathological and Molecular Aspects of Prostate Cancer. Lancet 2003, 361, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Herawi, M. Prostate Needle Biopsies Containing Prostatic Intraepithelial Neoplasia or Atypical Foci Suspicious for Carcinoma: Implications for Patient Care. J. Urol. 2006, 175, 820–834. [Google Scholar] [CrossRef]
- Morote, J.; Schwartzmann, I.; Celma, A.; Roche, S.; de Torres, I.M.; Mast, R.; Semidey, M.E.; Regis, L.; Santamaria, A.; Planas, J.; et al. The Current Recommendation for the Management of Isolated High-Grade Prostatic Intraepithelial Neoplasia. BJU Int. 2022, 129, 627–633. [Google Scholar] [CrossRef]
- Pallares, J.; Rojo, F.; Iriarte, J.; Morote, J.; Armadans, L.I.; de Torres, I. Study of Microvessel Density and the Expression of the Angiogenic Factors VEGF, BFGF and the Receptors Flt-1 and FLK-1 in Benign, Premalignant and Malignant Prostate Tissues. Histol. Histopathol. 2006, 21, 857–865. [Google Scholar] [CrossRef]
- Bettencourt, M.C.; Bauer, J.J.; Sesterhenn, I.A.; Connelly, R.R.; Moul, J.W. CD34 Immunohistochemical Assessment of Angiogenesis as a Prognostic Marker for Prostate Cancer Recurrence after Radical Prostatectomy. J. Urol. 1998, 160, 459–465. [Google Scholar] [CrossRef]
- Montironi, R.; Galluzzi, C.M.; Diamanti, L.; Taborro, R.; Scarpelli, M.; Pisani, E. Prostatic Intra-Epithelial Neoplasia. Qualitative and Quantitative Analyses of the Blood Capillary Architecture on Thin Tissue Sections. Pathol. Res. Pract. 1993, 189, 542–548. [Google Scholar] [CrossRef]
- Sinha, A.A.; Quast, B.J.; Reddy, P.K.; Lall, V.; Wilson, M.J.; Qian, J.; Bostwick, D.G. Microvessel Density as a Molecular Marker for Identifying High-Grade Prostatic Intraepithelial Neoplasia Precursors to Prostate Cancer. Exp. Mol. Pathol. 2004, 77, 153–159. [Google Scholar] [CrossRef]
- Miyata, Y.; Mitsunari, K.; Asai, A.; Takehara, K.; Mochizuki, Y.; Sakai, H. Pathological Significance and Prognostic Role of Microvessel Density, Evaluated Using CD31, CD34, and CD105 in Prostate Cancer Patients after Radical Prostatectomy with Neoadjuvant Therapy. Prostate 2015, 75, 84–91. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Figg, W.D.; Dahut, W.L. Angiogenesis Inhibitors in the Treatment of Prostate Cancer. Expert Opin. Pharmacother. 2010, 11, 233–247. [Google Scholar] [CrossRef]
- Taverna, G.; Giusti, G.; Seveso, M.; Hurle, R.; Colombo, P.; Stifter, S.; Grizzi, F. Mast Cells as a Potential Prognostic Marker in Prostate Cancer. Dis. Markers 2013, 35, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Cheng, H.-C.; Wang, J.; Wang, S.-W.; Tai, H.-C.; Lin, C.-W.; Tang, C.-H. Prostate Cancer-Derived CCN3 Induces M2 Macrophage Infiltration and Contributes to Angiogenesis in Prostate Cancer Microenvironment. Oncotarget 2014, 5, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Xavier, R.; Sugiura, T.; Chen, Y.; Park, E.C.; Lu, N.; Selig, M.; Nielsen, G.; Taksir, T.; Jain, R.K.; et al. Tumor Induction of VEGF Promoter Activity in Stromal Cells. Cell 1998, 94, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Cossigny, D.A.F.; Quan, G.M.Y. The Role of Vascular Endothelial Growth Factor in Metastatic Prostate Cancer to the Skeleton. Prostate Cancer 2013, 2013, 418340. [Google Scholar] [CrossRef]
- West, A.F.; O’Donnell, M.; Charlton, R.G.; Neal, D.E.; Leung, H.Y. Correlation of Vascular Endothelial Growth Factor Expression with Fibroblast Growth Factor-8 Expression and Clinico-Pathologic Parameters in Human Prostate Cancer. Br. J. Cancer 2001, 85, 576–583. [Google Scholar] [CrossRef]
- Aragon-Ching, J.B.; Dahut, W.L. VEGF Inhibitors and Prostate Cancer Therapy. Curr. Mol. Pharmacol. 2009, 2, 161–168. [Google Scholar] [CrossRef]
- Hwang, C.; Heath, E.I. Angiogenesis Inhibitors in the Treatment of Prostate Cancer. J. Hematol. Oncol. 2010, 3, 26. [Google Scholar] [CrossRef]
- Zurita, A.J.; George, D.J.; Shore, N.D.; Liu, G.; Wilding, G.; Hutson, T.E.; Kozloff, M.; Mathew, P.; Harmon, C.S.; Wang, S.L.; et al. Sunitinib in Combination with Docetaxel and Prednisone in Chemotherapy-Naive Patients with Metastatic, Castration-Resistant Prostate Cancer: A Phase 1/2 Clinical Trial. Ann. Oncol. 2012, 23, 688–694. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Armstrong, A.J. Emerging Therapeutic Approaches in the Management of Metastatic Castration-Resistant Prostate Cancer. Prostate Cancer Prostatic Dis. 2011, 14, 206–218. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Nordby, Y.; Andersen, S.; Richardsen, E.; Ness, N.; Al-Saad, S.; Melbø-Jørgensen, C.; Patel, H.R.H.; Dønnem, T.; Busund, L.-T.; Bremnes, R.M. Stromal Expression of VEGF-A and VEGFR-2 in Prostate Tissue Is Associated with Biochemical and Clinical Recurrence after Radical Prostatectomy. Prostate 2015, 75, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Woollard, D.J.; Opeskin, K.; Coso, S.; Wu, D.; Baldwin, M.E.; Williams, E.D. Differential Expression of VEGF Ligands and Receptors in Prostate Cancer. Prostate 2013, 73, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, L.; Hahnfeldt, P.; Folkman, J. Clinical Application of Antiangiogenic Therapy: Microvessel Density, What It Does and Doesn’t Tell Us. J. Natl. Cancer Inst. 2002, 94, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Carroll, P.R.; Flax, J.; Blumenfeld, W.; Folkman, J. Tumor Angiogenesis Correlates with Metastasis in Invasive Prostate Carcinoma. Am. J. Pathol. 1993, 143, 401–409. [Google Scholar] [PubMed]
- Chang, Y.; Li, H.; Guo, Z. Mesenchymal Stem Cell-like Properties in Fibroblasts. Cell. Physiol. Biochem. 2014, 34, 703–714. [Google Scholar] [CrossRef]
