A Promising Therapeutic Strategy of Combining Acoustically Stimulated Nanobubbles and Existing Cancer Treatments
Abstract
Simple Summary
Abstract
1. Introduction and Backgrounds
2. Structure and Composition of Nanobubbles
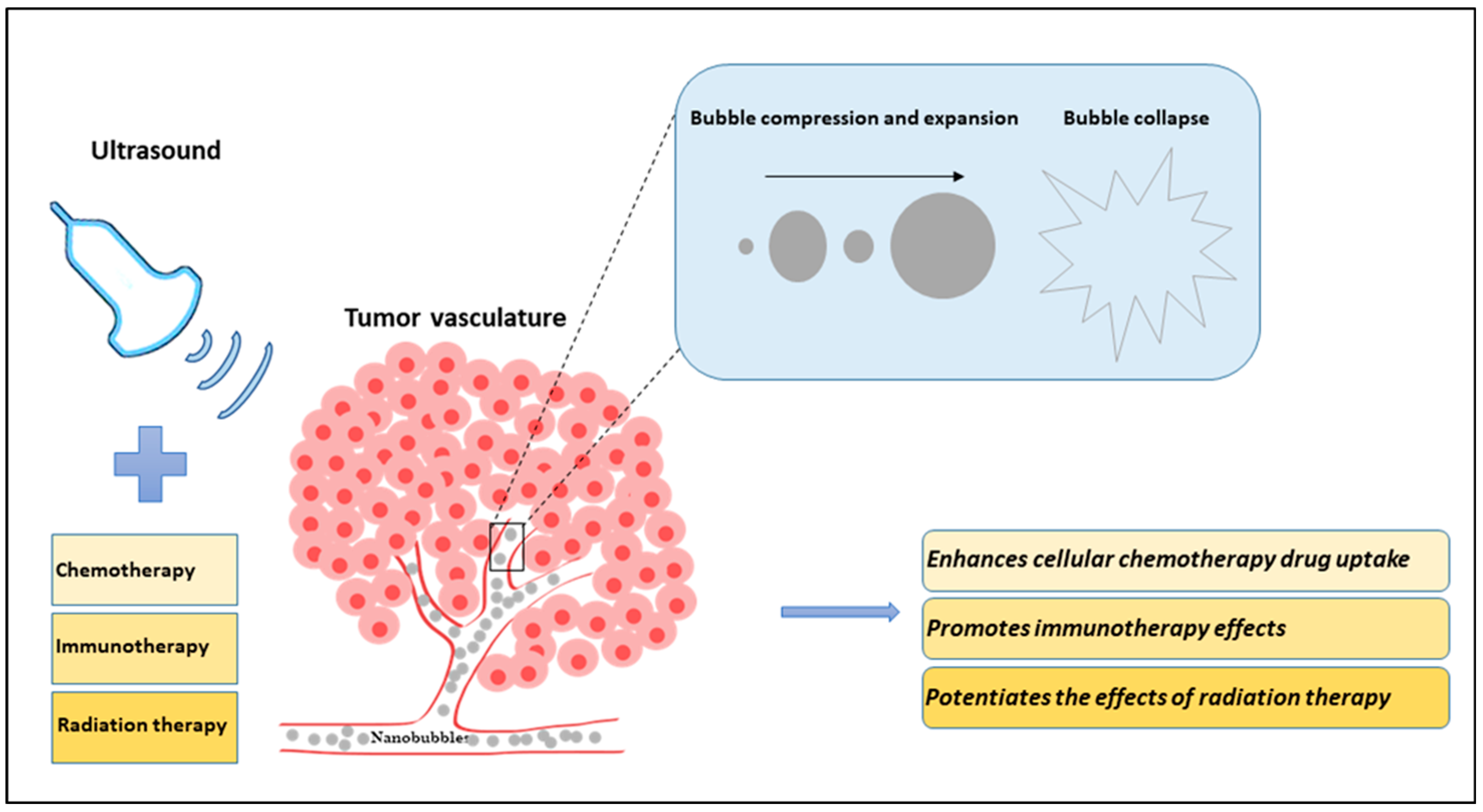
3. Combinatory Effect of USNBs and Cancer Therapies
| Experimental Model | Treatment Regimen | Ultrasound Parameters | Cellular/Tumor Response | Reference | |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| Human ovarian cancer cells (OVCAR-3) in vitro | USNB + Chemotherapy (doxorubicin) | Transducer frequency: 1 MHz Intensity: 1.7 W/cm2 Duty cycle: 100% for 1 min | Improved drug loading capacity and acoustic signal, decrease in cell viability | [77] | |
| Human lung cancer cells (A549) in vitro | USNB (survivin-siRNA bound) + Chemotherapy (paclitaxel) | Transducer frequency: 3 MHz E = 449 J, 5 min | Decreased survivin expression, increased siRNA delivered to target region, increased apoptosis | [95] | |
| Human colorectal cancer cells (LS-174T) in vitro and in vivo (mice) | USNB + Chemotherapy (doxorubicin) | Transducer frequency: 3 MHz Intensity: 2 W/cm2 Duty cycle: 20% for 1 min | Increased targeted drug accumulation and intracellular uptake, decreased cell viability | [76] | |
| Murine bladder cancer cells (MB49) in vitro and in vivo (mice) | USNB (Oxygen-bound) + Chemotherapy (mitomycin-C) | Transducer frequency: 40 MHz Duty cycle: 20% and 100% | Reduced tumor progression rates, increased cell death and enhanced re-oxygenation of hypoxic tumor regions, decreased level of HIF-1 and VEGF expression | [78] | |
| Human liver cancer cells (HepG2) in vitro and in vivo (mice) | USNB (siRNA-bound) + Chemotherapy (paclitaxel) | Transducer frequency: 1 MHz Pressure: 500 kPa Duty cycle: 50% | Enhanced drug and siRNA codelivery, cell apoptosis, reduced tumor volume, higher animal survival rates | [80] | |
| Human pancreatic cancer cells (Mia-Paca2) in vitro and in vivo (mice) | USNB + PTT + Chemotherapy (docetaxel) | Transducer frequency: 7.5 MHz Intensity: 2.5 W/cm2 PTT: 808 nm (1 W/cm2, 210 s) | Improved tumor tar-geting rates, increased apoptosis, reduction in tumor size and cellular proliferation | [75] | |
| Liver cancer (VX2) in vitro and in vivo (rabbits) | USNB + Chemotherapy (doxorubicin) | Transducer frequency: 1 MHz Intensity: 2 W/cm2 | Increased drug release decreased growth rate, reduced proliferation, and increased apoptosis, greater survival rates | [74] | |
| Immunotherapy | |||||
| Murine prostate (RM1, RM1-OVA), colon (MC38, MC38-OVA) and melanoma (B16) cancer cells in vitro and in vivo (mice) | USNB + Immunotherapy (anti-PD1) | Transducer frequency: 1 MHz Intensity: 1 W/cm2 | Decreased tumor growth and metastasis, increased immune response and immune memory | [37] | |
| Murine liver cancer cells (H22) in vivo (mice) | USNB + Immunotherapy (sPD-1 and Ce6) | Transducer frequency: 1.1 MHz Intensity: 1.8 W/cm2 Duty cycle: 50% | Decreased Bcl-2 mRNA, increased expression of Bax, CD80, CD86, IFN-γ, TNF-α, and IL-2, increased tumor apoptosis and necrosis | [96] | |
| Radiation Therapy | |||||
