External Validation of Risk Scores for Predicting Venous Thromboembolism in Ambulatory Patients with Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
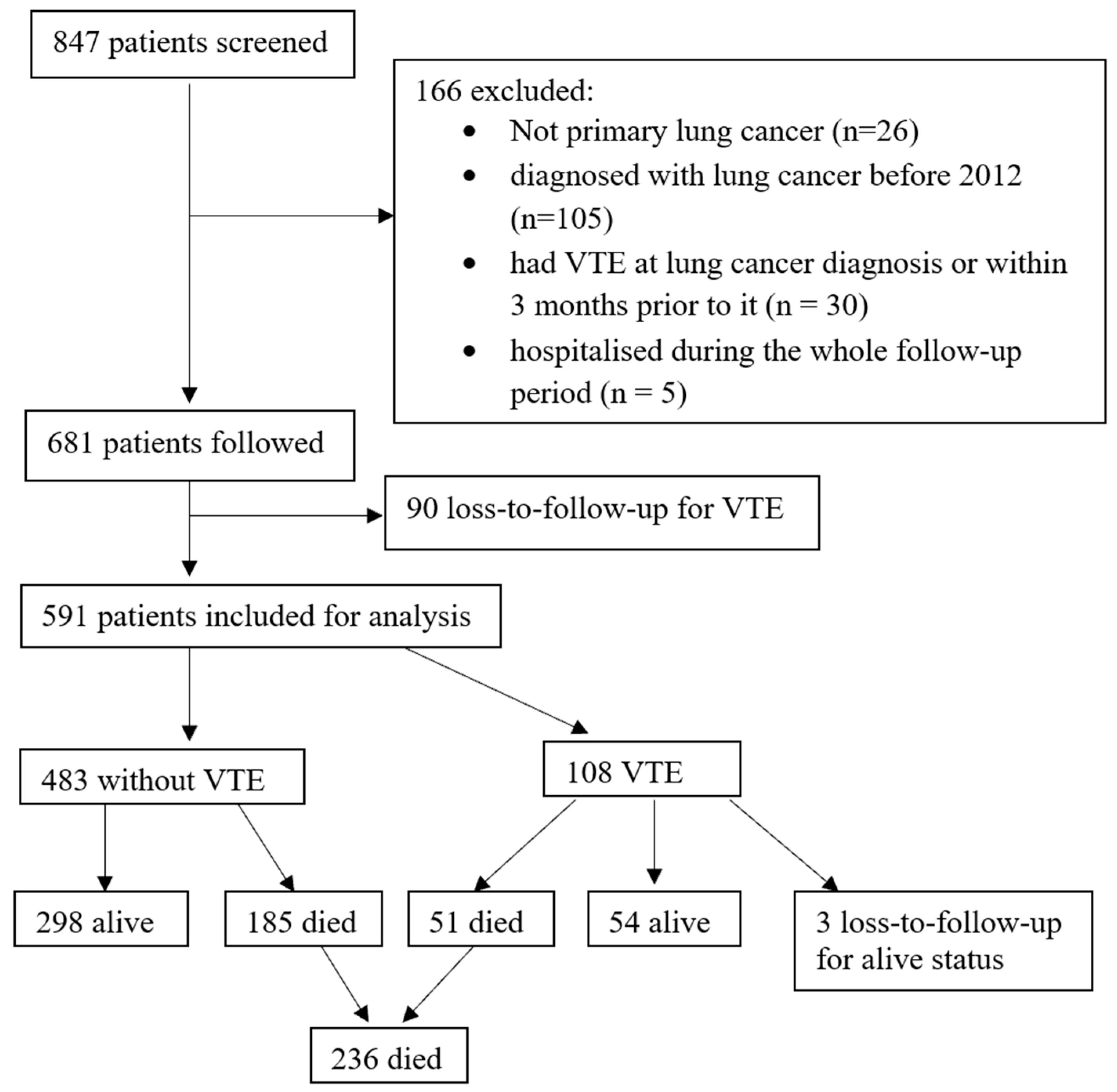
2. Materials and Methods
2.1. Study Design
2.2. Study Variables
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Performance of Risk Scores
3.3. Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L., Jr.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.-R.; Samarawickrema, I.; Naunton, M.; Peterson, G.M.; Yip, D.; Newman, P.; Mortazavi, R. Models for predicting venous thromboembolism in ambulatory patients with lung cancer: A systematic review and meta-analysis. Thromb. Res. 2024, 234, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Chan, W.-S.E.; Wiedemann, S.; Hoeller, C.; Tuchmann, F.; Aretin, M.-B.; Fuereder, T.; Zöchbauer-Müller, S.; Preusser, M.; Pabinger, I.; et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021, 137, 1669–1678. [Google Scholar] [CrossRef]
- Chew, H.K.; Davies, A.M.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. The incidence of venous thromboembolism among patients with primary lung cancer. J. Thromb. Haemost. 2008, 6, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Alexander, M.; Harris, S.; Underhill, C.; Torres, J.; Sharma, S.; Lee, N.; Wong, H.; Eek, R.; Michael, M.; Tie, J.; et al. Risk-Directed Ambulatory Thromboprophylaxis in Lung and Gastrointestinal Cancers: The TARGET-TP Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1536–1545. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Howlett, J.; Benzenine, E.; Cottenet, J.; Foucher, P.; Fagnoni, P.; Quantin, C. Could venous thromboembolism and major bleeding be indicators of lung cancer mortality? A nationwide database study. BMC Cancer 2020, 20, 1–11. [Google Scholar]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Gates, L.E.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3063–3071. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; van Es, N. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Poniewierski, M.S.; Culakova, E.; Lyman, G.H.; Khorana, A.A.; Pabinger, I.; Agnelli, G.; Liebman, H.A.; Vicaut, E.; Meyer, G.; et al. Predictors of Venous Thromboembolism and Early Mortality in Lung Cancer: Results from a Global Pro-spective Study (CANTARISK). Oncologist 2018, 23, 247–255. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Ventresca, M.; Di Nisio, M.; Zhou, Q.; Noble, S.; Crowther, M.; Briel, M.; Garcia, D.; Lyman, G.H.; Macbeth, F.; et al. The Khorana score for prediction of venous thromboembolism in cancer patients: An individual patient data meta-analysis. J. Thromb. Haemost. 2020, 18, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; LaBianca, R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chem-otherapy: The Protecht score. Intern. