Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies
Abstract
Simple Summary
Abstract
1. Introduction
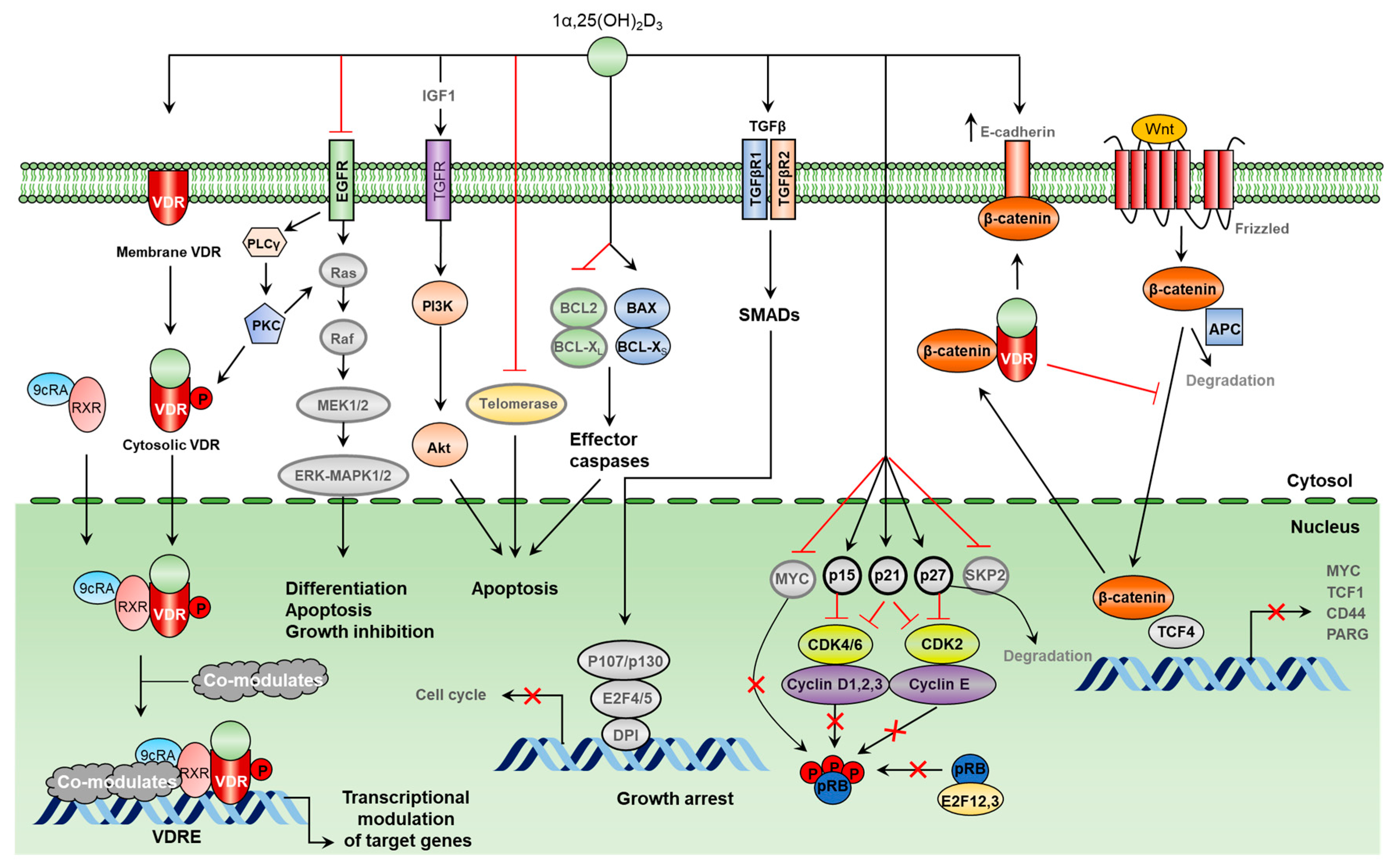
1.1. VDR Structure and Function: A Short Overview
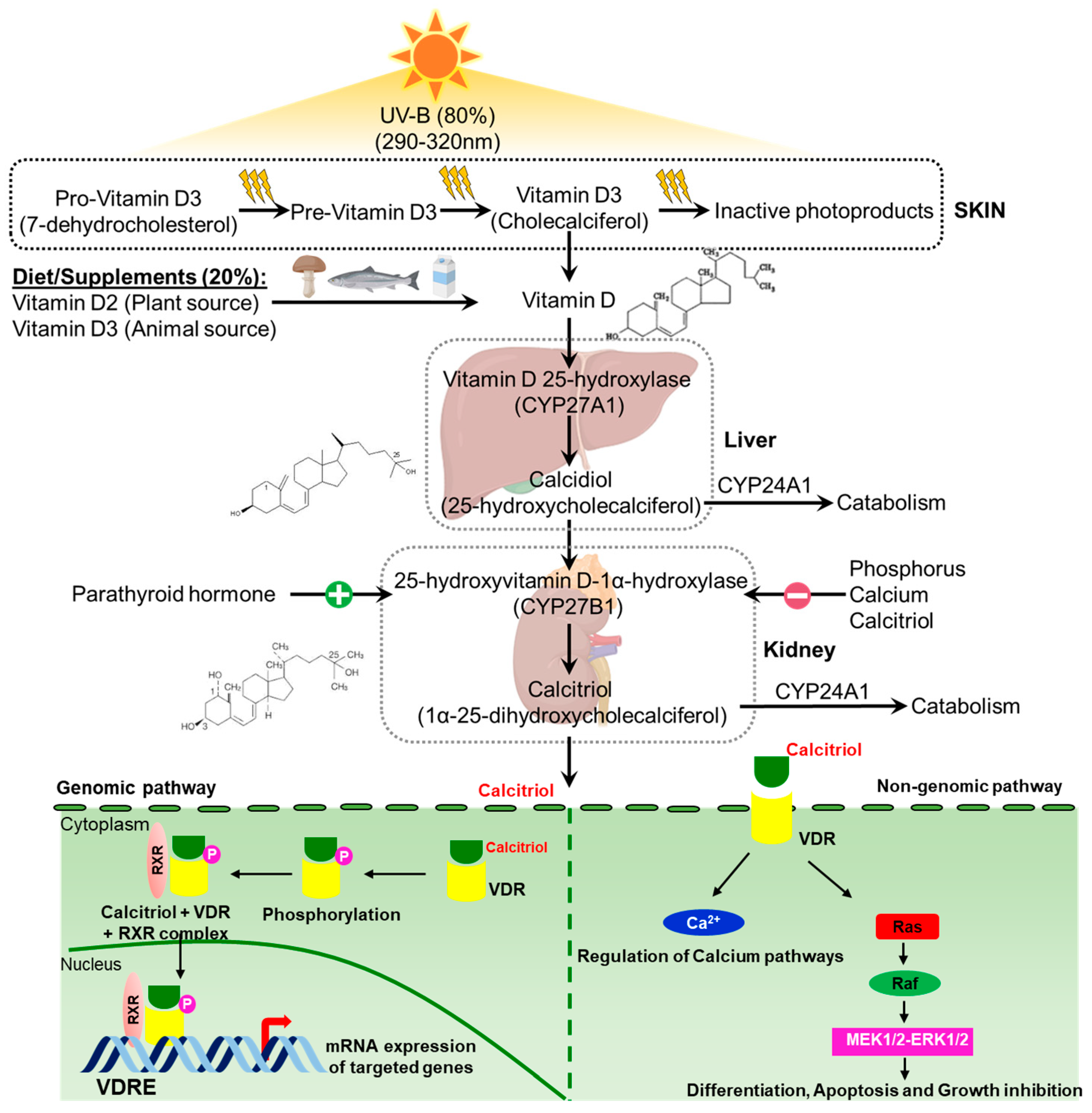
1.2. Vitamin D Metabolism and Mechanism: Short Overview
1.3. Is Vitamin D a Good Cancer Prevention Agent?
Vitamin D Supplementation and Cancer
1.4. Darker Side of the Sunshine Vitamin
1.5. A Promising Potential of Vitamin D Supplement: Anticancer Effects
1.6. Cancer Risk Reduction by Vitamin D Metabolite Calcitriol
2. Vitamin D Analogs for Cancer Treatment
2.1. EB-1089 (Seocalcitol)
2.2. HY-11
2.3. Tacalcitol
2.4. Inecalcitol
2.5. TX527
2.6. Paricalcitol
2.7. Doxercalciferol
2.8. Maxacalcitol
2.9. Calcipotriol
2.10. BGP-13
2.11. PRI-2205
2.12. PRI-1906
2.13. BXL-01-0126
2.14. BXL0124
2.15. Gemini0097
2.16. MART-10
2.17. 1,25-Dihydroxyvitamin D3-3-Bromoacetate
2.18. Ro26-2198
2.19. EM1
3. Molecular Mechanisms of Tumor Growth Inhibition by Vitamin D
3.1. Molecular Mechanisms of Vitamin D-Induced Apoptosis
3.2. Antiproliferative Mechanisms of Vitamin D
3.3. Vitamin D Inhibits Key Events in the Metastatic Spread of Cancer Cells
3.4. Vitamin D Induces Cancer Cell Differentiation
3.5. Vitamin D Inhibits Angiogenesis and Constrains Tumor Growth
4. VDR Gene Polymorphism in Cancers
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF2 | Activation function 2 |
| Akt | Protein kinase B |
| AML | Acute myeloid leukemia |
| AOM | Azoxymethane |
| AR | Androgen receptor |
| Bax | Bcl-2 Associated X-protein |
| BCC | Basal cell carcinoma |
| Bcl-2 | B-cell lymphoma 2 |
| C/EBP | CCAAT/enhancer binding protein |
| CAM | Chick chorioallantoic membrane |
| CD44 | Cluster of differentiation 44 |
| CDKs | Cyclin-dependent kinases |
| CKII | Casein kinase II |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| EMT | Epithelial-mesenchymal transition |
| EP2 | Prostaglandin E2 receptor |
| ERα | Estrogen receptor alpha |
| ERK | Extracellular signal-regulated kinase |
| ESRRα | Estrogen-related receptor alpha |
| ESRRγ | Estrogen-related receptor gamma |
| FAK | Focal adhesion kinase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GnRH | Gonadotropin-releasing hormone |
| GPCR | G protein-coupled receptor |
| HCC | Hepatocellular carcinoma |
| HER | Human epidermal growth factor receptor |
| hTERT | Human telomerase reverse transcriptase |
| HUVECs | Human umbilical vein endothelial cells |
| IGFBP3 | Insulin-like growth factor binding protein 3 |
| IL | Interleukin |
| IU | International units |
| JNK | c-Jun N-terminal kinase |
| KPS | Karnofsky Performance Scale |
| MAPK | Mitogen-activated protein kinase |
| MARRS | Membrane-associated, rapid response steroid-binding |
| MEK | Mitogen-activated protein kinase kinase |
| MMP | Matrix metalloproteinase |
| Myc | Myelocytomatosis |
| NCoA62–SKIP | Nuclear coactivator-62 kDa/Ski-interacting protein |
| NF-κB | Nuclear factor kappa B |
| NMU | N-methyl-N-nitrosourea |
| PARG | Poly(ADP-ribose) glycohydrolase |
| PGDH | 15-Hydroxyprostaglandin dehydrogenase |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| PTH | Parathyroid hormone |
| RCC | Renal cell carcinoma |
| RCTs | Randomized controlled trials |
| RDA | Recommended dietary allowance |
| ROS | Reactive oxygen species |
| RXR | Retinoic acid receptor |
| SCC | Squamous cell carcinoma |
| SMRT | Silencing mediator for retinoid and thyroid hormone receptors |
| SNAI1 | Snail family transcriptional repressor-1 |
| SNP | Single nucleotide polymorphism |
| SRCs | Steroid receptor coactivators |
| SVEC-vGPCR | Sarcoma-associated herpesvirus GPCR-transformed endothelial cells |
| Tcf-1 | T-cell factor 1 |
| TDECs | Tumor-derived endothelial cells |
| TGFβ | Transforming growth factor-β |
| TNBC | Triple-negative breast cancer |
| VDBP | Vitamin D-binding protein |
| VDR | Vitamin D receptor |
| VDRE | Vitamin D-response elements |
| VEGF | Vascular endothelial growth factor |
| ZEB1 | Zinc finger E-box binding homeobox-1 |
References
- Stamm, E.; Acchini, A.; Da Costa, A.; Besse, S.; Christou, F.; Launay, C.; Balmer, P.; Hamart, M.; Nguyun, S.; Major, K.; et al. Year in review: Geriatrics. Rev. Medicale Suisse 2019, 15, 50–52. [Google Scholar] [CrossRef]
- Tang, H.; Li, D.; Li, Y.; Zhang, X.; Song, Y.; Li, X. Effects of vitamin D supplementation on glucose and insulin homeostasis and incident diabetes among nondiabetic adults: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2018, 2018, 7908764. [Google Scholar] [CrossRef]
- Fink, C.; Peters, R.L.; Koplin, J.J.; Brown, J.; Allen, K.J. Factors affecting vitamin D status in infants. Children 2019, 6, 7. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef]
- Gupta, D.; Vashi, P.G.; Trukova, K.; Lis, C.G.; Lammersfeld, C.A. Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Exp. Ther. Med. 2011, 2, 181–193. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: A meta-analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef]
- Amiri, M. Vitamin D and cancer; a contradictory problem. Immunopathol. Persa 2018, 4, e13. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Kim, T.-K.; Tieu, E.W.; Tang, E.K.; Lin, Z.; Kovacic, D.; Miller, D.D.; Postlethwaite, A.; Tuckey, C.R.; et al. Novel vitamin D analogues as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014, 34, 2153–2163. [Google Scholar]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Shimizu, Y.; Minoshima, S.; Shimizu, N.; Haussler, C.A.; Jurutka, P.W.; Haussler, M.R. Novel nuclear localization signal between the two DNA-binding zinc fingers in the human vitamin D receptor. J. Cell. Biochem. 1998, 70, 94–109. [Google Scholar] [CrossRef]
- Michigami, T.; Suga, A.; Yamazaki, M.; Shimizu, C.; Cai, G.; Okada, S.; Ozono, K. Identification of amino acid sequence in the hinge region of human vitamin D receptor that transfers a cytosolic protein to the nucleus. J. Biol. Chem. 1999, 274, 33531–33538. [Google Scholar] [CrossRef]
- Yasmin, R.; Williams, R.M.; Xu, M.; Noy, N. Nuclear import of the retinoid X receptor, the vitamin D receptor, and their mutual heterodimer. J. Biol. Chem. 2005, 280, 40152–40160. [Google Scholar] [CrossRef]
- Uthaiah, C.A.; Beeraka, N.M.; Rajalakshmi, R.; Ramya, C.M.; Madhunapantula, S.V. Role of Neural Stem Cells and Vitamin D Receptor (VDR)-Mediated Cellular Signaling in the Mitigation of Neurological Diseases. Mol. Neurobiol. 2022, 59, 4065–4105. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Jurutka, P.W.; Galligan, M.A.; Terpening, C.M.; Haussler, C.A.; Samuels, D.S.; Shimizu, Y.; Haussler, M.R. Human vitamin D receptor is selectively phosphorylated by protein kinase C on serine 51, a residue crucial to its trans-activation function. Proc. Natl. Acad. Sci. USA 1991, 88, 9315–9319. [Google Scholar] [CrossRef]
- Jurutka, P.; Hsieh, J.C.; MacDonald, P.N.; Terpening, C.M.; Haussler, C.; Haussler, M.R.; Whitefield, G.K. Phosphorylation of serine 208 in the human vitamin D receptor. The predominant amino acid phosphorylated by casein kinase II, in vitro, and identification as a significant phosphorylation site in intact cells. J. Biol. Chem. 1993, 268, 6791–6799. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 186–199. [Google Scholar] [CrossRef]
- Takeyama, K.-I.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science 1997, 277, 1827–1830. [Google Scholar] [CrossRef]
- Brenza, H.L.; DeLuca, H.F. Regulation of 25-hydroxyvitamin D3 1α-hydroxylase gene expression by parathyroid hormone and 1, 25-dihydroxyvitamin D3. Archives of Biochemistry and Biophysics. Arch. Biochem. Biophys. 2000, 381, 143–152. [Google Scholar] [CrossRef]
- Hewison, M.; Zehnder, D.; Bland, R.; Stewart, P. 1alpha-Hydroxylase the action of vitamin, D. J. Mol. Endocrinol. 2000, 25, 141–148. [Google Scholar] [CrossRef]