- Chiarugi, P.; Paoli, P.; Cirri, P. Tumor Microenvironment and Metabolism in Prostate Cancer. Semin. Oncol. 2014, 41, 267–280. [Google Scholar] [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The Evolving Relationship of Wound Healing and Tumor Stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef]
- Levesque, C.; Nelson, P.S. Cellular Constituents of the Prostate Stroma: Key Contributors to Prostate Cancer Progression and Therapy Resistance. Cold Spring Harb. Perspect. Med. 2018, 8, a030510. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling. Clin. Cancer Res. 2002, 8, 2912–2923. [Google Scholar]
- Linxweiler, J.; Hajili, T.; Körbel, C.; Berchem, C.; Zeuschner, P.; Müller, A.; Stöckle, M.; Menger, M.D.; Junker, K.; Saar, M. Cancer-Associated Fibroblasts Stimulate Primary Tumor Growth and Metastatic Spread in an Orthotopic Prostate Cancer Xenograft Model. Sci. Rep. 2020, 10, 12575. [Google Scholar] [CrossRef]
- Pidsley, R.; Lawrence, M.G.; Zotenko, E.; Niranjan, B.; Statham, A.; Song, J.; Chabanon, R.M.; Qu, W.; Wang, H.; Richards, M.; et al. Enduring Epigenetic Landmarks Define the Cancer Microenvironment. Genome Res. 2018, 28, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Pidsley, R.; Niranjan, B.; Papargiris, M.; Pereira, B.A.; Richards, M.; Teng, L.; Norden, S.; Ryan, A.; Frydenberg, M.; et al. Alterations in the Methylome of the Stromal Tumour Microenvironment Signal the Presence and Severity of Prostate Cancer. Clin. Epigenetics 2020, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Tomas, D.; Kruslin, B. The Potential Value of (Myo)Fibroblastic Stromal Reaction in the Diagnosis of Prostatic Adenocarcinoma. Prostate 2004, 61, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.M.; DeMayo, F.; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate Cancer in a Transgenic Mouse. Proc. Natl. Acad. Sci. USA 1995, 92, 3439–3443. [Google Scholar] [CrossRef]
- Ittmann, M. Anatomy and Histology of the Human and Murine Prostate. Cold Spring Harb. Perspect. Med. 2018, 8, a030346. [Google Scholar] [CrossRef]
- Kasper, S.; Sheppard, P.C.; Yan, Y.; Pettigrew, N.; Borowsky, A.D.; Prins, G.S.; Dodd, J.G.; Duckworth, M.L.; Matusik, R.J. Development, Progression, and Androgen-Dependence of Prostate Tumors in Probasin-Large T Antigen Transgenic Mice: A Model for Prostate Cancer. Lab. Investig. 1998, 78, 319–334. [Google Scholar]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming Growth Factor-Beta 1 Induces Alpha-Smooth Muscle Actin Expression in Granulation Tissue Myofibroblasts and in Quiescent and Growing Cultured Fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Sun, D.-Y.; Wu, J.-Q.; He, Z.-H.; He, M.-F.; Sun, H.-B. Cancer-Associated Fibroblast Regulate Proliferation and Migration of Prostate Cancer Cells through TGF-β Signaling Pathway. Life Sci. 2019, 235, 116791. [Google Scholar] [CrossRef]
- Merz, V.W.; Miller, G.J.; Krebs, T.; Timme, T.L.; Kadmon, D.; Park, S.H.; Egawa, S.; Scardino, P.T.; Thompson, T.C. Elevated Transforming Growth Factor-Beta 1 and Beta 3 MRNA Levels Are Associated with Ras + Myc-Induced Carcinomas in Reconstituted Mouse Prostate: Evidence for a Paracrine Role during Progression. Mol. Endocrinol. 1991, 5, 503–513. [Google Scholar] [CrossRef][Green Version]
- Wu, T.; Wang, W.; Shi, G.; Hao, M.; Wang, Y.; Yao, M.; Huang, Y.; Du, L.; Zhang, X.; Ye, D.; et al. Targeting HIC1/TGF-β Axis-Shaped Prostate Cancer Microenvironment Restrains Its Progression. Cell Death Dis. 2022, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Barron, L.; Xia, L.; Huang, H.; Villarreal, M.M.; Zwaagstra, J.; Collins, C.; Yang, J.; Zwieb, C.; Kodali, R.; et al. A Novel Highly Potent Trivalent TGF-β Receptor Trap Inhibits Early-Stage Tumorigenesis and Tumor Cell Invasion in Murine Pten-Deficient Prostate Glands. Oncotarget 2016, 7, 86087–86102. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, M.J.; Larsen, M.; McBride, L.; Dang, T.D.; Lu, B.; Rowley, D.R. Localization of Transforming Growth Factor-Beta1 and Type II Receptor in Developing Normal Human Prostate and Carcinoma Tissues. J. Histochem. Cytochem. 1998, 46, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H.; Stapleton, A.M.; Yang, G.; Truong, L.D.; Rogers, E.; Timme, T.L.; Wheeler, T.M.; Scardino, P.T.; Thompson, T.C. Reduced Levels of Transforming Growth Factor Beta Receptor Type II in Human Prostate Cancer: An Immunohistochemical Study. Clin. Cancer Res. 1996, 2, 635–640. [Google Scholar]
- Reis, S.T.D.; Pontes-Júnior, J.; Antunes, A.A.; Sousa-Canavez, J.M.d; Abe, D.K.; Cruz, J.A.S.d; Dall’oglio, M.F.; Crippa, A.; Passerotti, C.C.; Ribeiro-Filho, L.A.; et al. Tgf-Β1 Expression as a Biomarker of Poor Prognosis in Prostate Cancer. Clinics 2011, 66, 1143–1147. [Google Scholar] [CrossRef]
- Ammirante, M.; Shalapour, S.; Kang, Y.; Jamieson, C.A.M.; Karin, M. Tissue Injury and Hypoxia Promote Malignant Progression of Prostate Cancer by Inducing CXCL13 Expression in Tumor Myofibroblasts. Proc. Natl. Acad. Sci. USA 2014, 111, 14776–14781. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/β-Catenin Signaling Suppresses Myofibroblast Differentiation of Lung Resident Mesenchymal Stem Cells and Pulmonary Fibrosis. Sci. Rep. 2018, 8, 13644. [Google Scholar] [CrossRef]
- Carthy, J.M.; Garmaroudi, F.S.; Luo, Z.; McManus, B.M. Wnt3a Induces Myofibroblast Differentiation by Upregulating TGF-β Signaling through SMAD2 in a β-Catenin-Dependent Manner. PLoS ONE 2011, 6, e19809. [Google Scholar] [CrossRef]