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, U.; Sinn, M.; Stieler, J.; Riess, H. Primary pharmacological prevention of thromboembolic events in ambulatory patients with advanced pan-creatic cancer treated with chemotherapy? Dtsch. Med. Wochenschr. 2013, 138, 2084–2088. [Google Scholar]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; on behalf of the COMPASS–CAT Working Group. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS–Cancer-Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef]
- Rupa-Matysek, J.; Lembicz, M.; Rogowska, E.K.; Gil, L.; Komarnicki, M.; Batura-Gabryel, H. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med. Oncol. 2018, 35, 63. [Google Scholar] [CrossRef]
- Syrigos, K.; Grapsa, D.; Sangare, R.; Evmorfiadis, I.; Larsen, A.K.; Van Dreden, P.; Boura, P.; Charpidou, A.; Kotteas, E.; Sergentanis, T.N.; et al. Prospective Assessment of Clinical Risk Factors and Biomarkers of Hypercoagulability for the Identification of Patients with Lung Adenocarcinoma at Risk for Cancer-Associated Thrombosis: The Observational ROADMAP-CAT Study. Oncologist 2018, 23, 1372–1381. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Di Nisio, M.; van Es, N.; Rotunno, L.; Anzoletti, N.; Falcone, L.; De Tursi, M.; Natoli, C.; Tinari, N.; Cavallo, I.; Valeriani, E.; et al. Long-term performance of risk scores for venous thromboembolism in ambulatory cancer patients. J. Thromb. Thrombolysis 2019, 48, 125–133. [Google Scholar] [CrossRef]
- van Es, N. Dynamic prediction modeling for cancer-associated venous thromboembolism. J. Thromb. Haemost. 2020, 18, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Martini, F.; Portarena, I.; Massimiani, G.; Riondino, S.; La Farina, F.; Mariotti, S.; Guadagni, F.; Roselli, M. Novel high-sensitive D-dimer determination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin. Lung Cancer 2012, 13, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Königsbrügge, O.; Posch, F.; Riedl, J.; Reitter, E.-M.; Zielinski, C.; Pabinger, I.; Ay, C. Association Between Decreased Serum Albumin With Risk of Venous Thromboembolism and Mortality in Cancer Patients. Oncologist 2016, 21, 252–257. [Google Scholar] [CrossRef]

| Variables | All (n = 591) | VTE (n = 108) | No VTE (n = 483) | p Value |
|---|---|---|---|---|
| Patient-related risk factors | ||||
| Age at diagnosis in years, median (p25–p75) | 67 (58–74) | 68 (61–74) | 67 (58–74) | 0.22 |
| Male, n (%) | 316 (53) | 58 (54) | 258 (53) | 0.96 |
| Indigenous, n (%) | 20 (3) | 6 (6) | 14 (3) | 0.17 |
| Ever smoker, n (%) | 471 (80) | 88 (83) | 383 (80) | 0.45 |
| Body Mass Index in kg/m2, median (p25–p75) | 25.5 (21.7–29.9) | 25.7 (21.5–29.5) | 25.5 (21.9–29.9) | 0.97 |