- Murayama, A.; Takeyama, K.-I.; Kitanaka, S.; Kodera, Y.; Hosoya, T.; Kato, S. The promoter of the human 25-hydroxyvitamin D31α-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1α, 25 (OH) 2D3. Biochemical and biophysical research communications. Biochem. Biophys. Res. Commun. 1998, 249, 11–16. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α, 25 (OH) 2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurukta, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Sannappa Gowda, N.G.; Shiragannavar, V.D.; Puttahanumantharayappa, L.D.; Shivakumar, A.T.; Dallavalasa, S.; Basavaraju, C.G.; Bhat, S.S.; Prasad, S.K.; Vamadevaiah, R.M.; Madhunapantula, S.V.; et al. Quercetin activates vitamin D receptor and ameliorates breast cancer induced hepatic inflammation and fibrosis. Front. Nutr. 2023, 10, 1158633. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. Fundamentals of vitamin D hormone-regulated gene expression. J. Steroid Biochem. Mol. Biol. 2014, 144, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Thadhani, R.; Slatopolsky, E. (Eds.) Vitamin D receptor and analogues. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1, 25D3-membrane-associated rapid response, steroid (1, 25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Di Majo, D.; La Guardia, M.; Aiello, S.; Crescimannno, M.; Flandina, C.; Tumminello, F.M.; Leto, G. Vitamin D in cancer chemoprevention. Pharm. Biol. 2015, 53, 1399–1434. [Google Scholar] [CrossRef]
- Zeeb, H. Vitamin D and cancer prevention. Curr. Nutr. Rep. 2012, 1, 24–29. [Google Scholar] [CrossRef][Green Version]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients 2023, 15, 2722. [Google Scholar] [CrossRef]
- Sha, S.; Chen, L.J.; Brenner, H.; Schottker, B. Associations of 25-hydroxyvitamin D status and vitamin D supplementation use with mortality due to 18 frequent cancer types in the UK Biobank cohort. Eur. J. Cancer 2023, 191, 113241. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhu, H.; Dai, Z. 25-hydroxyvitamin D concentration is positively associated with overall survival in advanced pancreatic cancer: A systematic review and meta-analysis. Nutr. Res. 2023, 117, 73–82. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, Y.; Cho, Y.; Chang, J.; Kim, K.; Park, D.I.; Park, S.K.; Joh, H.K.; Kim, M.K.; Kim, C.; et al. Serum 25-Hydroxyvitamin D Levels and Risk of Colorectal Cancer: An Age-Stratified Analysis. Gastroenterology 2023, 165, 920–931. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, L.; Deng, X.Q.; Fang, Y.J.; Zhang, C.X. Association of DNA methylation of vitamin D metabolic pathway related genes with colorectal cancer risk. Clin. Epigenetics 2023, 15, 140. [Google Scholar] [CrossRef]
- Chen, X.; Song, S.; Shi, J.; Wang, Z.; Song, W.; Wang, J.; Wang, G.; Wang, X. Evaluating the effect of body mass index and 25-hydroxy-vitamin D level on basal cell carcinoma using Mendelian randomization. Sci. Rep. 2023, 13, 16552. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Izurieta-Pacheco, A.C.; Sangros-Gimenez, A.; Martinez-Garcia, E.; Perez-Jaume, S.; Mora, J.; Gorostegui-Obanos, M. Vitamin D Status in Children with High-risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2023, 45, e953–e958. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.T.; Waterhouse, M.; Pham, H.; Duarte Romero, B.; Baxter, C.; McLeod, D.S.A.; English, D.R.; Ebeling, E.R.; Hartel, G.; Armstrong, B.K.; et al. Effects of Vitamin D Supplementation on Telomere Length: An Analysis of Data from the Randomised Controlled D-Health Trial. J. Nutr. Health Aging 2023, 27, 609–616. [Google Scholar] [CrossRef]
- Manocha, A.; Brockton, N.T.; Cook, L.; Kopciuk, K.A. Low Serum Vitamin D Associated with Increased Tumor Size and Higher Grade in Premenopausal Canadian Women with Breast Cancer. Clin. Breast Cancer 2023, 23, e368–e376. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional risk screening and assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef]
- Qadir, J.; Majid, S.; Khan, M.S.; Wani, M.D.; Naikoo, N.A. Vitamin D receptor gene variations and their haplotypic association: Possible impact on gastric cancer risk. J. Cancer Res. Ther. 2023, 19, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.A.; Fatima, M.; Shahid, A.; Bouaddi, O.; Elgenidy, A.; Rehman, A.U.; Kacimi, S.E.O.; Hasan, M.M.; Lee, K.Y. Vitamin D supplementation for the prevention of total cancer incidence and mortality: An updated systematic review and meta-analysis. Heliyon 2022, 8, e11290. [Google Scholar] [CrossRef]
- Woloszynska-Read, A.; Johnson, C.S.; Trump, D.L.; Endocrinology, R.C. Vitamin D and cancer: Clinical aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 605–615. [Google Scholar] [CrossRef]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef]
- Robsahm, T.E.; Tretli, S.; Torjesen, P.A.; Babigumira, R.; Schwartz, G.G. Serum 25-hydroxyvitamin D levels predict cancer survival: A prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin. Epidemiol. 2019, 11, 695–705. [Google Scholar] [CrossRef]
- Hernandez-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomas, N.; Fernandez de la Puente, M.; Babio, N.; Gonzalez, M.M.; Salvado, J.S. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliessan, A.H.; Jacquotte, A.Z.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Henn, M.; Martin-Gorgojo, V.; Martin-Moreno, J.M. Vitamin D in cancer prevention: Gaps in current knowledge and room for hope. Nutrients 2022, 14, 4512. [Google Scholar] [CrossRef]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Picrce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013, 139, 529–537. [Google Scholar] [CrossRef]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N.; Liu, Q.; Markward, N.J.; Montero, A.J.; Gluck, S.; Silva, O.; Ahn, E.R. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef]
- Yokosawa, E.B.; Arthur, A.E.; Rentschler, K.M.; Wolf, G.T.; Rozek, L.S.; Mondul, A.M.J.T.L. Vitamin D intake and survival and recurrence in head and neck cancer patients. Laryngoscope 2018, 128, E371–E376. [Google Scholar] [CrossRef]
- Mulpur, B.H.; Nabors, L.B.; Thompson, R.C.; Olson, J.J.; LaRocca, R.V.; Thompson, Z.; Egan, K.M. Complementary therapy and survival in glioblastoma. Neuro-Oncol. Pract. 2015, 2, 122–126. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lipsyc-Sharf, M.; Zong, X.; Wang, X.; Hur, J.; Song, M.; Wang, M.; Smith-Warner, S.A.; Fuchs, C.; Ogino, S.; et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 2021, 161, 1208–1217.e9. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am. J. Clin. Nutr. 2011, 94, 1144–1149. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnel, S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Zgaga, L. Heterogeneity of the effect of vitamin D supplementation in randomized controlled trials on cancer prevention. JAMA Netw. Open 2020, 3, e2027176. [Google Scholar] [CrossRef]
- Thacher, T.D.; Clarke, B.L. (Eds.) Vitamin D insufficiency. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordan, D.; Copeland, T.; Agistino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Johnson, K.C.; Kooperberg, C.; Pettinger, M.; Wactawski-Wende, J.; Rohan, T.; Rossouw, J.; Lane, D.; O’Sullivan, M.J.; Yasmeen, S. Calcium plus vitamin D supplementation and the risk of breast cancer. JNCI J. Natl. Cancer Inst. 2008, 100, 1581–1591. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.; Greenwood, D.; Manson, J.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hyppönen, E.; Kröger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mäntyselkä, P. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Garland, C.F.; French, C.B.; Baggerly, L.L.; Heaney, R.P. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011, 31, 607–611. [Google Scholar]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Mead, M.N. Benefits of sunlight: A bright spot for human health. Natl. Inst. Environ. Health Sci. 2008, 116, A160–A167. [Google Scholar] [CrossRef]
- Freedman, D.; Dosemeci, M.; McGlynn, K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: A composite death certificate based case-control study. Occup. Environ. Med. 2002, 59, 257–262. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Kohler, L.N.; Kunihiro, A.G.; Jurutka, P.W. Vitamin D and colorectal, breast, and prostate cancers: A review of the epidemiological evidence. J. Cancer 2016, 7, 232. [Google Scholar] [CrossRef]
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086. [Google Scholar] [CrossRef]
- Ahn, J.; Peters, U.; Albanes, D.; Purdue, M.P.; Abnet, C.C.; Chatterjee, N.; Horst, R.L.; Hollies, B.W.; Huang, W.; Shikany, J.M.; et al. Serum vitamin D concentration and prostate cancer risk: A nested case–control study. J. Natl. Cancer Inst. 2008, 100, 796–804. [Google Scholar] [CrossRef]