| Human prostate cancer cells (PC3) in vivo (mice) | USNB + Radiation therapy (8 Gy) | Transducer frequency: 500 kHz Pressure: 570 kPa Duty cycle: 0.24% or 720 ms | Increased cell death, reduced vessel counts, decreased oxygen saturation, reduced tumor size | [13] |
4. Clinical Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osei, E.; Al-Asady, A. A review of ultrasound-mediated microbubbles technology for cancer therapy: A vehicle for chemotherapeutic drug delivery. J. Radiother. Pract. 2020, 19, 291–298. [Google Scholar] [CrossRef]
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Nazeri, A.; Baek, H.; Ye, D.; Yang, Y.; Yuan, J.; Rubin, J.B.; Chen, H. A review of bioeffects induced by focused ultrasound combined with microbubbles on the neurovascular unit. J. Cereb. Blood Flow Metab. 2022, 42, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Leong, K.X.; Czarnota, G.J. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4393. [Google Scholar] [CrossRef] [PubMed]
- Abbondanza, D.; Gallo, M.; Casciola, C.M. Cavitation over solid surfaces: Microbubble collapse, shock waves, and elastic response. Meccanica 2023, 58, 1109–1119. [Google Scholar] [CrossRef]
- Tezel, A.; Mitragotri, S. Interactions of Inertial Cavitation Bubbles with Stratum Corneum Lipid Bilayers during Low-Frequency Sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Haworth, K.J.; Fakhri, N.H.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009, 56, 1006–1017. [Google Scholar] [CrossRef]
- Luo, J.; Niu, Z. Jet and Shock Wave from Collapse of Two Cavitation Bubbles. Sci. Rep. 2019, 9, 1352. [Google Scholar] [CrossRef]
- Zhao, X.; Pellow, C.; Goertz, D.E. Intravital imaging and cavitation monitoring of antivascular ultrasound in tumor microvasculature. Theranostics 2023, 13, 250–266. [Google Scholar] [CrossRef]
- Zhao, X.; Wright, A.; Goertz, D.E. An optical and acoustic investigation of microbubble cavitation in small channels under therapeutic ultrasound conditions. Ultrason. Sonochem. 2023, 93, 106291. [Google Scholar] [CrossRef]
- Czarnota, G.J.; Karshafian, R.; Burns, P.N.; Wong, S.; Al Mahrouki, A.; Lee, J.W.; Caissie, A.; Tran, W.; Kim, C.; Furukawa, M.; et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc. Natl. Acad. Sci. USA 2012, 109, E2033–E2041. [Google Scholar] [CrossRef] [PubMed]
- El Kaffas, A.; Al-Mahrouki, A.; Hashim, A.; Law, N.; Giles, A.; Czarnota, G.J. Role of acid sphingomyelinase and ceramide in mechano-acoustic enhancement of tumor radiation responses. J. Natl. Cancer Inst. 2018, 110, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Hysi, E.; Fadhel, M.N.; Wang, Y.; Sebastian, J.A.; Giles, A.; Czarnota, G.J.; Exner, A.A.; Kolios, M.C. Photoacoustic imaging biomarkers for monitoring biophysical changes during nanobubble-mediated radiation treatment. Photoacoustics 2020, 20, 100201. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef]
- Helfield, B.; Zou, Y.; Matsuura, N. Acoustically-Stimulated Nanobubbles: Opportunities in Medical Ultrasound Imaging and Therapy. Front. Phys. 2021, 9, 654374. [Google Scholar] [CrossRef]
- Myers, J.Z.; Navarro-Becerra, J.A.; Borden, M.A. Nanobubbles are Non-Echogenic for Fundamental-Mode Contrast-Enhanced Ultrasound Imaging. Bioconjug. Chem. 2022, 33, 1106–1113. [Google Scholar] [CrossRef]
- Chaurasia, G. Nanobubbles: An emerging science in nanotechnology. MGM J. Med. Sci. 2023, 10, 327. [Google Scholar] [CrossRef]