| ECOG PS, median (p25–p75) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.30 |
| Obesity, n (%) | 140 (24) | 25 (24) | 115 (24) | 0.88 |
| Hypertension, n (%) | 271 (46) | 48 (44) | 223 (46) | 0.75 |
| Hypercholesterolaemia/hyperlipidaemia/dyslipidaemia, n (%) | 196 (33) | 36 (33) | 160 (33) | 0.97 |
| Diabetes, n (%) | 95 (16) | 17 (16) | 78 (16) | 0.92 |
| Peripheral artery disease, n (%) | 23 (4) | 2 (2) | 21 (4) | 0.23 |
| Coronary artery disease, n (%) | 0.44 | |||
| No myocardial infarction | 38 (6) | 5 (5) | 33 (7) | |
| Had myocardial infarction # | 45 (8) | 6 (6) | 39 (8) | |
| Ischaemic stroke, n (%) | 34 (6) | 5 (5) | 29 (6) | 0.58 |
| Haemorrhagic stroke, n (%) | 1 (<1) | 0 (0) | 1 (<1) | -- |
| Heart failure, n (%) | 16 (3) | 2 (2) | 14 (3) | 0.55 |
| Atrial fibrillation, n (%) | 73 (12) | 11 (10) | 62 (13) | 0.45 |
| Chronic liver disease, n (%) | 8 (1) | 0 (0) | 8 (2) | 0.18 |
| Chronic kidney disease, n (%) | 28 (5) | 4 (4) | 24 (5) | 0.58 |
| Chronic obstruction pulmonary disease, n (%) | 189 (32) | 31 (29) | 158 (33) | 0.42 |
| History of VTE, n (%) | 26 (4) | 7 (6) | 19 (4) | 0.24 |
| Number of comorbidities *, median (p25–p75) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.65 |
| Medications during follow-up | ||||
| Anticoagulants, n (%) | 75 (13) | 9 (8) | 66 (14) | 0.13 |
| Antiplatelets, n (%) | 125 (21) | 19 (18) | 106 (22) | 0.32 |
| Selective estrogen receptor modulators, n (%) | 6 (1) | 1 (<1) | 5 (1) | -- |
| Hormone replacement therapy, n (%) | 9 (2) | 4 (4) | 5 (1) | 0.04 |
| Corticosteroids, n (%) | 262 (44) | 50 (46) | 212 (44) | 0.65 |
| Antipsychotics, n (%) | 172 (29) | 26 (24) | 146 (30) | 0.20 |
| Erythropoietin, n (%) | 43 (7) | 4 (4) | 39 (8) | 0.11 |
| Statins, n (%) | 187 (32) | 33 (31) | 154 (32) | 0.79 |
| Proton-pump inhibitors, n (%) | 226 (38) | 48 (44) | 178 (37) | 0.14 |
| Cancer-related risk factors | ||||
| Non-small-cell lung cancer (NSCLC), n (%) | 477 (81) | 91 (84) | 386 (80) | 0.32 |
| Poorly/undifferentiated, n (%) | 204 (67) | 34 (74) | 170 (66) | 0.27 |
| Advanced stage, n (%) | 389 (86) | 78 (92) | 311 (85) | 0.11 |
| Metastasis, n (%) | 439 (87) | 86 (91) | 353 (86) | 0.25 |
| Chemotherapy, n (%) | 486 (82) | 82 (76) | 404 (84) | 0.06 |
| Etoposide, n (%) | 115 (19) | 18 (17) | 97 (20) | 0.42 |
| Platinum, n (%) | 462 (78) | 78 (72) | 384 (80) | 0.10 |
| Carboplatin, n (%) | 356 (60) | 63 (58) | 293 (61) | 0.66 |
| Cisplatin, n (%) | 117 (20) | 16 (15) | 101 (21) | 0.15 |
| Gemcitabine, n (%) | 76 (13) | 16 (15) | 60 (12) | 0.50 |
| Vinorelbine, n (%) | 136 (23) | 21 (19) | 115 (24) | 0.33 |
| Docetaxel, n (%) | 13(2) | 5 (5) | 8 (2) | 0.06 |
| Paclitaxel, n (%) | 60 (10) | 8 (7) | 52 (11) | 0.30 |
| Pemetrexed, n (%) | 98 (17) | 16 (15) | 82 (17) | 0.59 |