- Banach-Petrosky, W.; Ouyang, X.; Gao, H.; Nader, K.; Ji, Y.; Suh, N.; Dipaola, R.S.; Shen, C.A. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3. 1; Pten mutant mice. Clin. Cancer Res. 2006, 12, 5895–5901. [Google Scholar] [CrossRef]
- Chung, I.; Han, G.; Seshadri, M.; Gillard, B.M.; Yu, W.-D.; Foster, B.A.; Trump, D.L.; Johnson, C.S. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009, 69, 967–975. [Google Scholar] [CrossRef]
- Colston, K.; James, S.; Ofori-Kuragu, E.; Binderup, L.; Grant, A. Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br. J. Cancer 1997, 76, 1017–1020. [Google Scholar] [CrossRef]
- Getzenberg, R.H.; Light, B.W.; Lapco, P.E.; Konety, B.R.; Nangia, A.K.; Acierno, J.S.; Dhir, R.; Shurin, Z.; Day, R.S.; Trump, D.L. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology 1997, 50, 999–1006. [Google Scholar] [CrossRef]
- Jiang, F.; Bao, J.; Li, P.; Nicosia, S.V.; Bai, W. Induction of ovarian cancer cell apoptosis by 1, 25-dihydroxyvitamin D3 through the down-regulation of telomerase. J. Biol. Chem. 2004, 279, 53213–53221. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Peehl, D.M.; Feldman, D. Inhibition of prostate cancer growth by vitamin D: Regulation of target gene expression. J. Cell. Biochem. 2003, 88, 363–371. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, W.D.; Trump, D.L.; Johnson, C.S. 1,25D3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer 2010, 116, 3294–3303. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kawaura, A.; Kato, S.; Takeda, E.; Okano, T. 1α, 25-Dihydroxyvitamin D 3 is a preventive factor in the metastasis of lung cancer. Carcinogenesis 2005, 26, 429–440. [Google Scholar] [CrossRef]
- Pálmer, H.G.; Sánchez-Carbayo, M.; Ordóñez-Morán, P.; Larriba MaJs Cordón-Cardó, C.; Muñoz, A. Genetic signatures of differentiation induced by 1α, 25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003, 63, 7799–7806. [Google Scholar]
- Zhang, X.; Jiang, F.; Li, P.; Li, C.; Ma, Q.; Nicosia, S.V.; Bai, W. Growth suppression of ovarian cancer xenografts in nude mice by vitamin D analogue EB1089. Clin. Cancer Res. 2005, 11, 323–328. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Reitsma, P.; Rothberg, P.; Astrin, S.; Trial, J.; Bar-Shavit, Z.; Hall, A.; Teitelbaum, S.L.; Khan, A.J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature 1983, 306, 492–494. [Google Scholar] [CrossRef]
- Fleet, J.C.; Desmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef]
- Moreno, J.; Krishnan, A.V.; Swami, S.; Nonn, L.; Peehl, D.M.; Feldman, D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005, 65, 7917–7925. [Google Scholar] [CrossRef]
- Sherman, M.H.; Ruth, T.Y.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Dyke, T.V.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Williams, J.; Aggarwal, A.; Albertelli, M.A.; Horst, R.L.; Feldman, B.J.; Feldman, D. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr.-Relat. Cancer 2016, 23, 251. [Google Scholar] [CrossRef]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef]
- James, S.Y.; Mackay, A.G.; Colston, K.W. Effects of 1, 25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J. Steroid Biochem. Mol. Biol. 1996, 58, 395–401. [Google Scholar] [CrossRef]
- Bao, B.-Y.; Yao, J.; Lee, Y.-F. 1α, 25-dihydroxyvitamin D 3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis 2006, 27, 1883–1893. [Google Scholar] [CrossRef]
- Li, H.; Stampfer, M.J.; Hollis, J.B.W.; Mucci, L.A.; Gaziano, J.M.; Hunter, D.; Giovannucci, E.L.; Ma, J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007, 4, e103. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef]
- Pereira, F.; Larriba, M.J.; Munoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, R51–R71. [Google Scholar] [CrossRef]
- Martinez-Reza, I.; Diaz, L.; Barrera, D.; Segovia-Mendoza, M.; Pedraza-Sanchez, S.; Soca-Chafre, G.; Larrea, F.; Garcia-Becerra, R. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Proinflammatory Cytokines IL-1beta and TNF-alpha. J. Immunol. Res. 2019, 2019, 6384278. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: Importance of mammary CYP27B1 for treatment and prevention. J. Steroid Biochem. Mol. Biol. 2013, 136, 289–295. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Friedrich, M.; Diesing, D.; Cordes, T.; Fischer, D.; Becker, S.; Chen, T.C.; Flanagnan, J.N.; Tangpricha, V.; Gherson, I.; Holick, M.F.; et al. Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in normal and malignant breast tissue. Anticancer Res. 2006, 26, 2615–2620. [Google Scholar]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef]
- Tangpricha, V.; Flanagan, J.N.; Whitlatch, L.W.; Tseng, C.C.; Chen, T.C.; Holt, P.R.; Lipkin, M.S.; Holick, M.F. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet 2001, 357, 1673–1674. [Google Scholar] [CrossRef]
- Hewison, M.; Kantorovich, V.; Liker, H.R.; Van Herle, A.J.; Cohan, P.; Zehnder, D.; Adams, J.S. Vitamin D-mediated hypercalcemia in lymphoma: Evidence for hormone production by tumor-adjacent macrophages. J. Bone Min. Res. 2003, 18, 579–582. [Google Scholar] [CrossRef]
- Maestro, M.A.; Molnar, F.; Carlberg, C. Vitamin D and its synthetic analogues. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L. Verstuyf AJFip. Future Vitam. D Analog. 2014, 5, 122. [Google Scholar]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J.J.C.R.i.O.H. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef]
- Hansen, C.M.; Hamberg, K.; Binderup, E.; Binderup, L. Seocalcitol (EB 1089) A vitamin D analogue of anti-cancer potential. Background, design, synthesis, pre-clinical and clinical evaluation. Curr. Pharm. Des. 2000, 6, 803–828. [Google Scholar] [CrossRef]
- James, S.Y.; Mercer, E.; Brady, M.; Binderup, L.; Colston, K.W. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. Br. J. Pharmacol. 1998, 125, 953–962. [Google Scholar] [CrossRef]
- Flanagan, L.; Packman, K.; Juba, B.; O’Neill, S.; Tenniswood, M.; Welsh, J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J. Steroid Biochem. Mol. Biol. 2003, 84, 181–192. [Google Scholar] [CrossRef]
- Prudencio, J.; Akutsu, N.; Benlimame, N.; Wang, T.; Bastien, Y.; Lin, R.; Black, M.J.; Jamali, M.A.A.; White, J.H. Action of low calcemic 1α, 25-dihydroxyvitamin D3 analogue EB1089 in head and neck squamous cell carcinoma. J. Natl. Cancer Inst. 2001, 93, 745–753. [Google Scholar] [CrossRef]
- Kasiappan, R.; Sun, Y.; Lungchukiet, P.; Quarni, W.; Zhang, X.; Bai, W. Vitamin D suppresses leptin stimulation of cancer growth through microRNA. Cancer Res. 2014, 74, 6194–6204. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Gao, Y.; Xie, D.; Wei, D.; Cui, J.; Mishra, L.; Haung, S.; Zhang, Y.; Xie, K. Activation of Vitamin D Receptor Signaling Downregulates the Expression of Nuclear FOXM1 Protein and Suppresses Pancreatic Cancer Cell StemnessVDR–FOXM1 Signaling in Pancreatic Cancer. Clin. Cancer Res. 2015, 21, 844–853. [Google Scholar] [CrossRef]
- Sundaram, S.; Sea, A.; Feldman, S.; Strawbridge, R.; Hoopes, P.J.; Demidenko, E.; Binderup, L.; Gewirtz, D.A. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin. Cancer Res. 2003, 9, 2350–2356. [Google Scholar]
- Ghous, Z.; Akhter, J.; Pourgholami, M.H.; Morris, D.L. Inhibition of hepatocellular cancer by EB1089: In vitro and in vivo study. Anticancer Res. 2008, 28, 3757–3761. [Google Scholar]
- Yoon, J.S.; Kim, J.Y.; Park, H.K.; Kim, E.S.; Ahn, K.S.; Yoon, S.S.; Cho, C.G.; Kim, B.K.; Lee, Y.Y. Antileukemic effect of a synthetic vitamin D3 analog, HY-11, with low potential to cause hypercalcemia. Int. J. Oncol. 2008, 32, 387–396. [Google Scholar] [CrossRef]
- Milczarek, M.; Rossowska, J.; Klopotowska, D.; Stachowicz, M.; Kutner, A.; Wietrzyk, J. Tacalcitol increases the sensitivity of colorectal cancer cells to 5-fluorouracil by downregulating the thymidylate synthase. J. Steroid Biochem. Mol. Biol. 2019, 190, 139–151. [Google Scholar] [CrossRef]
- Verlinden, L.; Verstuyf, A.; Van Camp, M.; Marcelis, S.; Sabbe, K.; Zhao, X.-Y.; Clercq, P.D.; Vandewalle, M.; Bouillon, R. Two novel 14-Epi-analogues of 1, 25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 2673–2679. [Google Scholar]