- Li, X.; Placencio, V.; Iturregui, J.M.; Uwamariya, C.; Sharif-Afshar, A.-R.; Koyama, T.; Hayward, S.W.; Bhowmick, N.A. Prostate Tumor Progression Is Mediated by a Paracrine TGF-Beta/Wnt3a Signaling Axis. Oncogene 2008, 27, 7118–7130. [Google Scholar] [CrossRef]
- Kato, M.; Placencio-Hickok, V.R.; Madhav, A.; Haldar, S.; Tripathi, M.; Billet, S.; Mishra, R.; Smith, B.; Rohena-Rivera, K.; Agarwal, P.; et al. Heterogeneous Cancer-Associated Fibroblast Population Potentiates Neuroendocrine Differentiation and Castrate Resistance in a CD105-Dependent Manner. Oncogene 2019, 38, 716–730. [Google Scholar] [CrossRef]
- Zong, Y.; Huang, J.; Sankarasharma, D.; Morikawa, T.; Fukayama, M.; Epstein, J.I.; Chada, K.K.; Witte, O.N. Stromal Epigenetic Dysregulation Is Sufficient to Initiate Mouse Prostate Cancer via Paracrine Wnt Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E3395–E3404. [Google Scholar] [CrossRef] [PubMed]
- Dakhova, O.; Rowley, D.; Ittmann, M. Genes Upregulated in Prostate Cancer Reactive Stroma Promote Prostate Cancer Progression in Vivo. Clin. Cancer Res. 2014, 20, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Carstens, J.L.; Shahi, P.; Van Tsang, S.; Smith, B.; Creighton, C.J.; Zhang, Y.; Seamans, A.; Seethammagari, M.; Vedula, I.; Levitt, J.M.; et al. FGFR1-WNT-TGF-β Signaling in Prostate Cancer Mouse Models Recapitulates Human Reactive Stroma. Cancer Res. 2014, 74, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kwabi-Addo, B.; Ozen, M.; Ittmann, M. The Role of Fibroblast Growth Factors and Their Receptors in Prostate Cancer. Endocr. Relat. Cancer 2004, 11, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Pecqueux, C.; Arslan, A.; Heller, M.; Falkenstein, M.; Kaczorowski, A.; Tolstov, Y.; Sültmann, H.; Grüllich, C.; Herpel, E.; Duensing, A.; et al. FGF-2 Is a Driving Force for Chromosomal Instability and a Stromal Factor Associated with Adverse Clinico-Pathological Features in Prostate Cancer. Urol. Oncol. 2018, 36, 365.e15–365.e26. [Google Scholar] [CrossRef]
- Yang, F.; Strand, D.W.; Rowley, D.R. Fibroblast Growth Factor-2 Mediates Transforming Growth Factor-Beta Action in Prostate Cancer Reactive Stroma. Oncogene 2008, 27, 450–459. [Google Scholar] [CrossRef]
- Thomson, A.A. Role of Androgens and Fibroblast Growth Factors in Prostatic Development. Reproduction 2001, 121, 187–195. [Google Scholar] [CrossRef]
- Wu, X.; Jin, C.; Wang, F.; Yu, C.; McKeehan, W.L. Stromal Cell Heterogeneity in Fibroblast Growth Factor-Mediated Stromal-Epithelial Cell Cross-Talk in Premalignant Prostate Tumors. Cancer Res. 2003, 63, 4936–4944. [Google Scholar]
- Yan, G.; Fukabori, Y.; McBride, G.; Nikolaropolous, S.; McKeehan, W.L. Exon Switching and Activation of Stromal and Embryonic Fibroblast Growth Factor (FGF)-FGF Receptor Genes in Prostate Epithelial Cells Accompany Stromal Independence and Malignancy. Mol. Cell. Biol. 1993, 13, 4513–4522. [Google Scholar] [CrossRef]
- Ruder, S.; Gao, Y.; Ding, Y.; Bu, P.; Miles, B.; De Marzo, A.; Wheeler, T.; McKenney, J.K.; Auman, H.; Fazli, L.; et al. Development and Validation of a Quantitative Reactive Stroma Biomarker (QRS) for Prostate Cancer Prognosis. Hum. Pathol. 2022, 122, 84–91. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, G.; Ling, Y.; Hsu, Y.-T.; Song, S.; Huang, J.T.-J.; Lang, S.; Wang, R.K.; Huang, Z.; Nabi, G. Detection and Characterisation of Biopsy Tissue Using Quantitative Optical Coherence Elastography (OCE) in Men with Suspected Prostate Cancer. Cancer Lett. 2015, 357, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.M.A.; Sun, Y.; Merajver, S.D.; Fu, J. Mechanotransduction-Induced Reversible Phenotypic Switching in Prostate Cancer Cells. Biophys. J. 2017, 112, 1236–1245. [Google Scholar] [CrossRef]
- Bianchi-Frias, D.; Damodarasamy, M.; Hernandez, S.A.; Gil da Costa, R.M.; Vakar-Lopez, F.; Coleman, I.M.; Reed, M.J.; Nelson, P.S. The Aged Microenvironment Influences the Tumorigenic Potential of Malignant Prostate Epithelial Cells. Mol. Cancer Res. 2019, 17, 321–331. [Google Scholar] [CrossRef]
- Bianchi-Frias, D.; Vakar-Lopez, F.; Coleman, I.M.; Plymate, S.R.; Reed, M.J.; Nelson, P.S. The Effects of Aging on the Molecular and Cellular Composition of the Prostate Microenvironment. PLoS ONE 2010, 5, e12501. [Google Scholar] [CrossRef]
- Ageeli, W.; Zhang, X.; Ogbonnaya, C.N.; Ling, Y.; Wilson, J.; Li, C.; Nabi, G. Characterisation of Collagen Re-Modelling in Localised Prostate Cancer Using Second-Generation Harmonic Imaging and Transrectal Ultrasound Shear Wave Elastography. Cancers 2021, 13, 5553. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen Reorganization at the Tumor-Stromal Interface Facilitates Local Invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-Associated Fibroblasts Promote Directional Cancer Cell Migration by Aligning Fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Ming, X.; Qiu, S.; Liu, X.; Li, S.; Wang, Y.; Zhu, M.; Li, N.; Luo, P.; Liang, C.; Tu, J. Prognostic Role of Tenascin-C for Cancer Outcome: A Meta-Analysis. Technol. Cancer Res. Treat. 2019, 18, 1533033818821106. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lin, S.-C.; Yu, G.; Zhu, M.; Song, J.H.; Rivera, K.; Pappin, D.J.; Logothetis, C.J.; Panaretakis, T.; Wang, G.; et al. Prostate Tumor-Induced Stromal Reprogramming Generates Tenascin C That Promotes Prostate Cancer Metastasis through YAP/TAZ Inhibition. Oncogene 2022, 41, 757–769. [Google Scholar] [CrossRef]