| Radiotherapy, n (%) | 287 (49) | 50 (46) | 237 (49) | 0.58 |
| Target therapy, n (%) | 24 (4) | 4 (4) | 20 (4) | 0.84 |
| Immune checkpoint inhibitors, n (%) | 214 (36) | 32 (30) | 182 (38) | 0.13 |
| Hospitalisation within 3 months prior to the diagnosis of lung cancer, n (%) | 168 (28) | 27 (25) | 141 (29) | 0.38 |
| Central venous catheter, n (%) | 39 (7) | 10 (10) | 29 (6) | 0.24 |
| Resection (lung surgery as cancer treatment), n (%) | 96 (16) | 7 (6) | 89 (18) | 0.002 |
| Biomarkers | ||||
| Haemoglobin in g/L, median (p25–p75) | 134 (121–144) | 135 (119–144) | 134 (123–145) | 0.54 |
| Haemoglobin < 100 g/L, n (%) | 26 (4) | 8 (7) | 18 (4) | 0.09 |
| White cell count in 109/L, median (p25–p75) | 9.0 (7.3–11.3) | 9.9 (7.9–12.6) | 8.8 (7.3–11.2) | 0.009 |
| White cell count ≥ 11 × 109/L, n (%) | 166 (28) | 40 (37) | 126 (26) | 0.02 |
| Platelet count in 109/L, median (p25–p75) | 287 (226–358) | 296 (246–365) | 286 (224–354) | 0.17 |
| Platelet count ≥ 350 × 109/L, n (%) | 157 (27) | 31 (29) | 126 (26) | 0.57 |
| Red cell distribution width in %, median (p25–p75) | 14.0 (13.3–14.9) | 14.2 (13.3–14.9) | 13.9 (13.3–14.9) | 0.47 |
| Neutrophil count in 109/L, median (p25–p75) | 6.11 (4.67–8.42) | 6.70 (5.10–9.94) | 6.01 (4.60–8.23) | 0.01 |
| Lymphocyte count in 109/L, median (p25–p75) | 1.58 (1.17–2.08) | 1.63 (1.22–2.15) | 1.57 (1.17–2.06) | 0.53 |
| Monocyte count in 109/L, median (p25–p75) | 0.73 (0.57–0.95) | 0.73 (0.60–1.00) | 0.73 (0.55–0.93) | 0.11 |
| Platelet to lymphocyte ratio (PLR), median (p25–p75) | 182 (126–264) | 182 (127–269) | 182 (126–262) | 0.92 |
| Neutrophil to lymphocyte ratio (NLR), median (p25–p75) | 3.77 (2.48–6.31) | 4.28 (2.82–6.25) | 3.69 (2.43–6.32) | 0.20 |
| Abnormal PT, n (%) | 25 (6) | 5 (6) | 20 (6) | 0.74 |
| Abnormal INR, n (%) | 14 (3) | 0 (0) | 14 (4) | 0.08 |
| Abnormal aPTT, n (%) | 26 (6) | 5 (6) | 21 (6) | 0.82 |
| Low eGFR (<60 mL/min/1.73m2), n (%) | 65 (11) | 13 (12) | 52 (11) | 0.67 |
| Total bilirubin above normal range, n (%) | 24 (4) | 3 (3) | 21 (4) | 0.46 |
| Alanine aminotransferase above normal range, n (%) | 87 (15) | 16 (15) | 71 (15) | 0.95 |
| Alkaline phosphatase above normal range, n (%) | 160 (27) | 32 (30) | 128 (27) | 0.48 |
| Gamma-glutamyl transferase above normal range, n (%) | 195 (33) | 38 (36) | 157 (33) | 0.55 |
| Albumin below normal range, n (%) | 57 (10) | 17 (16) | 40 (8) | 0.02 |
| Corrected total calcium above normal range, n (%) | 69 (12) | 13 (13) | 56 (12) | 0.84 |
| C-reactive protein in mg/L, median (p25–p75) | 28 (8–69) | 24 (10–52) | 32 (7–70) | 0.90 |
| All (n = 591) | VTE (n = 108) | No VTE (n = 483) | p Value | |
|---|---|---|---|---|
| Clinical outcomes | ||||
| Ischaemic stroke, n (%) | 11 (2) | 4 (4) | 7 (1) | 0.12 |