- Okamoto, R.; Delansorne, R.; Wakimoto, N.; Doan, N.B.; Akagi, T.; Shen, M.; Ho, Q.H.; Said, J.W.; Koeffler, H.P. Inecalcitol, an analog of 1α, 25 (OH) 2D3, induces growth arrest of androgen-dependent prostate cancer cells. Int. J. Cancer 2012, 130, 2464–2473. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, W.-D.; Hidalgo, A.A.; Luo, W.; Delansorne, R.; Johnson, C.S.; Trump, D.L. Inecalcitol, an analog of 1, 25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle 2013, 12, 743–752. [Google Scholar] [CrossRef]
- Eelen, G.; Verlinden, L.; Rochel, N.; Claessens, F.; De Clercq, P.; Vandewalle, M.; Valentini, G.T.; Moras, D.; Bouillon, R.; Verstuyf, A. Superagonistic action of 14-epi-analogues of 1, 25-dihydroxyvitamin D explained by vitamin D receptor-coactivator interaction. Mol. Pharmacol. 2005, 67, 1566–1573. [Google Scholar] [CrossRef]
- González-Pardo, V.; Verstuyf, A.; Boland, R.; de Boland, A.R. Vitamin D analogue TX 527 down-regulates the NF-κB pathway and controls the proliferation of endothelial cells transformed by K aposi sarcoma herpesvirus. Br. J. Pharmacol. 2013, 169, 1635–1645. [Google Scholar] [CrossRef]
- Molnár, I.; Kute, T.; Willingham, M.C.; Schwartz, G.G.J.; Biology, M. 19-Nor-1α, 25-dihydroxyvitamin D2 (paricalcitol) exerts anticancer activity against HL-60 cells in vitro at clinically achievable concentrations. J. Steroid Biochem. Mol. Biol. 2004, 89, 539–543. [Google Scholar] [CrossRef]
- Bae, W.K.; Lee, J.H.; Park, M.S.; Ahn, J.S.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Chung, I.J. 19-nor-1α-25-Dihydroxyvitamin D2 (Paricalcitol) Induces Apoptosis in Gastric Cancer Cells. J. Cancer Prev. 2009, 14, 329–334. [Google Scholar]
- El-Shemi, A.G.; Refaat, B.; Kensara, O.A.; Mohamed, A.M.; Idris, S.; Ahmad, J.J.C.P.R. Paricalcitol enhances the chemopreventive efficacy of 5-fluorouracil on an intermediate-term model of azoxymethane-induced colorectal tumors in rats. Cancer Prev. Res. 2016, 9, 491–501. [Google Scholar] [CrossRef]
- Liu, Y.; Shin, D.-Y.; Oh, S.; Kim, S.; Koh, Y.; Kim, I. KML001 and doxercalciferol induce synergistic antileukemic effect in acute lymphoid leukemia cells. Oncol. Rep. 2017, 38, 481–487. [Google Scholar] [CrossRef][Green Version]
- Kawa, S.; Yoshizawa, K.; Nikaido, T.; Kiyosawa, K.J.T.J.o.S.B.; Biology, M. Inhibitory effect of 22-oxa-1, 25-dihydroxyvitamin D3, maxacalcitol, on the proliferation of pancreatic cancer cell lines. J. Steroid Biochem. Mol. Biol. 2005, 97, 173–177. [Google Scholar] [CrossRef]
- Arensman, M.D.; Nguyen, P.; Kershaw, K.M.; Lay, A.R.; Ostertag-Hill, C.A.; Sherman, M.H.; Downes, M.; Liddle, C.; Evans, R.M.; Dawson, D.W. Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic CancerCalcipotriol Targets LRP6. Mol. Cancer Res. 2015, 13, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Berkovich, L.; Ben-Shabat, S. Inhibition of cancer growth and induction of apoptosis by BGP-13 and BGP-15, new calcipotriene-derived vitamin D 3 analogues, in-vitro and in-vivo studies. Investig. New Drugs 2013, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Filip-Psurska, B.; Psurski, M.; Anisiewicz, A.; Libako, P.; Zbrojewicz, E.; Maciejewska, M.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Vitamin d compounds pri-2191 and pri-2205 enhance anastrozole activity in human breast cancer models. Int. J. Mol. Sci. 2021, 22, 2781. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Chodyński, M.; Filip-Psurska, B.; Martowicz, A.; Krupa, M.; Krajewski, K.; Kutner, A.; Wietrzyk, J. Synthesis and biological activity of diastereomeric and geometric analogues of calcipotriol, PRI-2202 and PRI-2205, against human HL-60 leukemia and MCF-7 breast cancer cells. Cancers 2013, 5, 1355–1378. [Google Scholar] [CrossRef]
- Piatek, K.; Kutner, A.; Cacsire Castillo-Tong, D.; Manhardt, T.; Kupper, N.; Nowak, U.; Chodynski, M.; Marcinkowska, E.; Kallay, E.; Schepelmann, M. Vitamin D analogues regulate the vitamin d system and cell viability in ovarian cancer cells. Int. J. Mol. Sci. 2021, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Gery, S.; Kuwayama, Y.; Borregaard, N.; Ho, Q.; Alvarez, R.; Akagi, T.; Liu, G.Y.; Uskokovic, M.R.; Koeffler, H.P. Novel Gemini vitamin D3 analogues: Large structure/function analysis and ability to induce antimicrobial peptide. Int. J. Cancer 2014, 134, 207–217. [Google Scholar] [CrossRef] [PubMed]
- So, J.Y.; Lee, H.J.; Smolarek, A.K.; Paul, S.; Wang, C.-X.; Maehr, H.; Uskovic, M.; Zheng, X.; Conney, A.H.; Cai, L.; et al. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol. Pharmacol. 2011, 79, 360–367. [Google Scholar] [CrossRef]
- So, J.Y.; Wahler, J.E.; Yoon, T.; Smolarek, A.K.; Lin, Y.; Shih, W.J.; Maehr, H.; Uskokovic, M.; Liby, K.T.; Sporn, M.B.; et al. Oral Administration of a Gemini Vitamin D Analog, a Synthetic Triterpenoid and the Combination Prevents Mammary Tumorigenesis Driven by ErbB2 OverexpressionInhibition of Mammary Tumorigenesis by Vitamin D Analog and Synthetic Triterpenoid. Cancer Prev. Res. 2013, 6, 959–970. [Google Scholar] [CrossRef]
- So, J.Y.; Smolarek, A.K.; Salerno, D.M.; Maehr, H.; Uskokovic, M.; Liu, F.; Suh, N. Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS ONE 2013, 8, e54020. [Google Scholar] [CrossRef]
- Wahler, J.; So, J.Y.; Kim, Y.C.; Liu, F.; Maehr, H.; Uskokovic, M.; Suh, N. Inhibition of the Transition of Ductal Carcinoma In Situ to Invasive Ductal Carcinoma by a Gemini Vitamin D AnalogInhibition of Breast Cancer Progression with a Vitamin D Analog. Cancer Prev. Res. 2014, 7, 617–626. [Google Scholar] [CrossRef]
- Huet, T.; Maehr, H.; Lee, H.J.; Uskokovic, M.R.; Suh, N.; Moras, D.; Rochel, N. Structure–function study of gemini derivatives with two different side chains at C-20, Gemini-0072 and Gemini-0097. MedChemComm 2011, 2, 424–429. [Google Scholar] [CrossRef]
- Lee, H.J.; Paul, S.; Atalla, N.; Thomas, P.E.; Lin, X.; Yang, I.; Buckley, B.; Lu, G.; Zheng, X.; Lou, Y.R.; et al. Gemini vitamin D analogues inhibit estrogen receptor–positive and estrogen receptor–negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev. Res. 2008, 1, 476–484. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Yeh, T.-S.; Chen, S.-C.; Pang, J.-H.S.; Yeh, C.-N.; Hsu, J.-T.; Chen, L.W.; Kuo, S.F.; Takano, M.; Kittaka, A.; et al. The vitamin D analog, MART-10, attenuates triple negative breast cancer cells metastatic potential. Int. J. Mol. Sci. 2016, 17, 606. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Eddy, V.J.; Young, C.D.; Persons, K.S.; Sarkar, S.; Kelly, J.A.; Genova, E.; Lucia, M.S.; Faller, D.V.; Ray, R. A vitamin D receptor-alkylating derivative of 1α, 25-dihydroxyvitamin D3 inhibits growth of human kidney cancer cells and suppresses tumor growth. Cancer Prev. Res. 2010, 3, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Fichera, A.; Little, N.; Dougherty, U.; Mustafi, R.; Cerda, S.; Li, Y.C.; Delgado, J.; Arora, A.; Campbell, L.K.; Joseph, L.; et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J. Surg. Res. 2007, 142, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, M.R.; Norman, A.W.; Manchand, P.S.; Studzinski, G.P.; Campbell, M.J.; Koeffler, H.P.; Takeuchi, A.; Siu-Caldera, M.L.; Rao, D.S.; Reddy, G.S. Highly active analogues of 1α, 25-dihydroxyvitamin D3 that resist metabolism through C-24 oxidation and C-3 epimerization pathways. Steroids 2001, 66, 463–471. [Google Scholar] [CrossRef]
- Salomón, D.G.; Grioli, S.M.; Buschiazzo, M.; Mascaró, E.; Vitale, C.; Radivoy, G.; Perez, M.; Fall, Y.; Mesri, E.A.; Curino, A.C.; et al. Novel alkynylphosphonate analogue of calcitriol with potent antiproliferative effects in cancer cells and lack of calcemic activity. ACS Med. Chem. Lett. 2011, 2, 503–508. [Google Scholar] [CrossRef]
- Ferronato, M.J.; Obiol, D.J.; Fermento, M.E.; Gandini, N.A.; Alonso, E.N.; Salomón, D.G.; Vitale, C.; Mascaro, E.; Fall, Y.; Raimondi, R.; et al. The alkynylphosphonate analogue of calcitriol EM1 has potent anti-metastatic effects in breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 154, 285–293. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Tosetti, F.; Ferrari, N.; De Flora, S.; Albini, A. ‘Angioprevention’: Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Guzey, M.; Kitada, S.; Reed, J.C. Apoptosis induction by 1α, 25-dihydroxyvitamin D3 in prostate cancer. Mol. Cancer Ther. 2002, 1, 667–677. [Google Scholar]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Welsh, J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J. Biol. Chem. 2001, 276, 9101–9107. [Google Scholar] [CrossRef] [PubMed]