- Ni, W.-D.; Yang, Z.-T.; Cui, C.-A.; Cui, Y.; Fang, L.-Y.; Xuan, Y.-H. Tenascin-C Is a Potential Cancer-Associated Fibroblasts Marker and Predicts Poor Prognosis in Prostate Cancer. Biochem. Biophys. Res. Commun. 2017, 486, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Rochette, A.; Boufaied, N.; Scarlata, E.; Hamel, L.; Brimo, F.; Whitaker, H.C.; Ramos-Montoya, A.; Neal, D.E.; Dragomir, A.; Aprikian, A.; et al. Asporin Is a Stromally Expressed Marker Associated with Prostate Cancer Progression. Br. J. Cancer 2017, 116, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Hurley, P.J.; Sundi, D.; Shinder, B.; Simons, B.W.; Hughes, R.M.; Miller, R.M.; Benzon, B.; Faraj, S.F.; Netto, G.J.; Vergara, I.A.; et al. Germline Variants in Asporin Vary by Race, Modulate the Tumor Microenvironment, and Are Differentially Associated with Metastatic Prostate Cancer. Clin. Cancer Res. 2016, 22, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Tischler, V.; Fritzsche, F.R.; Wild, P.J.; Stephan, C.; Seifert, H.-H.; Riener, M.-O.; Hermanns, T.; Mortezavi, A.; Gerhardt, J.; Schraml, P.; et al. Periostin Is Up-Regulated in High Grade and High Stage Prostate Cancer. BMC Cancer 2010, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Doldi, V.; Lecchi, M.; Ljevar, S.; Colecchia, M.; Campi, E.; Centonze, G.; Marenghi, C.; Rancati, T.; Miceli, R.; Verderio, P.; et al. Potential of the Stromal Matricellular Protein Periostin as a Biomarker to Improve Risk Assessment in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 7987. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Riddick, A.C.P.; Shukla, C.J.; Pennington, C.J.; Bass, R.; Nuttall, R.K.; Hogan, A.; Sethia, K.K.; Ellis, V.; Collins, A.T.; Maitland, N.J.; et al. Identification of Degradome Components Associated with Prostate Cancer Progression by Expression Analysis of Human Prostatic Tissues. Br. J. Cancer 2005, 92, 2171–2180. [Google Scholar] [CrossRef]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Membrane-Type-1 Matrix Metalloproteinase, Matrix Metalloproteinase 2, and Tissue Inhibitor of Matrix Proteinase 2 in Prostate Cancer: Identification of Patients with Poor Prognosis by Immunohistochemistry. Hum. Pathol. 2008, 39, 731–739. [Google Scholar] [CrossRef]
- Miyake, H.; Muramaki, M.; Kurahashi, T.; Takenaka, A.; Fujisawa, M. Expression of Potential Molecular Markers in Prostate Cancer: Correlation with Clinicopathological Outcomes in Patients Undergoing Radical Prostatectomy. Urol. Oncol. 2010, 28, 145–151. [Google Scholar] [CrossRef]
- Samaržija, I. The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines 2023, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chippada-Venkata, U.D.; Oh, W.K. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Prostate Cancer Progression. Cancers 2014, 6, 1298–1327. [Google Scholar] [CrossRef]
- Medina-González, A.; Eiró-Díaz, N.; Fernández-Gómez, J.M.; Ovidio-González, L.; Jalón-Monzón, A.; Casas-Nebra, J.; Escaf-Barmadah, S. Comparative Analysis of the Expression of Metalloproteases (MMP-2, MMP-9, MMP-11 and MMP-13) and the Tissue Inhibitor of Metalloprotease 3 (TIMP-3) between Previous Negative Biopsies and Radical Prostatectomies. Actas Urol. Esp. 2020, 44, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Boxler, S.; Djonov, V.; Kessler, T.M.; Hlushchuk, R.; Bachmann, L.M.; Held, U.; Markwalder, R.; Thalmann, G.N. Matrix Metalloproteinases and Angiogenic Factors: Predictors of Survival after Radical Prostatectomy for Clinically Organ-Confined Prostate Cancer? Am. J. Pathol. 2010, 177, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Oguić, R.; Mozetič, V.; Cini Tešar, E.; Fučkar Čupić, D.; Mustać, E.; Dorđević, G. Matrix Metalloproteinases 2 and 9 Immunoexpression in Prostate Carcinoma at the Positive Margin of Radical Prostatectomy Specimens. Patholog. Res. Int. 2014, 2014, 262195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, S.; He, J.; Yin, L.; Zhou, J.; Lian, J.; Ren, Y.; Zhang, X.; Yuan, J.; Wang, G.; Li, X. Matrix Metalloproteinases Targeting in Prostate Cancer. Urol. Oncol. 2024, 42, 275–287. [Google Scholar] [CrossRef]
- González, L.O.; Eiro, N.; Fraile, M.; Beridze, N.; Escaf, A.R.; Escaf, S.; Fernández-Gómez, J.M.; Vizoso, F.J. Prostate Cancer Tumor Stroma: Responsibility in Tumor Biology, Diagnosis and Treatment. Cancers 2022, 14, 4412. [Google Scholar] [CrossRef]
- Guccini, I.; Revandkar, A.; D’Ambrosio, M.; Colucci, M.; Pasquini, E.; Mosole, S.; Troiani, M.; Brina, D.; Sheibani-Tezerji, R.; Elia, A.R.; et al. Senescence Reprogramming by TIMP1 Deficiency Promotes Prostate Cancer Metastasis. Cancer Cell 2021, 39, 68–82.e9. [Google Scholar] [CrossRef]
- El-Chaer, W.K.; Moraes, C.F.; Nóbrega, O.T. Diagnosis and Prognosis of Prostate Cancer from Circulating Matrix Metalloproteinases and Inhibitors. J. Aging Res. 2018, 2018, 7681039. [Google Scholar] [CrossRef]
- Pu, T.; Wang, J.; Wei, J.; Zeng, A.; Zhang, J.; Chen, J.; Yin, L.; Li, J.; Lin, T.-P.; Melamed, J.; et al. Stromal-Derived MAOB Promotes Prostate Cancer Growth and Progression. Sci. Adv. 2024, 10, eadi4935. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A Comprehensive Review of the Role of Zinc in Normal Prostate Function and Metabolism; and Its Implications in Prostate Cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Chetta, P.; Zadra, G. Metabolic Reprogramming as an Emerging Mechanism of Resistance to Endocrine Therapies in Prostate Cancer. Cancer Drug Resist. 2021, 4, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic Reprogramming in Prostate Cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Beier, A.M.K.; Puhr, M.; Stope, M.B.; Thomas, C.; Erb, H.H.H. Metabolic Changes during Prostate Cancer Development and Progression. J. Cancer Res. Clin. Oncol. 2022, 149, 2259–2270. [Google Scholar] [CrossRef]