- Hansen, H.H.; Cha, H.; Ouyang, L.; Zhang, J.; Jin, B.; Stratton, H.; Nguyen, N.-T.; An, H. Nanobubble technologies: Applications in therapy from molecular to cellular level. Biotechnol. Adv. 2023, 63, 108091. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Jang, J.; Kwon, Y.; Hong, J.W.; Choi, J. Surface Composition and Preparation Method for Oxygen Nanobubbles for Drug Delivery and Ultrasound Imaging Applications. Nanomaterials 2019, 9, 48. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.-J.; Hong, J.W.; Choi, J. Oxygen-Carrying Micro/Nanobubbles: Composition, Synthesis Techniques and Potential Prospects in Photo-Triggered Theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Du, M.; Chen, Z. Nanosized Contrast Agents in Ultrasound Molecular Imaging. Front. Bioeng. Biotechnol. 2021, 9, 758084. [Google Scholar] [CrossRef] [PubMed]
- Pellow, C.; Cherin, E.; Abenojar, E.C.; Exner, A.A.; Zheng, G.; Demore, C.E.M.; Goertz, D.E. High-Frequency Array-Based Nanobubble Nonlinear Imaging in a Phantom and in Vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xi, A.; Qiao, H.; Liu, Z. Ultrasound-mediated diagnostic imaging and advanced treatment with multifunctional micro/nanobubbles. Cancer Lett. 2020, 475, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Lee, J.; Moon, Y.; Pradhan, D.; Kim, S.-H.; Lee, H.-Y.; Lee, J. Experimental Investigation of Cavitation Bulk Nanobubbles Characteristics: Effects of pH and Surface-Active Agents. Langmuir 2023, 39, 1968–1986. [Google Scholar] [CrossRef]
- Fournier, L.; de La Taille, T.; Chauvierre, C. Microbubbles for human diagnosis and therapy. Biomaterials 2023, 294, 122025. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, B.; Shang, H.; Sun, Y.; Tian, H.; Yang, H.; Wang, C.; Wang, X.; Cheng, W. Sono-Immunotherapy Mediated Controllable Composite Nano Fluorescent Probes Reprogram the Immune Microenvironment of Hepatocellular Carcinoma. Int. J. Nanomed. 2023, 18, 6059–6073. [Google Scholar] [CrossRef]
- Jose, A.D.; Wu, Z.; Thakur, S.S. A comprehensive update of micro- and nanobubbles as theranostics in oncology. Eur. J. Pharm. Biopharm. 2022, 172, 123–133. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Ma, X.; He, W.; Liu, C.; Liu, Z. Functional micro/nanobubbles for ultrasound medicine and visualizable guidance. Sci. China Chem. 2021, 64, 899–914. [Google Scholar] [CrossRef]
- Exner, A.A.; Kolios, M.C. Bursting microbubbles: How nanobubble contrast agents can enable the future of medical ultrasound molecular imaging and image-guided therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101463. [Google Scholar] [CrossRef]
- Pellow, C.; Abenojar, E.C.; Exner, A.A.; Zheng, G.; Goertz, D.E. Concurrent visual and acoustic tracking of passive and active delivery of nanobubbles to tumors. Theranostics 2020, 10, 11690–11706. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef] [PubMed]