- Veeresh, P.K.M.; Basavaraju, C.G.; Dallavalasa, S.; Anantharaju, P.G.; Natraj, S.M.; Sukocheva, O.A.; Madhunapantula, S.V. Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers 2023, 15, 4833. [Google Scholar] [CrossRef]
- Mathiasen, I.S.; Sergeev, I.N.; Bastholm, L.; Elling, F.; Norman, A.W.; Jäättelä, M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J. Biol. Chem. 2002, 277, 30738–30745. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Bastholm, L.; Mathiasen, I.; Elling, F.; Jäättelä, M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005, 12, 1297–1309. [Google Scholar] [CrossRef]
- Darío Díaz, G.; Paraskeva, C.; Thomas, M.; Binderup, L.; Hague, A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: Possible implications for prevention and therapy. Cancer Res. 2000, 60, 2304–2312. [Google Scholar]
- McGuire, T.F.; Trump, D.L.; Johnson, C.S. Vitamin D3-induced apoptosis of murine squamous cell carcinoma cells: Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J. Biol. Chem. 2001, 276, 26365–26373. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, K.A.; Johannes, W.U.; Hedlund, T.E.; Miller, G.J. Growth inhibitory effects of 1α, 25-dihydroxyvitamin D3 are mediated by increased levels of p21 in the prostatic carcinoma cell line ALVA-31. Cancer Res. 2001, 61, 7122–7129. [Google Scholar]
- Liu, M.; Lee, M.-H.; Cohen, M.; Bommakanti, M.; Freedman, L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996, 10, 142–153. [Google Scholar] [CrossRef]
- Saramäki, A.; Banwell, C.M.; Campbell, M.J.; Carlberg, C. Regulation of the human p21 (waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D 3 receptor. Nucleic Acids Res. 2006, 34, 543–554. [Google Scholar] [CrossRef]
- Kawa, S.; Nikaido, T.; Aoki, Y.; Zhai, Y.; Kumagai, T.; Furihata, K.; Fujii, S.; Kiyosawa, K. Vitamin D analogues up-regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. Br. J. Cancer 1997, 76, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.A.; Modzelewski, R.A.; Shurin, Z.R.; Rueger, R.M.; Trump, D.L.; Johnson, C.S. 1,25-Dihydroxycholecalciferol (1, 25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21Waf1/Cip1 in vitro and in vivo. Cancer Res. 1999, 59, 2644–2649. [Google Scholar]
- Jiang, F.; Li, P.; Fornace, A.J.; Nicosia, S.V.; Bai, W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J. Biol. Chem. 2003, 278, 48030–48040. [Google Scholar] [CrossRef]
- Bernardi, R.J.; Trump, D.L.; Yu, W.D.; McGuire, T.F.; Hershberger, P.A.; Johnson, C.S. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: Role of nuclear receptor cross-talk and Erk/Akt signaling. Clin. Cancer Res. 2001, 7, 4164–4173. [Google Scholar] [PubMed]
- Liu, W.; Asa, S.L.; Fantus, I.G.; Walfish, P.G.; Ezzat, S. Vitamin D arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through PTEN/akt-dependent and-independent pathways. Am. J. Pathol. 2002, 160, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kemmochi, S.; Fujimoto, H.; Woo, G.-H.; Hirose, M.; Nishikawa, A.; Mitsumori, K.; Shibutani, M. Preventive effects of calcitriol on the development of capsular invasive carcinomas in a rat two-stage thyroid carcinogenesis model. J. Vet. Med. Sci. 2011, 73, 655–664. [Google Scholar] [CrossRef]
- Gonzalez-Pardo, V.; D’Elia, N.; Verstuyf, A.; Boland, R.; de Boland, A.R. NFκB pathway is down-regulated by 1α, 25 (OH) 2-vitamin D3 in endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein coupled receptor. Steroids 2012, 77, 1025–1032. [Google Scholar] [CrossRef]
- Capiati, D.A.; Rossi, A.M.; Picotto, G.; Benassati, S.; Boland, R.L. Inhibition of serum-stimulated mitogen activated protein kinase by 1α, 25 (OH) 2-vitamin D3 in MCF-7 breast cancer cells. J. Cell. Biochem. 2004, 93, 384–397. [Google Scholar] [CrossRef]
- Colston, K.; Perks, C.; Xie, S.; Holly, J. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J. Mol. Endocrinol. 1998, 20, 157–162. [Google Scholar] [CrossRef]
- Koli, K.; Keski-Oja, J. 1,25-Dihydroxyvitamin D3 enhances the expression of transforming growth factor β1 and its latent form binding protein in cultured breast carcinoma cells. Cancer Res. 1995, 55, 1540–1546. [Google Scholar]
- Alvarez-Díaz, S.; Valle, N.; Ferrer-Mayorga, G.; Lombardía, L.; Herrera, M.; Domínguez, O.; Segura, M.F.; Bonilla, F.; Hernando, E.; Munoz, A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 2012, 21, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Liu, C.; Chen, Z.; Wang, L.; Li, C.; Zhao, J.; Yu, Y.; Zhang, P.; Chen, W.; Jiang, A. 1, 25-Dihydroxyvitamin D3 up-regulates expression of hsa-let-7a-2 through the interaction of VDR/VDRE in human lung cancer A549 cells. Gene 2013, 522, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Sporn, M.B. The war on cancer. Lancet 1996, 347, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Asa, S.L.; Ezzat, S. 1α, 25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol. Endocrinol. 2005, 19, 2349–2357. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, W.D.; Su, B.; Seshadri, M.; Luo, W.; Trump, D.L.; Johnson, C.S. Regulation of motility, invasion, and metastatic potential of squamous cell carcinoma by 1α, 25-dihydroxycholecalciferol. Cancer 2013, 119, 563–574. [Google Scholar] [CrossRef]
- Bao, B.-Y.; Yeh, S.-D.; Lee, Y.-F. 1α, 25-dihydroxyvitamin D 3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis 2005, 27, 32–42. [Google Scholar] [CrossRef]
- Hsu, J.-W.; Yasmin-Karim, S.; King, M.R.; Wojciechowski, J.C.; Mickelsen, D.; Blair, M.L.; Ting, H.J.; Ma, W.L.; Lee, Y.F. Suppression of prostate cancer cell rolling and adhesion to endothelium by 1α, 25-dihydroxyvitamin D3. Am. J. Pathol. 2011, 178, 872–880. [Google Scholar] [CrossRef]
- Pálmer, H.G.; González-Sancho, J.M.; Espada, J.; Berciano, M.T.; Puig, I.; Baulida, J.; Quintanilla, M.; Cano, A.; Herreros, A.G.; Lafarga, M.; et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 2001, 154, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Halpin, J.; Hussain, R.; Lozano, Y.; Djordjevic, A.; Devata, S.; Matthews, J.P.; Wright, M.A. Inhibition of tumor production of granulocyte-macrophage colony-stimulating factor by 1 alpha, 25-dihydroxyvitamin D3 reduces tumor motility and metastasis. Invasion Metastasis 1993, 13, 169–177. [Google Scholar]
- Young, M.R.I.; Lozano, Y. Inhibition of tumor invasiveness by 1-alpha-25-dihydroxy-vitamin D coupled to a decline in protein kinase A activity and an increase in cytoskeletal organization. Clin. Exp. Metastasis 1997, 15, 102–110. [Google Scholar] [CrossRef]
- Pendás-Franco, N.; García, J.M.; Peña, C.; Valle, N.; Pálmer, H.G.; Heinäniemi, M.; Carlberg, C.; Jimenez, B.; Bonilla, F.; Munoz, A.; et al. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α, 25-dihydroxyvitamin D3. Oncogene 2008, 27, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.-C.; Yeh, C.-N.; Hsu, J.-T.; Jan, Y.-Y.; Chen, L.-W.; Kuo, S.-F.; Takano, M.; Kittaka, A.; Chen, T.C.; Chen, W.T.; et al. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial–mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014, 354, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Rebsamen, M.C.; Sun, J.; Norman, A.W.; Liao, J.K. 1α, 25-Dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ. Res. 2002, 91, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Matsuno, H.; Kimura, T. 1α, 25-Dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J. Lab. Clin. Med. 1999, 133, 120–128. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sasaki, Y.; Kato, S.; Kubodera, N.; Okano, T. 22-Oxa-1α, 25-dihydroxyvitamin D 3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis 2005, 26, 1044–1054. [Google Scholar] [CrossRef]
- Bhatia, V.; Saini, M.K.; Shen, X.; Bi, L.X.; Qiu, S.; Weigel, N.L.; Falzon, M. EB1089 inhibits the parathyroid hormone–related protein–enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1787–1798. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Brackman, D.; Lund-Johansen, F.; Aarskog, D. Expression of cell surface antigens during the differentiation of HL-60 cells induced by 1, 25-ihydroxyvitamin D3, retinoic acid and DMSO. Leuk. Res. 1995, 19, 57–64. [Google Scholar] [CrossRef]