- Bader, D.A.; Hartig, S.M.; Putluri, V.; Foley, C.; Hamilton, M.P.; Smith, E.A.; Saha, P.K.; Panigrahi, A.; Walker, C.; Zong, L.; et al. Mitochondrial Pyruvate Import Is a Metabolic Vulnerability in Androgen Receptor-Driven Prostate Cancer. Nat. Metab. 2019, 1, 70–85. [Google Scholar] [CrossRef]
- Audet-Walsh, É.; Dufour, C.R.; Yee, T.; Zouanat, F.Z.; Yan, M.; Kalloghlian, G.; Vernier, M.; Caron, M.; Bourque, G.; Scarlata, E.; et al. Nuclear MTOR Acts as a Transcriptional Integrator of the Androgen Signaling Pathway in Prostate Cancer. Genes Dev. 2017, 31, 1228–1242. [Google Scholar] [CrossRef]
- Scaglia, N.; Frontini-López, Y.R.; Zadra, G. Prostate Cancer Progression: As a Matter of Fats. Front. Oncol. 2021, 11, 719865. [Google Scholar] [CrossRef]
- Audet-Walsh, É.; Yee, T.; McGuirk, S.; Vernier, M.; Ouellet, C.; St-Pierre, J.; Giguère, V. Androgen-Dependent Repression of ERRγ Reprograms Metabolism in Prostate Cancer. Cancer Res. 2017, 77, 378–389. [Google Scholar] [CrossRef]
- Swinnent, J.V.; Van Veldhoven, P.P.; Esquenet, M.; Heyns, W.; Verhoeven, G. Androgens Markedly Stimulate the Accumulation of Neutral Lipids in the Human Prostatic Adenocarcinoma Cell Line LNCaP. Endocrinology 1996, 137, 4468–4474. [Google Scholar] [CrossRef]
- Liu, I.J.; Zafar, M.B.; Lai, Y.H.; Segall, G.M.; Terris, M.K. Fluorodeoxyglucose Positron Emission Tomography Studies in Diagnosis and Staging of Clinically Organ-Confined Prostate Cancer. Urology 2001, 57, 108–111. [Google Scholar] [CrossRef]
- Fox, J.J.; Gavane, S.C.; Blanc-Autran, E.; Nehmeh, S.; Gonen, M.; Beattie, B.; Vargas, H.A.; Schoder, H.; Humm, J.L.; Fine, S.W.; et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgenreceptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Wang, B.; Lin, G.; Wei, Y.; Abudurexiti, M.; Zhu, W.; Liu, C.; Qin, X.; Dai, B.; et al. GLUT1 Is an AR Target Contributing to Tumor Growth and Glycolysis in Castration-Resistant and Enzalutamide-Resistant Prostate Cancers. Cancer Lett. 2020, 485, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Strmiska, V.; Michalek, P.; Eckschlager, T.; Stiborova, M.; Adam, V.; Krizkova, S.; Heger, Z. Prostate Cancer-Specific Hallmarks of Amino Acids Metabolism: Towards a Paradigm of Precision Medicine. Biochim. Biophys. Acta. Rev. Cancer 2019, 1871, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Haldar, S.; Placencio, V.; Madhav, A.; Rohena-Rivera, K.; Agarwal, P.; Duong, F.; Angara, B.; Tripathi, M.; Liu, Z.; et al. Stromal Epigenetic Alterations Drive Metabolic and Neuroendocrine Prostate Cancer Reprogramming. J. Clin. Investig. 2018, 128, 4472–4484. [Google Scholar] [CrossRef]
- Schöpf, B.; Weissensteiner, H.; Schäfer, G.; Fazzini, F.; Charoentong, P.; Naschberger, A.; Rupp, B.; Fendt, L.; Bukur, V.; Giese, I.; et al. OXPHOS Remodeling in High-Grade Prostate Cancer Involves MtDNA Mutations and Increased Succinate Oxidation. Nat. Commun. 2020, 11, 1487. [Google Scholar] [CrossRef]
- Chan, S.C.; Selth, L.A.; Li, Y.; Nyquist, M.D.; Miao, L.; Bradner, J.E.; Raj, G.V.; Tilley, W.D.; Dehm, S.M. Targeting Chromatin Binding Regulation of Constitutively Active AR Variants to Overcome Prostate Cancer Resistance to Endocrine-Based Therapies. Nucleic Acids Res. 2015, 43, 5880–5897. [Google Scholar] [CrossRef]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de Novo Lipogenesis Targets Androgen Receptor Signaling in Castration-Resistant Prostate Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 631–640. [Google Scholar] [CrossRef]
- Han, W.; Gao, S.; Barrett, D.; Ahmed, M.; Han, D.; MacOska, J.A.; He, H.H.; Cai, C. Reactivation of Androgen Receptor-Regulated Lipid Biosynthesis Drives the Progression of Castration-Resistant Prostate Cancer. Oncogene 2018, 37, 710–721. [Google Scholar] [CrossRef]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal Metabolic Reprogramming through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef]
- Pertega-Gomes, N.; Felisbino, S.; Massie, C.E.; Vizcaino, J.R.; Coelho, R.; Sandi, C.; Simoes-Sousa, S.; Jurmeister, S.; Ramos-Montoya, A.; Asim, M.; et al. A Glycolytic Phenotype Is Associated with Prostate Cancer Progression and Aggressiveness: A Role for Monocarboxylate Transporters as Metabolic Targets for Therapy. J. Pathol. 2015, 236, 517–530. [Google Scholar] [CrossRef]
- Pértega-Gomes, N.; Vizcaíno, J.R.; Attig, J.; Jurmeister, S.; Lopes, C.; Baltazar, F. A Lactate Shuttle System between Tumour and Stromal Cells Is Associated with Poor Prognosis in Prostate Cancer. BMC Cancer 2014, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Boidot, R.; Veǵran, F.; Meulle, A.; Le Breton, A.; Dessy, C.; Sonveaux, P.; Lizard-Nacol, S.; Feron, O. Regulation of Monocarboxylate Transporter MCT1 Expression by P53 Mediates Inward and Outward Lactate Fluxes in Tumors. Cancer Res. 2012, 72, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Solstad, Ø.; Moi, L.; Donnem, T.; Eilertsen, M.; Nordby, Y.; Ness, N.; Richardsen, E.; Busund, L.-T.; Bremnes, R.M. Organized Metabolic Crime in Prostate Cancer: The Coexpression of MCT1 in Tumor and MCT4 in Stroma Is an Independent Prognosticator for Biochemical Failure. Urol. Oncol. 2015, 33, 338.e9–338.e17. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Taddei, M.L.; Morandi, A.; Comito, G.; Calvani, M.; Bianchini, F.; Richichi, B.; Raugei, G.; Wong, N.; Tang, D.; et al. Targeting Stromal-Induced Pyruvate Kinase M2 Nuclear Translocation Impairs OXPHOS and Prostate Cancer Metastatic Spread. Oncotarget 2015, 6, 24061. [Google Scholar] [CrossRef]