- Huynh, E.; Leung, B.Y.C.; Helfield, B.L.; Shakiba, M.; Gandier, J.-A.; Jin, C.S.; Master, E.R.; Wilson, B.C.; Goertz, D.E.; Zheng, G. In situ conversion of porphyrin microbubbles to nanoparticles for multimodality imaging. Nat. Nanotechnol. 2015, 10, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, D.V.B.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; McLaughlan, J.R.; Coletta, P.L.; Evans, S.D. The Influence of Nanobubble Size and Stability on Ultrasound Enhanced Drug Delivery. Langmuir 2022, 38, 13943–13954. [Google Scholar] [CrossRef]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and applications of nanobubbles: A review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Batchelor, D.V.B.; Abou-Saleh, R.H.; Coletta, P.L.; McLaughlan, J.R.; Peyman, S.A.; Evans, S.D. Nested Nanobubbles for Ultrasound-Triggered Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 29085–29093. [Google Scholar] [CrossRef]
- Hu, J.; He, J.; Wang, Y.; Zhao, Y.; Fang, K.; Dong, Y.; Chen, Y.; Zhang, Y.; Zhang, C.; Wang, H.; et al. Ultrasound combined with nanobubbles promotes systemic anticancer immunity and augments anti-PD1 efficacy. J. Immunother. Cancer 2022, 10, e003408. [Google Scholar] [CrossRef]
- Qiu, Y.; Ren, H.; Edwards, M.A.; Gao, R.; Barman, K.; White, H.S. Electrochemical Generation of Individual Nanobubbles Comprising H2, D2, and HD. Langmuir 2020, 36, 6073–6078. [Google Scholar] [CrossRef]
- Yadav, G.; Nirmalkar, N.; Ohl, C.-D. Electrochemically reactive colloidal nanobubbles by water splitting. J. Colloid Interface Sci. 2024, 663, 518–531. [Google Scholar] [CrossRef]
- de Leon, A.; Perera, R.; Hernandez, C.; Cooley, M.; Jung, O.; Jeganathan, S.; Abenojar, E.; Fishbein, G.; Sojahrood, A.J.; Emerson, C.C.; et al. Contrast enhanced ultrasound imaging by nature-inspired ultrastable echogenic nanobubbles. Nanoscale 2019, 11, 15647–15658. [Google Scholar] [CrossRef]
- Zafar, M.N.; Abuwatfa, W.H.; Husseini, G.A. Acoustically-Activated Liposomal Nanocarriers to Mitigate the Side Effects of Conventional Chemotherapy with a Focus on Emulsion-Liposomes. Pharmaceutics 2023, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochem. 2021, 76, 105629. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Guo, Z.; Tan, T.; Wang, Y.; Zhang, L.; Hu, J.; Zhang, Y. Generating Bulk Nanobubbles in Alcohol Systems. ACS Omega 2021, 6, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, A.A.; Zalloum, I.O.; Karshafian, R.; Kolios, M.C.; Tsai, S.S.H. High throughput microfluidic nanobubble generation by microporous membrane integration and controlled bubble shrinkage. J. Colloid Interface Sci. 2024, 653, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, A.A.; Kerr, L.; Wong, D.A.; Kolios, M.C.; Tsai, S.S.H. Biomedical nanobubbles and opportunities for microfluidics. RSC Adv. 2021, 11, 32750–32774. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Wang, X.; Hu, J.; Li, P.; Lin, G.; Gao, Y.; Zhang, L.; Wang, C. Collective Dynamics of Bulk Nanobubbles with Size-Dependent Surface Tension. Langmuir 2021, 37, 7986–7994. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Generation methods, stability, detection techniques, and applications of bulk nanobubbles in agro-food industries: A review and future perspective. Crit. Rev. Food Sci. Nutr. 2023, 63, 9262–9281. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Xu, X.; Sun, C. Coupling Effects of Ionic Surfactants and Electrolytes on the Stability of Bulk Nanobubbles. Nanomaterials 2022, 12, 3450. [Google Scholar] [CrossRef]