- Zakaria Hmama, D.N.; Sly, L.; Knutson, K.L.; Herrera-velit, P.; Reiner, N.E. 1,25-Dihydroxyvitamin D 3–induced Myeloid Cell Differentiation Is Regulated by a Vitamin D Receptor–Phosphatidylinositol 3-Kinase Signaling Complex. J. Exp. Med. 1999, 190, 1583–1594. [Google Scholar] [CrossRef]
- Wang, X.; Studzinski, G.P. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1, 25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J. Cell. Biochem. 2001, 80, 471–482. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Studzinski, G.P. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1, 25-dihydroxyvitamin D3. J. Cell. Biochem. 2003, 89, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Studzinski, G.P. Retinoblastoma protein and CCAAT/enhancer-binding protein β are required for 1, 25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004, 64, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, E.; Garay, E.; Gocek, E.; Chrobak, A.; Wang, X.; Studzinski, G.P. Regulation of C/EBPβ isoforms by MAPK pathways in HL60 cells induced to differentiate by 1, 25-dihydroxyvitamin D3. Exp. Cell Res. 2006, 312, 2054–2065. [Google Scholar] [CrossRef]
- Chen, A.; Davis, B.H.; Bissonnette, M.; Scaglione-Sewell, B.; Brasitus, T.A. 1,25-Dihydroxyvitamin D3 stimulates activator protein-1-dependent Caco-2 cell differentiation. J. Biol. Chem. 1999, 274, 35505–35513. [Google Scholar] [CrossRef]
- Larriba, M.J.; Garcia de Herreros, A.; Munoz, A. Vitamin D and the Epithelial to Mesenchymal Transition. Stem Cells Int. 2016, 2016, 6213872. [Google Scholar] [CrossRef] [PubMed]
- Pendas-Franco, N.; Gonzalez-Sancho, J.M.; Suarez, Y.; Aguilera, O.; Steinmeyer, A.; Gamallo, C.; Berciano, M.T.; Lafarga, M.; Monoz, A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation 2007, 75, 193–207. [Google Scholar] [CrossRef]
- Lazzaro, G.; Agadir, A.; Qing, W.; Poria, M.; Mehta, R.; Moriarty, R.; Gupta, T.K.D.; Zhang, X.K.; Mehta, R.G. Induction of differentiation by 1α-hydroxyvitamin D5 in T47D human breast cancer cells and its interaction with vitamin D receptors. Eur. J. Cancer 2000, 36, 780–786. [Google Scholar] [CrossRef]
- Wang, Q.; Lee, D.; Sysounthone, V.; Chandraratna, R.A.; Christakos, S.; Korah, R.; Wieder, R. 1, 25-dihydroxyvitamin D3 and retonic acid analogues induce differentiation in breast cancer cells with function-and cell-specific additive effects. Breast Cancer Res. Treat. 2001, 67, 157–168. [Google Scholar] [CrossRef]
- Haselberger, M.; Springwald, A.; Konwisorz, A.; Lattrich, C.; Goerse, R.; Ortmann, O.; Treeck, O. Silencing of the icb-1 gene inhibits the induction of differentiation-associated genes by vitamin D3 and all-trans retinoic acid in gynecological cancer cells. Int. J. Mol. Med. 2011, 28, 121–127. [Google Scholar]
- Beer, T.M.; Garzotto, M.; Park, B.; Mori, M.; Myrthue, A.; Janeba, N.; Sauer, D.; Eilers, K. Effect of calcitriol on prostate-specific antigen in vitro and in humans. Clin. Cancer Res. 2006, 12, 2812–2816. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Folkman, J. (Ed.) Role of angiogenesis in tumor growth and metastasis. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Bernardi, R.J.; Johnson, C.S.; Modzelewski, R.A.; Trump, D.L. Antiproliferative effects of 1α, 25-dihydroxyvitamin D3 and vitamin D analogues on tumor-derived endothelial cells. Endocrinology 2002, 143, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Merke, J.; Milde, P.; Lewicka, S.; Hügel, U.; Klaus, G.; Mangelsdorf, D.; Haussler, M.R.; Rauterburg, E.W.; Ritz, E. Identification and regulation of 1, 25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1, 25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J. Clin. Investig. 1989, 83, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Wong, M.K.; Flynn, G.; Yu, W.-D.; Johnson, C.S.; Trump, D.L. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res. 2006, 66, 8565–8573. [Google Scholar] [CrossRef]
- Gonzalez-Pardo, V.; Martin, D.; Gutkind, J.S.; Verstuyf, A.; Bouillon, R.; de Boland, A.R.; Boland, R.L. 1α, 25-dihydroxyvitamin D3 and its TX527 analog inhibit the growth of endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein-coupled receptor in vitro and in vivo. Endocrinology 2010, 151, 23–31. [Google Scholar] [CrossRef]
- Mantell, D.; Owens, P.; Bundred, N.; Mawer, E.; Canfield, A. 1α, 25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef]
- Oikawa, T.; Hirotani, K.; Ogasawara, H.; Katayama, T.; Nakamura, O.; Iwaguchi, T.; Hiragun, A. Inhibition of angiogenesis by vitamin D3 analogues. Eur. J. Pharmacol. 1990, 178, 247–250. [Google Scholar] [CrossRef]
- Suzuki, T.; Sano, Y.; Kinoshita, S. Effects of 1α, 25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 154–158. [Google Scholar]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Chana, R.S.; Wheeler, D.C.; Howie, A.J.; Williams, M.C.; Stewart, P.M.; Hewison, M. Synthesis of 1, 25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002, 13, 621–629. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1, 25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting MicroRNA-155–SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Eisman, J.A.; Barkla, D.H.; Tutton, P.J. Suppression of in vivo growth of human cancer solid tumor xenografts by 1, 25-dihydroxyvitamin D3. Cancer Res. 1987, 47, 21–25. [Google Scholar] [PubMed]
- Evans, S.R.; Houghton, A.M.; Schumaker, L.; Brenner, R.V.; Buras, R.R.; Davoodi, F.; Nauta, R.J.; Shabahang, M. Vitamin D Receptor and Growth Inhibition by 1, 25-Dihydroxyvitamin D3in Human Malignant Melanoma Cell Lines. J. Surg. Res. 1996, 61, 127–133. [Google Scholar] [CrossRef]
- Fu, B.; Wang, H.; Wang, J.; Barouhas, I.; Liu, W.; Shuboy, A.; Bushinsky, D.A.; Zhou, D.; Favus, M.J. Epigenetic regulation of BMP2 by 1, 25-dihydroxyvitamin D3 through DNA methylation and histone modification. PLoS ONE 2013, 8, e61423. [Google Scholar] [CrossRef]
- Hansen, C.M.; Madsen, M.W.; Arensbak, B.; Skak-Nielsen, T.; Latini, S.; Binderup, L. Down-regulation of Laminin-Binding Integrins by 1α, 25= Dihydroxyvitamin D3 in Human Melanoma Cells in Vitro. Cell Adhes. Commun. 1998, 5, 109–120. [Google Scholar] [CrossRef]
- Newton-Bishop, J.A.; Beswick, S.; Randerson-Moor, J.; Chang, Y.-M.; Affleck, P.; Elliott, F.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J. Clin. Oncol. 2009, 27, 5439. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.; Hutchinson, P. Vitamin D and systemic cancer: Is this relevant to malignant melanoma? Br. J. Dermatol. 2002, 147, 197–213. [Google Scholar] [CrossRef]
- Elzehery, R.R.; Baiomy, A.A.; Hegazy, M.A.-F.; Fares, R.; El-Gilany, A.-H.; Hegazi, R. Vitamin D status, receptor gene BsmI (A/G) polymorphism and breast cancer in a group of Egyptian females. Egypt. J. Med. Hum. Genet. 2017, 18, 269–273. [Google Scholar] [CrossRef]
- Zhang, K.; Song, L. Association between vitamin D receptor gene polymorphisms and breast cancer risk: A meta-analysis of 39 studies. PLoS ONE 2014, 9, e96125. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.-P.; Chang, S.-C.; Linet, M.S.; Sigurdson, A.J. Sunlight, polymorphisms of vitamin D-related genes and risk of breast cancer. Anticancer Res. 2013, 33, 543–551. [Google Scholar]
- Rai, V.; Abdo, J.; Agrawal, S.; Agrawal, D.K. Vitamin D receptor polymorphism and cancer: An update. Anticancer Res. 2017, 37, 3991–4003. [Google Scholar]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Vitamin D, cancer risk, and mortality. Adv. Food Nutr. Res. 2015, 75, 1–52. [Google Scholar] [PubMed]
- Raimondi, S.; Johansson, H.; Maisonneuve, P.; Gandini, S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009, 30, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, V.M.; Silva, T.D.; de Oliveira, J.; Pimenta, C.A.M.; Felipe, A.V.; Forones, N.M. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int. J. Biol. Markers 2017, 32, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Gnagnarella, P.; Raimondi, S.; Gandini, S. Meta-analysis on vitamin D receptor and cancer risk: Focus on the role of TaqI, ApaI, and Cdx2 polymorphisms. Eur. J. Cancer Prev. 2016, 25, 85. [Google Scholar] [CrossRef]