- Ippolito, L.; Morandi, A.; Taddei, M.L.; Parri, M.; Comito, G.; Iscaro, A.; Raspollini, M.R.; Magherini, F.; Rapizzi, E.; Masquelier, J.; et al. Cancer-Associated Fibroblasts Promote Prostate Cancer Malignancy via Metabolic Rewiring and Mitochondrial Transfer. Oncogene 2019, 38, 5339–5355. [Google Scholar] [CrossRef]
- Valencia, T.; Kim, J.Y.; Abu-Baker, S.; Moscat-Pardos, J.; Ahn, C.S.; Reina-Campos, M.; Duran, A.; Castilla, E.A.; Metallo, C.M.; Diaz-Meco, M.T.; et al. Metabolic Reprogramming of Stromal Fibroblasts through P62-MTORC1 Signaling Promotes Inflammation and Tumorigenesis. Cancer Cell 2014, 26, 121–135. [Google Scholar] [CrossRef]
- Kolonin, M.G.; DiGiovanni, J. The Role of Adipose Stroma in Prostate Cancer Aggressiveness. Transl. Androl. Urol. 2019, 8, S348–S350. [Google Scholar] [CrossRef]
- Zhang, R.; Zong, J.; Peng, Y.; Shi, J.; Du, X.; Liu, H.; Shen, Y.; Cao, J.; Jia, B.; Liu, F.; et al. GPR30 Knockdown Weakens the Capacity of CAF in Promoting Prostate Cancer Cell Invasion via Reducing Macrophage Infiltration and M2 Polarization. J. Cell. Biochem. 2021, 122, 1173–1191. [Google Scholar] [CrossRef]
- Maeda, M.; Takeshima, H.; Iida, N.; Hattori, N.; Yamashita, S.; Moro, H.; Yasukawa, Y.; Nishiyama, K.; Hashimoto, T.; Sekine, S.; et al. Cancer Cell Niche Factors Secreted from Cancer-Associated Fibroblast by Loss of H3K27me3. Gut 2020, 69, 243–251. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 Promotes Lineage Plasticity and Antiandrogen Resistance in TP53-and RB1-Deficient Prostate Cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef]
- Davies, A.H.; Beltran, H.; Zoubeidi, A. Cellular Plasticity and the Neuroendocrine Phenotype in Prostate Cancer. Nat. Rev. Urol. 2018, 15, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Campbell, B.K.; Stylli, S.S.; Corcoran, N.M.; Hovens, C.M. The Prostate Cancer Immune Microenvironment, Biomarkers and Therapeutic Intervention. Uro 2022, 2, 74–92. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in Prostate Carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlomm, T.; Köllermann, J.; Sekulic, N.; Huland, H.; Mirlacher, M.; Sauter, G.; Simon, R.; Erbersdobler, A. Immunological Microenvironment in Prostate Cancer: High Mast Cell Densities Are Associated with Favorable Tumor Characteristics and Good Prognosis. Prostate 2009, 69, 976–981. [Google Scholar] [CrossRef]
- Flammiger, A.; Bayer, F.; Cirugeda-Kühnert, A.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; et al. Intratumoral T but Not B Lymphocytes Are Related to Clinical Outcome in Prostate Cancer. APMIS 2012, 120, 901–908. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Davidsson, S.; Ohlson, A.-L.; Andersson, S.-O.; Fall, K.; Meisner, A.; Fiorentino, M.; Andrén, O.; Rider, J.R. CD4 Helper T Cells, CD8 Cytotoxic T Cells, and FOXP3(+) Regulatory T Cells with Respect to Lethal Prostate Cancer. Mod. Pathol. 2013, 26, 448–455. [Google Scholar] [CrossRef]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of Tumor-Infiltrating T-Cell Density with Molecular Subtype, Racial Ancestry and Clinical Outcomes in Prostate Cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef]
- Gollapudi, K.; Galet, C.; Grogan, T.; Zhang, H.; Said, J.W.; Huang, J.; Elashoff, D.; Freedland, S.J.; Rettig, M.; Aronson, W.J. Association between Tumor-Associated Macrophage Infiltration, High Grade Prostate Cancer, and Biochemical Recurrence after Radical Prostatectomy. Am. J. Cancer Res. 2013, 3, 523–529. [Google Scholar] [PubMed]
- Shiao, S.L.; Chu, G.C.-Y.; Chung, L.W.K. Regulation of Prostate Cancer Progression by the Tumor Microenvironment. Cancer Lett. 2016, 380, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, J.; Wang, Y.; Zhang, J.; Ji, C.; Cong, R.; Wang, Y.; Song, N. Tumor Infiltrating M2 Macrophages Could Predict Biochemical Recurrence of Localized Prostate Cancer after Radical Prostatectomy. Exp. Cell Res. 2019, 384, 111588. [Google Scholar] [CrossRef]
- Guan, W.; Hu, J.; Yang, L.; Tan, P.; Tang, Z.; West, B.L.; Bollag, G.; Xu, H.; Wu, L. Inhibition of TAMs Improves the Response to Docetaxel in Castration-Resistant Prostate Cancer. Endocr. Relat. Cancer 2019, 26, 131–140. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 Macrophages and Regulatory T Cells in Lethal Prostate Cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Xu, N.; Dong, R.-N.; Lin, T.-T.; Lin, T.; Lin, Y.-Z.; Chen, S.-H.; Zhu, J.-M.; Ke, Z.-B.; Huang, F.; Chen, Y.-H.; et al. Development and Validation of Novel Biomarkers Related to M2 Macrophages Infiltration by Weighted Gene Co-Expression Network Analysis in Prostate Cancer. Front. Oncol. 2021, 11, 634075. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-De-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-Associated Fibroblasts and M2-Polarized Macrophages Synergize during Prostate Carcinoma Progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, E.; Fujimori, M.; Kodama, H.; Felsen, D.; Chen, J.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; Srimathveeravalli, G. Macrophage-Secreted TGF-Β1 Contributes to Fibroblast Activation and Ureteral Stricture after Ablation Injury. Am. J. Physiol. Renal Physiol. 2019, 317, F52–F64. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-Associated Fibroblasts Promote an Immunosuppressive Microenvironment through the Induction and Accumulation of Protumoral Macrophages. Oncotarget 2017, 8, 8633–8647. [Google Scholar] [CrossRef] [PubMed]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer Associated Fibroblasts Sculpt Tumour Microenvironment by Recruiting Monocytes and Inducing Immunosuppressive PD-1+ TAMs. Sci. Rep. 2019, 9, 3172. [Google Scholar] [CrossRef]