- Counil, C.; Abenojar, E.; Perera, R.; Exner, A.A. Extrusion: A New Method for Rapid Formulation of High-Yield, Monodisperse Nanobubbles. Small 2022, 18, e2200810. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk nanobubbles: Production and investigation of their formation/stability mechanism. J. Colloid Interface Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Pfeiffer, P.; Eisener, J.; Ohl, C.-D.; Sun, C. Ion adsorption stabilizes bulk nanobubbles. J. Colloid Interface Sci. 2022, 606, 1380–1394. [Google Scholar] [CrossRef] [PubMed]
- Sennoga, C.A.; Yeh, J.S.; Alter, J.; Stride, E.; Nihoyannopoulos, P.; Seddon, J.M.; Haskard, D.O.; Hajnal, J.V.; Tang, M.-X.; Eckersley, R.J. Evaluation of Methods for Sizing and Counting of Ultrasound Contrast Agents. Ultrasound Med. Biol. 2012, 38, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, E.C.; Bederman, I.; de Leon, A.C.; Zhu, J.; Hadley, J.; Kolios, M.C.; Exner, A.A. Theoretical and Experimental Gas Volume Quantification of Micro- and Nanobubble Ultrasound Contrast Agents. Pharmaceutics 2020, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tonggu, L.; Zhan, X.; Mega, T.L.; Wang, L. Cryo-EM Visualization of Nanobubbles in Aqueous Solutions. Langmuir 2016, 32, 11111–11115. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Gulati, S.; Fioravanti, G.; Stewart, P.L.; Exner, A.A. Cryo-EM Visualization of Lipid and Polymer-Stabilized Perfluorocarbon Gas Nanobubbles—A Step Towards Nanobubble Mediated Drug Delivery. Sci. Rep. 2017, 7, 13517. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Craig, V.S.J. Differentiating between Nanoparticles and Nanobubbles by Evaluation of the Compressibility and Density of Nanoparticles. J. Phys. Chem. C 2018, 122, 21998–22007. [Google Scholar] [CrossRef]
- Jin, J.; Wang, R.; Tang, J.; Yang, L.; Feng, Z.; Xu, C.; Yang, F.; Gu, N. Dynamic tracking of bulk nanobubbles from microbubbles shrinkage to collapse. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124430. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Foreman-Ortiz, I.U.; Ma, T.F.; Hoover, B.M.; Wu, M.; Murphy, C.J.; Murphy, R.M.; Pedersen, J.A. Nanoparticle tracking analysis and statistical mixture distribution analysis to quantify nanoparticle–vesicle binding. J. Colloid Interface Sci. 2022, 615, 50–58. [Google Scholar] [CrossRef]
- Ma, X.-T.; Li, M.-B.; Sun, C. Measurement and characterization of bulk nanobubbles by nanoparticle tracking analysis method. J. Hydrodyn. 2022, 34, 1121–1133. [Google Scholar] [CrossRef]
- Han, Z.; Chen, H.; He, C.; Dodbiba, G.; Otsuki, A.; Wei, Y.; Fujita, T. Nanobubble size distribution measurement by interactive force apparatus under an electric field. Sci. Rep. 2023, 13, 3663. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.-A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. Ganti AK, editor. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Katta, B.; Vijayakumar, C.; Dutta, S.; Dubashi, B.; Nelamangala Ramakrishnaiah, V.P. The Incidence and Severity of Patient-Reported Side Effects of Chemotherapy in Routine Clinical Care: A Prospective Observational Study. Cureus 2023, 15, e38301. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; Dhanjal, J.K.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Wu, C.-H.; Jin, Q.-F.; Lin, C.-Y.; Chiang, P.-H.; Wu, N.; Fan, C.-H.; Yang, C.-M.; Yeh, C.-K. Superhydrophobic drug-loaded mesoporous silica nanoparticles capped with β-cyclodextrin for ultrasound image-guided combined antivascular and chemo-sonodynamic therapy. Biomaterials 2020, 232, 119723. [Google Scholar] [CrossRef]