- Von Schuckmann, L.A.; Law, M.H.; Montgomery, G.W.; Green, A.C.; van der Pols, J.C. Vitamin D pathway gene polymorphisms and keratinocyte cancers: A nested case-control study and meta-analysis. Anticancer. Res. 2016, 36, 2145–2152. [Google Scholar]
- Wang, K.; Wu, G.; Li, J.; Song, W. Role of vitamin D receptor gene Cdx2 and Apa1 polymorphisms in prostate cancer susceptibility: A meta-analysis. BMC Cancer 2016, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhao, Y.; Liu, J.; Wang, L.; Zhao, G.; Chen, X.; Yao, A.; Zhang, L.; Zhang, X.; Li, X. Association of Vitamin D receptor Fok I polymorphism with the risk of prostate cancer: A meta-analysis. Oncotarget 2016, 7, 77878. [Google Scholar] [CrossRef]
- Mi, Y.-Y.; Chen, Y.-Z.; Chen, J.; Zhang, L.-F.; Zuo, L.; Zou, J.-G. Updated analysis of vitamin D receptor gene FokI polymorphism and prostate cancer susceptibility. Arch. Med. Sci. 2017, 13, 1449–1458. [Google Scholar] [CrossRef]
- Lu, D.; Jing, L.; Zhang, S. Vitamin D receptor polymorphism and breast cancer risk: A meta-analysis. Medicine 2016, 95, e3535. [Google Scholar] [CrossRef]
- Laczmanski, L.; Lwow, F.; Osina, A.; Kepska, M.; Laczmanska, I.; Witkiewicz, W. Association of the vitamin D receptor FokI gene polymorphism with sex-and non-sex-associated cancers: A meta-analysis. Tumor Biol. 2017, 39, 1010428317727164. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, H.; Cheng, W.; Zhang, H.; Pan, Z.; Wang, D. Association of VDR polymorphisms (Taq I and Bsm I) with prostate cancer: A new meta-analysis. J. Int. Med. Res. 2017, 45, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.u.N.; Khan, T.A. Association between vitamin D receptor (Cdx2, Fok1, Bsm1, Apa1, Bgl1, Taq1, and Poly (A)) gene polymorphism and breast cancer: A systematic review and meta-analysis. Tumor Biol. 2017, 39, 1010428317731280. [Google Scholar] [CrossRef]
- Sheng, S.; Chen, Y.; Shen, Z. Correlation between polymorphism of vitamin D receptor TaqI and susceptibility to colorectal cancer: A meta-analysis. Medicine 2017, 96, e7242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, J.; Zhang, S.; Lou, Z.; Wang, X.; Ren, Y.; Qi, H.; Xie, Z.; Chen, Y.; Chen, F.; et al. Association of VDR gene TaqI polymorphism with the susceptibility to prostate cancer in Asian population evaluated by an updated systematic meta-analysis. OncoTargets Ther. 2018, 11, 3267. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Chen, M.; Zhang, Q.-Q.; Hu, X. The association of vitamin d receptor gene polymorphism with lung cancer risk: An update meta-analysis. Comb. Chem. High Throughput Screen. 2018, 21, 704–710. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Vitamin D receptor FokI polymorphism and the risks of colorectal cancer, inflammatory bowel disease, and colorectal adenoma. Sci. Rep. 2018, 8, 12899. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, M.; Hu, X.; Wang, H.; Yang, J.; Zhang, C.; Pan, F.; Sun, G. Associations between VDR gene polymorphisms and colorectal cancer susceptibility: An updated meta-analysis based on 39 case-control studies. Oncotarget 2018, 9, 13068. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Zhu, J. Vitamin D receptor rs2228570 polymorphism and susceptibility to ovarian cancer: An updated meta-analysis. J. Obstet. Gynaecol. Res. 2018, 44, 556–565. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Jiang, Q.; Zhang, Y.; Liu, A.; Wang, H.; Zhang, J.; Qin, Q.; Hong, Z.; Li, B.A. Do genetic polymorphisms of the vitamin D receptor contribute to breast/ovarian cancer? A systematic review and network meta-analysis. Gene 2018, 677, 211–227. [Google Scholar] [CrossRef]
- Mun, M.-J.; Kim, T.-H.; Hwang, J.-Y.; Jang, W.-C. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas 2015, 81, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Zhang, X.-L.; Yang, Z.-S.; She, X.-Y.; Xie, Y.; Xie, W.-J. Relationship between Vitamin D receptor gene polymorphism and renal cell carcinoma susceptibility. J. Cancer Res. Ther. 2018, 14, 820. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, N.; Yang, T.; Shi, P.; He, R.; Chen, M. Association between polymorphisms of Vitamin D receptor and lung cancer susceptibility: Evidence from an updated Meta-analysis. J. Cancer 2019, 10, 3639. [Google Scholar] [CrossRef] [PubMed]
- Laczmanski, L.; Laczmanska, I.; Lwow, F. Association of select vitamin D receptor gene polymorphisms with the risk of tobacco-related cancers—A meta-analysis. Sci. Rep. 2019, 9, 16026. [Google Scholar] [CrossRef] [PubMed]
- Birke, M.; Schoepe, J.; Wagenpfeil, S.; Vogt, T.; Reichrath, J. Association of vitamin D receptor gene polymorphisms with melanoma risk: A meta-analysis and systematic review. Anticancer Res. 2020, 40, 583–595. [Google Scholar] [CrossRef]
- Duan, G.-Q.; Zheng, X.; Li, W.-K.; Zhang, W.; Li, Z.; Tan, W. The association between VDR and GC polymorphisms and lung cancer risk: A systematic review and meta-analysis. Genet. Test. Mol. Biomark. 2020, 24, 285–295. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.A.; Bellerba, F.; Corso, F.; Gandini, S. Vitamin D receptor polymorphisms and cancer. In Sunlight, Vitamin D and Skin Cancer; Springer: Berlin/Heidelberg, Germany, 2020; pp. 53–114. [Google Scholar]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.; Bellerba, F.; Corso, F.; Angelis, S.P.D.; Belloni, P.; Caini, S.; Gandini, S. Ethnicity as modifier of risk for Vitamin D receptors polymorphisms: Comprehensive meta-analysis of all cancer sites. Crit. Rev. Oncol./Hematol. 2021, 158, 103202. [Google Scholar] [CrossRef]

| Sl. No. | Study Design | Year | Sample Size | Conclusion | References |
|---|---|---|---|---|---|
| 1 | Case–Control Study | 2023 | 293 (143 gastric cancer patients and 150 controls) | VDR Fok1 polymorphism is significantly associated with GC risk in the Kashmiri population | [48] |
| 2 | Ancillary Study | 2023 | 1519 participants (vitamin D: n = 744; placebo: n = 775) | Vitamin D supplementation in older adults with vitamin D deficiency has no effect on the telomere length | [45] |
| 3 | Case–Control Study | 2023 | 204 (cases—102; controls—102) | Methylation levels of significant CpG sites in VDRs, CYP24A1, and CYP2R1 are inversely associated with CRC risk | [41] |
| 4 | Cohort Study | 2023 | 236,382 participants | Study showed the beneficial association of Serum 25(OH)D with risk of developing CRC. | [40] |
| 5 | Prospective Cohort Study | 2023 | 476 women with incident stage I–III breast cancer (BC) | Women with sufficient vitamin D had smaller and lower-grade tumors compared to the women with insufficient vitamin D | [46] |
| S. No. | Name | Structure * | Type of Cancer | Animal Type | Induction Method | Results Obtained |
|---|---|---|---|---|---|---|
| 1 | EB-1089 (Seocalcitol) | 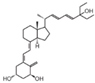 | Breast cancer HCC | Mice (I) Mice (I) | Subcutaneously Subcutaneously | Tumor growth inhibition |
| 2 | HY-11 | 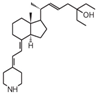 | Mice were inoculated with leukemia cells | Mice (I) | Intraperitoneally | |
| 3 | Tacalcitol (PRI-2191) | 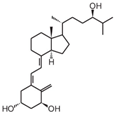 | Colorectal cancer | Mice (I) | Subcutaneously | Tumor growth inhibition |
| 4 | Inecalcitol | 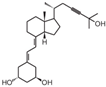 | Squamous cell carcinoma | Mice (I) | Subcutaneously | Inhibition of tumor growth, increased apoptosis, and decreased proliferation |
| 5 | TX527 | 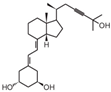 | Kaposi’s sarcoma | Mice (I) | Subcutaneously | Tumor growth Inhibition |
| 6 | Paricalcitol | 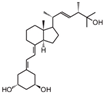 | Metastatic breast cancer | Mice (I) | Subcutaneously | Tumor inhibition was accompanied by in vivo upregulation of p21 and p27 expression |
| 7 | Doxercalciferol | 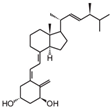 | Neuroblastoma | Mice (I) | Flanks | Tumor growth inhibition |
| 8 | Maxacalcitol | 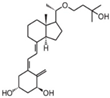 | Cholangial carcinoma | Mice (I) | Subcutaneously | Inhibition of tumor growth and inhibition of proliferation |
| 9 | Calcipotriol |  | Non-melanoma skin cancer | Mice (I) | Subcutaneously | Tumor growth inhibition |