- Di Somma, S.; Napolitano, F.; Portella, G.; Malfitano, A.M. Cross Talk of Macrophages with Tumor Microenvironment Cells and Modulation of Macrophages in Cancer by Virotherapy. Biomedicines 2021, 9, 1309. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-KappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Augsten, M.; Sjöberg, E.; Frings, O.; Vorrink, S.U.; Frijhoff, J.; Olsson, E.; Borg, Å.; Östman, A. Cancer-Associated Fibroblasts Expressing CXCL14 Rely upon NOS1-Derived Nitric Oxide Signaling for Their Tumor-Supporting Properties. Cancer Res. 2014, 74, 2999–3010. [Google Scholar] [CrossRef]
- Vickman, R.E.; Broman, M.M.; Lanman, N.A.; Franco, O.E.; Sudyanti, P.A.G.; Ni, Y.; Ji, Y.; Helfand, B.T.; Petkewicz, J.; Paterakos, M.C.; et al. Heterogeneity of Human Prostate Carcinoma-Associated Fibroblasts Implicates a Role for Subpopulations in Myeloid Cell Recruitment. Prostate 2020, 80, 173–185. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Franks, J.M.; Cai, G.; Mehta, B.K.; Wood, T.A.; Archambault, K.; Pioli, P.A.; Simms, R.W.; Orzechowski, N.; Arron, S.; et al. Microbiome Dysbiosis Is Associated with Disease Duration and Increased Inflammatory Gene Expression in Systemic Sclerosis Skin. Arthritis Res. Ther. 2019, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Kondoh, E.; Chigusa, Y.; Kawamura, Y.; Mogami, H.; Takeda, S.; Horie, A.; Baba, T.; Matsumura, N.; Mandai, M.; et al. Metabolomic Profiles of Placenta in Preeclampsia. Hypertens 2019, 73, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, D.; Li, J.; Zhao, G.; Tang, W.; Cheng, H. Database Mining of Genes of Prognostic Value for the Prostate Adenocarcinoma Microenvironment Using the Cancer Gene Atlas. Biomed Res. Int. 2020, 2020, 5019793. [Google Scholar] [CrossRef] [PubMed]
- Dysthe, M.; Parihar, R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 117–140. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Wen, J.; Huang, G.; Liu, S.; Wan, J.; Wang, X.; Zhu, Y.; Kaliney, W.; Zhang, C.; Cheng, L.; Wen, X.; et al. Polymorphonuclear MDSCs Are Enriched in the Stroma and Expanded in Metastases of Prostate Cancer. J. Pathol. Clin. Res. 2020, 6, 171–177. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The Role of STAT3 in Leading the Crosstalk between Human Cancers and the Immune System. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Ishii, K.; Sasaki, T.; Iguchi, K.; Kajiwara, S.; Kato, M.; Kanda, H.; Hirokawa, Y.; Arima, K.; Mizokami, A.; Sugimura, Y. Interleukin-6 Induces VEGF Secretion from Prostate Cancer Cells in a Manner Independent of Androgen Receptor Activation. Prostate 2018, 78, 849–856. [Google Scholar] [CrossRef]
- Bruzzese, F.; Hägglöf, C.; Leone, A.; Sjöberg, E.; Roca, M.S.; Kiflemariam, S.; Sjöblom, T.; Hammarsten, P.; Egevad, L.; Bergh, A.; et al. Local and Systemic Protumorigenic Effects of Cancer-Associated Fibroblast-Derived GDF15. Cancer Res. 2014, 74, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-ΚB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Vecchiotti, D.; Verzella, D.; Di Vito Nolfi, M.; D’Andrea, D.; Flati, I.; Di Francesco, B.; Cornice, J.; Alesse, E.; Capece, D.; Zazzeroni, F. Elevated NF-ΚB/SHh/GLI1 Signature Denotes a Worse Prognosis and Represent a Novel Potential Therapeutic Target in Advanced Prostate Cancer. Cells 2022, 11, 2118. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-ΚB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Li, J.; Yadav, S.S.; Tewari, A.K. Recent Insights into NF-ΚB Signalling Pathways and the Link between Inflammation and Prostate Cancer. BJU Int. 2014, 114, 168–176. [Google Scholar] [CrossRef]
- Karan, D.; Dubey, S. From Inflammation to Prostate Cancer: The Role of Inflammasomes. Adv. Urol. 2016, 2016, 3140372. [Google Scholar] [CrossRef]
- Sethi, S.; Macoska, J.; Chen, W.; Sarkar, F.H. Molecular Signature of Epithelial-Mesenchymal Transition (EMT) in Human Prostate Cancer Bone Metastasis. Am. J. Transl. Res. 2010, 3, 90–99. [Google Scholar]
- Stikbakke, E.; Richardsen, E.; Knutsen, T.; Wilsgaard, T.; Giovannucci, E.L.; McTiernan, A.; Eggen, A.E.; Haugnes, H.S.; Thune, I. Inflammatory Serum Markers and Risk and Severity of Prostate Cancer: The PROCA-Life Study. Int. J. Cancer 2020, 147, 84–92. [Google Scholar] [CrossRef]
- Di Minno, A.; Aveta, A.; Gelzo, M.; Tripodi, L.; Pandolfo, S.D.; Crocetto, F.; Imbimbo, C.; Castaldo, G. 8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to Assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP). J. Clin. Med. 2022, 11, 6102. [Google Scholar] [CrossRef]
- Schillaci, O.; Scimeca, M.; Trivigno, D.; Chiaravalloti, A.; Facchetti, S.; Anemona, L.; Bonfiglio, R.; Santeusanio, G.; Tancredi, V.; Bonanno, E.; et al. Prostate Cancer and Inflammation: A New Molecular Imaging Challenge in the Era of Personalized Medicine. Nucl. Med. Biol. 2019, 68, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Ding, Y.; Xu, N. A Double-Edged Sword Role of Cytokines in Prostate Cancer Immunotherapy. Front. Oncol. 2021, 11, 688489. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, L.; Lin, H.-K.; Kan, P.-Y.; Xie, S.; Tsai, M.-Y.; Wang, P.-H.; Chen, Y.-T.; Chang, C. Interleukin-6 Differentially Regulates Androgen Receptor Transactivation via PI3K-Akt, STAT3, and MAPK, Three Distinct Signal Pathways in Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2003, 305, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Response to Androgens and Androgen Receptor Antagonists in the Presence of Cytokines in Prostate Cancer. Cancers 2021, 13, 2944. [Google Scholar] [CrossRef]