| Peripheral artery disease, n (%) | 4 (<1) | 1 (<1) | 3 (<1) | -- |
| Coronary artery disease, n (%) | 12 (2) | 4 (4) | 8 (2) | 0.16 |
| Death within one year since cancer diagnosis, n (%) | 236 (40) | 51 (49) | 185 (38) | 0.05 |
| Risk scores | ||||
| Khorana score (n = 570), median (p25–p75) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.13 |
| Khorana score ≥ 2 points, n (%) | 298 (52) | 63 (61) | 235 (50) | 0.06 |
| Khorana score ≥ 3 points, n (%) | 101 (18) | 19 (18) | 82 (18) | 0.87 |
| PROTECHT score (n = 570), median (p25–p75) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.49 |
| PROTECHT score ≥ 2 points, n (%) | 526 (92) | 97 (93) | 429 (92) | 0.68 |
| PROTECHT score ≥ 3 points, n (%) | 248 (44) | 50 (48) | 198 (42) | 0.30 |
| CONKO score (n = 370), median (p25–p75) | 2 (1–2) | 2 (1–3) | 2 (1–2) | 0.02 |
| CONKO score ≥ 2 points, n (%) | 197 (53) | 41 (68) | 156 (50) | 0.01 |
| CONKO score ≥ 3 points, n (%) | 85 (23) | 17 (28) | 68 (22) | 0.28 |
| COMPASS-CAT score (n = 429), median (p25–p75) | 11 (6–12) | 9 (6–11) | 11 (6–13) | 0.84 |
| COMPASS-CAT score ≥ 7, n (%) | 298 (69) | 57 (70) | 241 (69) | 0.99 |
| COMPASS-CAT score ≥ 11, n (%) | 219 (51) | 40 (49) | 179 (52) | 0.65 |
| VTE Prevalence % High Risk vs. Low Risk, p Value | Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | Positive Predictive Value (%, 95% CI) | Negative Predictive Value (%, 95% CI) | AUC (95% CI) | Odds Ratio (95% CI) | Hosmer-Lemeshow Test, p value | ||
| 6 months | Khorana score Threshold of 2 points Threshold of 3 points | 18.1% vs. 11.0%, p = 0.02 16.8% vs. 14.3%, p = 0.51 | 64.3 (53.1, 74.4) 20.2 (12.3, 30.4) | 49.8 (45.3, 54.3) 82.7 (79.1, 86.0) | 17.4 (13.6, 21.8) 16.8 (10.1, 25.6) | 89.5 (84.7, 93.3) 85.7 (82.2, 88.8) | 0.57 (0.51, 0.63) 0.52 (0.47, 0.56) | 1.80 (1.11, 2.88) 1.21 (0.68, 2.16) | 0.09 |
| PROTECHET score Threshold of 2 points Threshold of 3 points | 14.8% vs. 13.6%, p = 0.83 16.9% vs. 13.0%, p = 0.19 | 92.9 (85.1, 97.3) 50.0 (38.9, 61.1) | 7.8 (5.6, 10.6) 57.6 (53.1, 62.1) | 14.7 (11.8, 18.0) 16.9 (12.5, 22.2) | 84.8 (68.1, 94.9) 87.0 (82.8, 90.4) | 0.50 (0.47, 0.53) 0.54 (0.48, 0.60) | 1.10 (0.46, 2.63) 1.36 (0.86, 2.16) | 0.12 | |
| CONKO score Threshold of 2 points Threshold of 3 points | 18.3% vs. 6.9%, p = 0.001 18.8% vs. 11.2%, p = 0.07 | 75.0 (60.4, 86.4) 33.3 (20.4, 48.4) | 50.0 (44.4, 55.6) 78.6 (73.7, 82.9) | 18.3 (13.1, 24.4) 18.8 (11.2, 28.8) | 93.1 (88.2, 96.4) 88.8 (84.5, 92.2) | 0.63 (0.56, 0.69) 0.56 (0.49, 0.63) | 3.00 (1.52, 5.91) 1.83 (0.96, 3.51) | 0.12 | |
| COMPASS-CAT score Threshold of 7 points Threshold of 11 points | 14.8% vs. 15.3%, p = 0.89 13.7% vs. 16.2%, p = 0.47 | 68.8 (55.9, 79.8) 46.9 (34.3, 59.8) | 30.4 (25.7, 35.4) 48.2 (43.0, 53.5) | 14.8 (10.9, 19.3) 13.7 (9.4, 19.0) | 84.7 (77.4, 90.4) 83.8 (78.1, 88.5) | 0.50 (0.43, 0.56) 0.48 (0.41, 0.54) | 0.96 (0.54, 1.70) 0.82 (0.48, 1.39) | 0.36 | |