| 10 | BGP-13 | 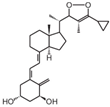 | Colorectal cancer (CRC) | Mice (I) | Subcutaneously | Inhibition of growth of HT-29 tumors in mice |
| 11 | PRI-2205 | 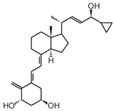 | Breast cancer | Mice (I) | Subcutaneously | Lowering the expression of estrogen receptors and aromatase activity |
| 12 | PRI-1906 | 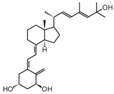 | Breast cancer | Mice (I) | Orthotopically | Tumor growth and metastases inhibition |
| 13 | BXL-01-0126 | 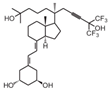 | Acute myeloid leukemia | Mice (I) | Intrahepatic (IH) or facial (FV) vein | Activation of apoptosis |
| 14 | BXL0124 | 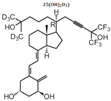 | Breast cancer | Mice (I) | Mammary fat pads | Proliferation, angiogenesis, invasion, and metastasis |
| 15 | Gemini0097 |  | Breast cancer | Mice (I) | Mammary fat pads | Suppressed tumor growth and inhibition of tumor burden |
| 16 | MART-10 | 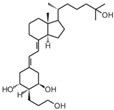 | Pancreatic cancer | Mice (I) | Subcutaneously | Inhibition of tumor growth |
| 17 | (1,25(OH)2D3-3-BE) |  | Kidney cancer | Mice (I) | Subcutaneously to the flanks | Inhibition of tumor growth and increase in apoptosis |
| 18 | Ro26-2198 | 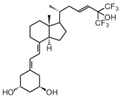 | Colorectal cancer (CRC) | Mice (C) | Administration of Dextran sulfate sodium (DSS) | Inhibition of dysplasia progression and inhibition of proliferation and pro-inflammatory signals |
| 19 | EM1 | 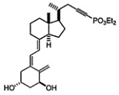 | Breast cancer | Mice (I) | Subcutaneously | Reduced the formation of metastasis |
| S. No. | VDR Polymorphism | Type of Study | Cancer Types | Study Outcome or Key Finding | Reference |
|---|---|---|---|---|---|
| 1 | Apa1 (rs7975232), Cdx2 (rs11568820), and Taq1 (rs731236) | Meta-analysis | 23 cancer types | Cdx2 showed increased risk of cancer. Taq1 was associated with increased risk of CRC. Apa1 was not associated with cancer risk. | [229] |
| 2 | Apa1 (rs7975232), Bsm1 (rs1544410), Bgl1 (rs739837), and Fok1 (rs2228570) | Nested case–control study and meta-analysis | Keratinocyte cancers | VDR polymorphisms may be associated with the risk of keratinocyte cancers. | [230] |
| 3 | ApaI1 (rs7975232) and Cdx2 (rs11568820) | Meta-analysis | Prostate cancer | VDR Cdx2 and Apa1 polymorphisms were not associated with prostate cancer. | [231] |
| 4 | Fok1 (rs10735810) | Meta-analysis | Prostate cancer | VDR Fok1 polymorphism could be a promising target and might be capable of causing prostate cancer risk. | [232] |
| 5 | Fok1 (rs10735810) | Meta-analysis | Prostate cancer | VDR Fok1 polymorphism may contribute to the risk of developing prostate cancer in Caucasian and population-based studies. | [233] |
| 6 | Apa1 (rs7975232), Bsm1 (rs1544410), Fok1 (rs2228570), and Taq1 (rs731236) | Meta-analysis | Breast cancer | VDR Fok1, Bsm1, Taq1, and Apa1 polymorphisms were not associated with the risk of breast cancer in the general as well as Caucasian population. | [234] |
| 7 | Fok1 (rs10735810) | Meta-analysis | Sex- and non-sex-associated cancers | Fok1 polymorphism was associated with breast and ovarian cancers. | [235] |
| 8 | Bsm1 (rs1544410) and Taq1 (rs731236) | Meta-analysis | Prostate cancer | Taq1 was significantly associated with risk of prostate cancer in Asians and African Americans but not Bsm 1 polymorphism. | [236] |
| 9 | Apa1, Bsm1, BgI1, Cdx2, Fok1, Taq1, and Poly (A) | Systematic review and meta-analysis | Breast cancer | VDR gene polymorphisms (Bsm1, Apa1, Fok1, and Poly (A)) may increase susceptibility to breast cancer development. | [237] |
| 10 | Taq1 | Meta-analysis | CRC | There was no correlation between Taq1 polymorphisms and susceptibility to CRC. | [238] |
| 11 | Taq1 | Systematic meta-analysis | Prostate cancer | The VDR Taq1 polymorphism might be associated with risk of prostate cancer in Asian (especially Japanese) populations. | [239] |
| 12 | Apa1 (rs7975232), Bsm1 (rs1544410), Fok1 (rs10735810), and Taq1 (rs731236) | Meta-analysis | Lung cancer | VDR genetic polymorphism may be correlated with the risk of lung cancer. | [240] |
| 13 | Fok1 (rs2228570) | Systematic meta-analysis | CRC | Role of VDR Fok1 polymorphism may differ based on the type and severity of colorectal disease. | [241] |
| 14 | Apa1, Fok1, Bsm1, Taq1, and Cdx2 | Meta-analysis | CRC | Bsm1 polymorphism was associated with CRC risk, and Fok1 might be a risk factor for CRC. | [242] |
| 15 | Fok1 (rs2228570) | Meta-analysis | Ovarian cancer | Fok1 polymorphism increased the risk of ovarian cancer in Caucasian populations in a dominant genetic model. | [243] |
| 16 | Bsm1 (rs1544410), Cdx2, and Fok1 (rs2228570) | A systematic review and network meta-analysis | Breast and ovarian cancers | Fok1 and Bsm1 polymorphism are likely the best genetic model for detecting the risk of breast and ovarian cancers, respectively, in Caucasian patients. | [244] |
| 17 | Apa1, Fok1, Bsm1, Taq1, and Cdx2 | Meta-analysis | Female reproductive cancers | Fok1 and Bsm1 VDR gene polymorphisms may be significantly associated with gynecological cancers. | [245] |
| 18 | Apa1 (rs7975232), Fok1 (rs2228570), Bsm1 (rs1544410), and Taq1 (rs731236) | Meta-analysis | RCC | ApaI gene polymorphism and Fok1 FF genotype were associated with RCC susceptibility in Asians. | [246] |
| 19 | Apa1 (rs7975232 C > A), Bsm1 (rs1544410 G > A), Cdx2 (rs11568820 T > C), and Taq1 (rs731236 T > C) | Meta-analysis | Lung cancer | Bsm1, Taq1, and Cdx-2 polymorphisms may contribute to lung cancer susceptibility. | [247] |
| 20 | Apa1 (rs7975232), Bsm1 (rs1544410), Fok1 (rs10735810), and Taq1 (rs731236) | Meta-analysis | Tobacco-related cancers | Taq1 polymorphism and the risk of tobacco-related cancers were correlated with each other. | [248] |
| 21 | A-1012G (rs4516035), Apa1 (rs7975232), Bsm1 (rs1544410), BgI1 (rs739837), Cdx2 (rs11568820), Fok1 (rs2228570), and Taq1 (rs731236) | Systematic review and meta-analysis | Melanoma | Apa1, Bsm1, and Fok1 polymorphisms may influence the development of melanoma. | [249] |
| 22 | Apa1 (rs7975232), Bsm1 (rs1544410, A/G), Cdx2 (rs11568820, C/T), Fok1 (rs2228570, T/C), and Taq1 (rs731236, T/C) | Systematic review and meta-analysis | Lung cancer | Bsm1 and Cdx2 polymorphisms decreased lung cancer risk, while Taq1 increased it. | [250] |
| 23 | Apa1, Bsm1, Cdx2, Fok1, and Taq1 | Systematic review and meta-analysis | 18 cancer types | Significant associations with VDR polymorphisms have been reported for prostate (Fok1, Bsm1, Taq1, Apa1, and Cdx2), breast (Fok1, Bsm1, Taq1, Apa1, and Cdx2), colorectal (Fok1, Bsm1, Taq1, and Apa1), and skin cancer (Fok1, Bsm1, and Taq1). | [251] |
| 24 | Apa1 (rs7975232), Bsm1 (rs1544410), Cdx2 (rs11568820), Fok1 (rs10735810), and Taq1 (rs731236) | Comprehensive meta-analysis | 22 cancer types | VDR polymorphisms were linked to cancer susceptibility. Ethnicity may be a modifier of cancer risk, in particular for hormone-dependent cancers. | [252] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallavalasa, S.; Tulimilli, S.V.; Bettada, V.G.; Karnik, M.; Uthaiah, C.A.; Anantharaju, P.G.; Nataraj, S.M.; Ramashetty, R.; Sukocheva, O.A.; Tse, E.; et al. Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers 2024, 16, 3211. https://doi.org/10.3390/cancers16183211
Dallavalasa S, Tulimilli SV, Bettada VG, Karnik M, Uthaiah CA, Anantharaju PG, Nataraj SM, Ramashetty R, Sukocheva OA, Tse E, et al. Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers. 2024; 16(18):3211. https://doi.org/10.3390/cancers16183211
Chicago/Turabian StyleDallavalasa, Siva, SubbaRao V. Tulimilli, Vidya G. Bettada, Medha Karnik, Chinnappa A. Uthaiah, Preethi G. Anantharaju, Suma M. Nataraj, Rajalakshmi Ramashetty, Olga A. Sukocheva, Edmund Tse, and et al. 2024. "Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies" Cancers 16, no. 18: 3211. https://doi.org/10.3390/cancers16183211
APA StyleDallavalasa, S., Tulimilli, S. V., Bettada, V. G., Karnik, M., Uthaiah, C. A., Anantharaju, P. G., Nataraj, S. M., Ramashetty, R., Sukocheva, O. A., Tse, E., Salimath, P. V., & Madhunapantula, S. V. (2024). Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers, 16(18), 3211. https://doi.org/10.3390/cancers16183211