- Okamoto, M.; Lee, C.; Oyasu, R. Interleukin-6 as a Paracrine and Autocrine Growth Factor in Human Prostatic Carcinoma Cells in Vitro. Cancer Res. 1997, 57, 141–146. [Google Scholar]
- Natani, S.; Sruthi, K.K.; Asha, S.M.; Khilar, P.; Lakshmi, P.S.V.; Ummanni, R. Activation of TGF-β—SMAD2 Signaling by IL-6 Drives Neuroendocrine Differentiation of Prostate Cancer through P38MAPK. Cell. Signal. 2022, 91, 110240. [Google Scholar] [CrossRef]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.; Wheeler, T.M.; Slawin, K.M. Plasma Levels of Interleukin-6 and Its Soluble Receptor Are Associated with Prostate Cancer Progression and Metastasis. Urology 2001, 58, 1008–1015. [Google Scholar] [CrossRef]
- Merkens, L.; Sailer, V.; Lessel, D.; Janzen, E.; Greimeier, S.; Kirfel, J.; Perner, S.; Pantel, K.; Werner, S.; von Amsberg, G. Aggressive Variants of Prostate Cancer: Underlying Mechanisms of Neuroendocrine Transdifferentiation. J. Exp. Clin. Cancer Res. 2022, 41, 46. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Cui, Y.; Nadiminty, N.; Lou, W.; Gao, A.C. Interleukin-6 Induces Neuroendocrine Differentiation (NED) through Suppression of RE-1 Silencing Transcription Factor (REST). Prostate 2014, 74, 1086–1094. [Google Scholar] [CrossRef]
- de Kouchkovsky, I.; Chan, E.; Schloss, C.; Poehlein, C.; Aggarwal, R. Diagnosis and Management of Neuroendocrine Prostate Cancer. Prostate 2024, 84, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, C.; Cancila, V.; Ferri, R.; Sulsenti, R.; Fischetti, I.; Milani, M.; Ostano, P.; Gregnanin, I.; Mello-Grand, M.; Berrino, E.; et al. Castration-Induced Downregulation of SPARC in Stromal Cells Drives Neuroendocrine Differentiation of Prostate Cancer. Cancer Res. 2021, 81, 4257–4274. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Frierson, H.F.; Sanchez-Carbayo, M.; Brekken, R.A.; Theodorescu, D. Loss of SPARC in Bladder Cancer Enhances Carcinogenesis and Progression. J. Clin. Investig. 2013, 123, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a New Cytokine That Induces IFN-Gamma Production by T Cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cheon, S.; Cho, D. The Dual Effects of Interleukin-18 in Tumor Progression. Cell. Mol. Immunol. 2007, 4, 329–335. [Google Scholar]
- Yao, Z.; Zhao, M.; Gao, G.; Yang, J.; Wang, Z.; Liu, Y. Prognostic Role of IL-18 in Various Human Cancers and Radiation Injuries: A Meta-Analysis. Dose Response 2020, 18, 1559325820931360. [Google Scholar] [CrossRef]
- Maynard, J.P.; Ertunc, O.; Kulac, I.; Baena-Del Valle, J.A.; De Marzo, A.M.; Sfanos, K.S. IL8 Expression Is Associated with Prostate Cancer Aggressiveness and Androgen Receptor Loss in Primary and Metastatic Prostate Cancer. Mol. Cancer Res. 2020, 18, 153–165. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Zhang, F.; Lee, J.; Lu, S.; Pettaway, C.A.; Dong, Z. Blockade of Transforming Growth Factor-Beta Signaling Suppresses Progression of Androgen-Independent Human Prostate Cancer in Nude Mice. Clin. Cancer Res. 2005, 11, 4512–4520. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, B.; Chen, C.-K.; Mahajan, S.D.; Reynolds, J.L.; Zhang, H.; Sun, H.; Sykes, D.E.; Chadha, K.C.; Turowski, S.G.; et al. Nanotherapy Silencing the Interleukin-8 Gene Produces Regression of Prostate Cancer by Inhibition of Angiogenesis. Immunology 2016, 148, 387–406. [Google Scholar] [CrossRef]
- MacManus, C.F.; Pettigrew, J.; Seaton, A.; Wilson, C.; Maxwell, P.J.; Berlingeri, S.; Purcell, C.; McGurk, M.; Johnston, P.G.; Waugh, D.J.J. Interleukin-8 Signaling Promotes Translational Regulation of Cyclin D in Androgen-Independent Prostate Cancer Cells. Mol. Cancer Res. 2007, 5, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zang, Y.; Lv, L.; Cai, F.; Qian, T.; Zhang, G.; Feng, Q. IL-8 Promotes Proliferation and Inhibition of Apoptosis via STAT3/AKT/NF-κB Pathway in Prostate Cancer. Mol. Med. Rep. 2017, 16, 9035–9042. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gray, K.P.; Harshman, L.C.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated IL-8, TNF-α, and MCP-1 in Men with Metastatic Prostate Cancer Starting Androgen-Deprivation Therapy (ADT) Are Associated with Shorter Time to Castration-Resistance and Overall Survival. Prostate 2014, 74, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Scullin, P.; Maxwell, P.J.; Wilson, C.; Pettigrew, J.; Gallagher, R.; O’Sullivan, J.M.; Johnston, P.G.; Waugh, D.J.J. Interleukin-8 Signaling Promotes Androgen-Independent Proliferation of Prostate Cancer Cells via Induction of Androgen Receptor Expression and Activation. Carcinogenesis 2008, 29, 1148–1156. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Dwivedi, S.; Goel, A.; Natu, S.M.; Mandhani, A.; Khattri, S.; Pant, K.K. Diagnostic and Prognostic Significance of Prostate Specific Antigen and Serum Interleukin 18 and 10 in Patients with Locally Advanced Prostate Cancer: A Prospective Study. Asian Pac. J. Cancer Prev. 2011, 12, 1843–1848. [Google Scholar]
- Rasmussen, M.; Fredsøe, J.; Salachan, P.V.; Blanke, M.P.L.; Larsen, S.H.; Ulhøi, B.P.; Jensen, J.B.; Borre, M.; Sørensen, K.D. Stroma-Specific Gene Expression Signature Identifies Prostate Cancer Subtype with High Recurrence Risk. Npj Precis. Oncol. 2024, 8, 48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).