| 12 months | Khorana score Threshold of 2 points Threshold of 3 points | 21.1% vs. 15.1%, p = 0.06 18.8% vs. 18.1%, p = 0.87 | 60.6 (50.5, 70.0) 18.3 (11.4, 27.1) | 49.6 (44.9, 54.2) 82.4 (78.6, 85.8) | 21.1 (16.6, 26.2) 18.8 (11.7, 27.8) | 84.9 (80.1, 89.0) 81.9 (78.1, 85.3) | 0.55 (0.50, 0.60) 0.50 (0.46, 0.54) | 1.51 (0.98, 2.32) 1.05 (0.61, 1.81) | 0.10 |
| PROTECHET score Threshold of 2 points Threshold of 3 points | 18.4% vs. 15.9%, p = 0.68 20.1% vs. 16.8%, p = 0.30 | 93.3 (86.6, 97.3) 48.1 (38.2, 58.1) | 7.9 (5.7, 10.8) 57.5 (52.9, 62.0) | 18.4 (15.2, 22.0) 20.2 (15.4, 25.7) | 84.1 (69.9, 93.4) 83.2 (78.7, 87.1) | 0.51 (0.48, 0.53) 0.53 (0.48, 0.58) | 1.20 (0.53, 2.70) 1.25 (0.82, 1.92) | 0.12 | |
| CONKO score Threshold of 2 points Threshold of 3 points | 20.8% vs. 11.0%, p = 0.01 20.0% vs. 15.1%, p = 0.28 | 68.3 (55.0, 79.7) 28.3 (17.5, 41.4) | 49.7 (44.0, 55.4) 78.1 (73.0, 82.5) | 20.8 (15.4, 27.2) 20.0 (12.1, 30.1) | 89.0 (83.4, 93.3) 84.9 (80.2, 88.9) | 0.59 (0.52, 0.66) 0.53 (0.47, 0.59) | 2.13 (1.19, 3.81) 1.41 (0.76, 2.61) | 0.17 | |
| COMPASS-CAT score Threshold of 7 points Threshold of 11 points | 19.1% vs. 19.1%, p = 0.99 18.3% vs. 20.0%, p = 0.65 | 69.5 (58.4, 79.2) 48.8 (37.6, 60.1) | 30.5 (25.7, 35.7) 48.4 (43.0, 53.8) | 19.1 (14.8, 24.1) 18.3 (13.4, 24.0) | 80.9 (73.1, 87.3) 80.0 (73.9, 85.2) | 0.50 (0.45, 0.56) 0.49 (0.43, 0.55) | 1.00 (0.60, 1.68) 0.89 (0.55, 1.44) | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.-R.; Yip, D.; Peterson, G.M.; Samarawickrema, I.; Naunton, M.; Newman, P.; Mortazavi, R. External Validation of Risk Scores for Predicting Venous Thromboembolism in Ambulatory Patients with Lung Cancer. Cancers 2024, 16, 3165. https://doi.org/10.3390/cancers16183165
Yan A-R, Yip D, Peterson GM, Samarawickrema I, Naunton M, Newman P, Mortazavi R. External Validation of Risk Scores for Predicting Venous Thromboembolism in Ambulatory Patients with Lung Cancer. Cancers. 2024; 16(18):3165. https://doi.org/10.3390/cancers16183165
Chicago/Turabian StyleYan, Ann-Rong, Desmond Yip, Gregory M. Peterson, Indira Samarawickrema, Mark Naunton, Phillip Newman, and Reza Mortazavi. 2024. "External Validation of Risk Scores for Predicting Venous Thromboembolism in Ambulatory Patients with Lung Cancer" Cancers 16, no. 18: 3165. https://doi.org/10.3390/cancers16183165
APA StyleYan, A.-R., Yip, D., Peterson, G. M., Samarawickrema, I., Naunton, M., Newman, P., & Mortazavi, R. (2024). External Validation of Risk Scores for Predicting Venous Thromboembolism in Ambulatory Patients with Lung Cancer. Cancers, 16(18), 3165. https://doi.org/10.3390/cancers16183165