- Zhong, S.; Ling, Z.; Zhou, Z.; He, J.; Ran, H.; Wang, Z.; Zhang, Q.; Song, W.; Zhang, Y.; Luo, J. Herceptin-decorated paclitaxel-loaded poly(lactide-co-glycolide) nanobubbles: Ultrasound-facilitated release and targeted accumulation in breast cancers. Pharm. Dev. Technol. 2020, 25, 454–463. [Google Scholar] [CrossRef]
- Wang, F.; Dong, L.; Liang, S.; Wei, X.; Wang, Y.; Chang, L.; Guo, K.; Wu, H.; Chang, Y.; Yin, Y.; et al. Ultrasound-triggered drug delivery for glioma therapy through gambogic acid-loaded nanobubble-microbubble complexes. Biomed. Pharmacother. 2022, 150, 113042. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Abenojar, E.; Cooley, M.B.; Berg, F.M.; Counil, C.; Sojahrood, A.J.; Khan, M.S.; Yang, C.; Berndl, E.; Golczak, M.; et al. Efficient ultrasound-mediated drug delivery to orthotopic liver tumors—Direct comparison of doxorubicin-loaded nanobubbles and microbubbles. J. Control. Release 2024, 367, 135–147. [Google Scholar] [CrossRef]
- Batchelor, D.V.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; Mclaughlan, J.R.; Coletta, P.L.; Evans, S.D. Nanobubbles for therapeutic delivery: Production, stability and current prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101456. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chen, F.; Gu, N. Drug delivery system based on nanobubbles. Interdiscip. Mater. 2022, 1, 471–494. [Google Scholar] [CrossRef]
- Wu, R.; Yang, X.; Li, X.; Dong, N.; Liu, Y.; Zhang, P. Nanobubbles for tumors: Imaging and drug carriers. J. Drug Deliv. Sci. Technol. 2021, 65, 102749. [Google Scholar] [CrossRef]
- Edwards, I.A.; De Carlo, F.; Sitta, J.; Varner, W.; Howard, C.M.; Claudio, P.P. Enhancing Targeted Therapy in Breast Cancer by Ultrasound-Responsive Nanocarriers. Int. J. Mol. Sci. 2023, 24, 5474. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Gao, J.; Wu, C.; Zhou, X.; Zang, X.; Lin, X.; Liu, H.; Wang, C.; Su, H.; Liu, K.; et al. Doxorubicin nanobubble for combining ultrasonography and targeted chemotherapy of rabbit with VX2 liver tumor. Tumor. Biol. 2016, 37, 8673–8680. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, P.; Zhou, Y.; Li, Q.; Cai, W.; Zhao, Z.; Shen, J.; Yao, K.; Duan, Y. Preparation of multifunctional nanobubbles and their application in bimodal imaging and targeted combination therapy of early pancreatic cancer. Sci. Rep. 2021, 11, 6254. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Yuan, H.X.; Hernandez, C.; Bielecki, P.; Zhou, H.; Exner, A.A. Enhancing Tumor Drug Distribution with Ultrasound-Triggered Nanobubbles. J. Pharm. Sci. 2019, 108, 3091–3098. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Abenojar, E.; De Leon, A.; Wegierak, D.; Exner, A.A. Increasing Doxorubicin Loading in Lipid-Shelled Perfluoropropane Nanobubbles via a Simple Deprotonation Strategy. Front. Pharmacol. 2020, 11, 644. [Google Scholar] [CrossRef]
- Bhandari, P.; Novikova, G.; Goergen, C.J.; Irudayaraj, J. Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy. Sci. Rep. 2018, 8, 3112. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Li, J.; Zheng, R.; Zheng, B.; Cheng, D.; Li, R.; Lai, J.; Shuai, X. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 2013, 34, 4532–4543. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Li, J.; Wang, Y.; Zheng, B.; Zheng, R.; Cheng, D.; Shuai, X. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials 2014, 35, 5932–5943. [Google Scholar] [CrossRef]
- Cheng, B.; Bing, C.; Xi, Y.; Shah, B.; Exner, A.A.; Chopra, R. Influence of Nanobubble Concentration on Blood–Brain Barrier Opening Using Focused Ultrasound Under Real-Time Acoustic Feedback Control. Ultrasound Med. Biol. 2019, 45, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Bing, C.; Chopra, R. The effect of transcranial focused ultrasound target location on the acoustic feedback control performance during blood-brain barrier opening with nanobubbles. Sci. Rep. 2019, 9, 20020. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Oda, Y.; Omata, D.; Nishiie, N.; Koshima, R.; Shiono, Y.; Sawaguchi, Y.; Unga, J.; Naoi, T.; Negishi, Y.; et al. Tumor growth suppression by the combination of nanobubbles and ultrasound. Cancer Sci. 2016, 107, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, M.; Katz, S.; Mano, T.; Aronovich, R.; Hershkovitz, D.; Exner, A.A.; Ilovitsh, T. Low frequency nanobubble-enhanced ultrasound mechanotherapy for noninvasive cancer surgery. Nanoscale 2022, 14, 13614–13627. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.; Brenner, J.; Prada, F.; Klibanov, A.L. Theranostics in the vasculature: Bioeffects of ultrasound and microbubbles to induce vascular shutdown. Theranostics 2023, 13, 4079–4101. [Google Scholar] [CrossRef]
- Fuks, Z.; Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef]
- Sharma, D.; Czarnota, G.J. Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. Int. J. Mol. Sci. 2022, 23, 6671. [Google Scholar] [CrossRef]
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- El Kaffas, A.; Giles, A.; Czarnota, G.J. Dose-dependent response of tumor vasculature to radiation therapy in combination with Sunitinib depicted by three-dimensional high-frequency power Doppler ultrasound. Angiogenesis 2013, 16, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahrouki, A.A.; Iradji, S.; Tran, W.T.; Czarnota, G.J. Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model. DMM Dis. Model. Mech. 2014, 7, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Al-Mahrouki, A.; Gorjizadeh, A.; Sadeghi-Naini, A.; Karshafian, R.; Czarnota, G.J. Quantitative Ultrasound Characterization of Tumor Cell Death: Ultrasound-Stimulated Microbubbles for Radiation Enhancement. PLoS ONE 2014, 9, e102343. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahrouki, A.; Giles, A.; Hashim, A.; Kim, H.C.; El-Falou, A.; Rowe-Magnus, D.; Farhat, G.; Czarnota, G.J. Microbubble-based enhancement of radiation effect: Role of cell membrane ceramide metabolism. PLoS ONE 2017, 12, e0181951. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, H.; Erel-Akbaba, G.; Kotmakçı, M.; Başpınar, Y. Enhanced Cellular Uptake and Gene Silencing Activity of Survivin-siRNA via Ultrasound-Mediated Nanobubbles in Lung Cancer Cells. Pharm. Res. 2020, 37, 165. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, S.; Ma, Y.; Li, J.; Xie, Q.; Liu, C.; Zhao, Y. Nanobubbles Containing sPD-1 and Ce6 Mediate Combination Immunotherapy and Suppress Hepatocellular Carcinoma in Mice. Int. J. Nanomed. 2021, 16, 3241–3254. [Google Scholar] [CrossRef]
- Willmann, J.K.; Bonomo, L.; Testa, A.C.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound molecular imaging with BR55 in patients with breast & ovarian lesions: First-in-human results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef]
- Zhou, B.; Lian, Q.; Jin, C.; Lu, J.; Xu, L.; Gong, X.; Zhou, P. Human clinical trial using diagnostic ultrasound and microbubbles to enhance neoadjuvant chemotherapy in HER2- negative breast cancer. Front. Oncol. 2022, 12, 992774. [Google Scholar] [CrossRef]
- Meacock, L.M.; Sellars, M.E.; Sidhu, P.S. Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. Br. J. Radiol. 2010, 83, 615–627. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, L.; Chien, C.-Y.; Yang, Y.; Yue, Y.; Fadera, S.; Stark, A.H.; Schwetye, K.E.; Nazeri, A.; Desai, R.; et al. First-in-human prospective trial of sonobiopsy in high-grade glioma patients using neuronavigation-guided focused ultrasound. NPJ Precis. Oncol. 2023, 7, 92. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Forsberg, F.; Wessner, C.E.; Delaney, L.J.; Bradigan, K.; Gummadi, S.; Tantawi, M.; Lyshchik, A.; O’kane, P.; Liu, J.-B.; et al. US-triggered Microbubble Destruction for Augmenting Hepatocellular Carcinoma Response to Transarterial Radioembolization: A Randomized Pilot Clinical Trial. Radiology 2021, 298, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, F.; Itoh, K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: Phase 3 multicenter clinical trial. Am. J. Roentgenol. 2009, 193, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin. J. Cancer Res. 2018, 30, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Dimcevski, G.; Mc Cormack, E.; Postema, M.; Gjertsen, B.T.; Gilja, O.H. Ultrasound- and microbubble-enhanced chemotherapy for treating pancreatic cancer: A phase I clinical trial. J. Acoust. Soc. Am. 2016, 139, 2092. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Petchiny, T.N.; Czarnota, G.J. A Promising Therapeutic Strategy of Combining Acoustically Stimulated Nanobubbles and Existing Cancer Treatments. Cancers 2024, 16, 3181. https://doi.org/10.3390/cancers16183181
Sharma D, Petchiny TN, Czarnota GJ. A Promising Therapeutic Strategy of Combining Acoustically Stimulated Nanobubbles and Existing Cancer Treatments. Cancers. 2024; 16(18):3181. https://doi.org/10.3390/cancers16183181
Chicago/Turabian StyleSharma, Deepa, Tera N. Petchiny, and Gregory J. Czarnota. 2024. "A Promising Therapeutic Strategy of Combining Acoustically Stimulated Nanobubbles and Existing Cancer Treatments" Cancers 16, no. 18: 3181. https://doi.org/10.3390/cancers16183181
APA StyleSharma, D., Petchiny, T. N., & Czarnota, G. J. (2024). A Promising Therapeutic Strategy of Combining Acoustically Stimulated Nanobubbles and Existing Cancer Treatments. Cancers, 16(18), 3181. https://doi.org/10.3390/cancers16183181








