Simple Summary
This study examined the link between metabolic dysfunction-associated steatotic liver disease (MASLD) and kidney cancer risk. Over 8.8 million participants (aged 20–79) were followed for a median of 13.3 years. The study found that participants with MASLD and those with MASLD plus increased alcohol intake (MetALD) had a significantly higher risk of developing kidney cancer compared to those without MASLD. The risk was especially elevated in younger patients. A cumulative relationship between metabolic dysfunction and kidney cancer risk was also observed. The findings highlight the need for a comprehensive approach to metabolic health, particularly focusing on younger individuals.
Abstract
Background: This study investigated the association between metabolic dysfunction-associated steatotic liver disease (MASLD) and Kidney Cancer Risk, as the incidence of both diseases gradually increases owing to metabolic health issues. Methods: Participants (aged 20–79) undergoing a national health examination between 2009 and 2010 were monitored for new-onset kidney cancer. The MASLD spectrum was classified as non-MASLD, MASLD, or MASLD with increased alcohol uptake (MetALD). Kidney Cancer Risk associated with the MASLD spectrum was estimated using multivariate Cox proportional hazard models. Age- and sex-stratified analyses were also performed. Results: Among 8,829,510 participants (median follow-up 13.3 years), the proportion of non-MASLD, MASLD, and MetALD was 64.9%, 30.3%, and 4.7%, respectively, with newly developed kidney cancer in 17,555 participants. Kidney cancer was significantly increased with MASLD (adjusted hazard ratio [aHR] 1.51, 95% confidence interval [CI] 1.46–1.56) and MetALD (aHR 1.51, 95% CI 1.42–1.61), compared with the non-MASLD group. Kidney Cancer Risk was the highest among young populations (aHR 1.93, 95% CI 1.77–2.11 for MASLD and aHR 1.91, 95% CI 1.65–2.22 for MetALD), according to stratification analysis. Furthermore, the cumulative relationship between metabolic dysfunction and Kidney Cancer Risk was confirmed across all MASLD spectra. Conclusions: Our study highlights the positive association between MASLD and Kidney Cancer Risk, emphasizing a comprehensive approach to metabolic health. This also serves as a call to devote closer attention to the metabolic health of younger patients.
1. Introduction
The global incidence of kidney cancer has been increasing, making this disease a significant oncological concern [1]. Kidney cancer is typically diagnosed at an advanced stage, leading to limited treatment options and a poor prognosis. Furthermore, it is partly attributed to the increasing prevalence of metabolic syndrome influenced by Westernized dietary habits and lifestyles [2]. Previous studies have identified components of metabolic dysfunction, such as increased waist circumference, triglycerides, fasting blood glucose, and blood pressure, as risk and prognostic factors for kidney cancer [3,4]. Such findings prompt the need for further studies to identify high-risk patients for effective screening and appropriate treatment.
Hepatic steatosis has been increasingly recognized as a critical global metabolic health concern as the prevalence of metabolic syndrome and/or obesity simultaneously increases [5,6,7]. The terminology for hepatic steatosis has undergone significant changes. Initially defined as non-alcoholic fatty liver disease (NAFLD), this classification failed to address metabolic factors, alcohol consumption, and other etiologies, complicating risk stratification [8,9,10,11]. Later, metabolic dysfunction-associated fatty liver disease (MAFLD) was introduced to include metabolically complex liver conditions that overlapped with chronic liver diseases of various etiologies not covered by NAFLD [1,12,13]. However, MAFLD could not account for mixed etiologies involving both metabolic factors and alcohol consumption, nor could it include lean patients with hepatic steatosis [14]. Consequently, a new classification system, steatotic liver disease (SLD), was introduced, recognizing the interaction between metabolic conditions and alcohol consumption. This led to the development of new terms: metabolic dysfunction-associated steatotic liver disease (MASLD) and MASLD with increased alcohol consumption (MetALD) [15,16,17,18].
Previous studies assessing the effects of metabolic components on kidney outcomes have primarily focused on chronic kidney disease (CKD) rather than kidney cancer [19,20,21,22]. Moreover, although various studies have investigated the association between hepatic steatosis and extrahepatic malignancies, few have assessed its association with kidney cancer [23,24,25,26].
Hence, this study aimed to evaluate the association between MASLD and the risk of kidney cancer by incorporating demographic stratification to ascertain the effects of metabolic dysfunction on the risk of kidney cancer.
2. Materials and Methods
This nationwide cohort study used data from the National Health Insurance Service (NHIS) database in the Republic of Korea, which contains information on 97.2% of the entire population [27]. The database contains an array of demographic and socioeconomic characteristics, information on outpatient visits or hospitalizations, diagnostic codes, health checkup data, and comprehensive drug prescriptions. The NHIS conducts a comprehensive biennial health examination for adults. This examination encompasses clinical and biochemical tests, along with the collection of lifestyle information through structured questionnaires. Study subjects ranging in age from 20 to 79 years who underwent a national health examination between 2009 and 2010 were included. The date of the health examination was designated as the index date for each participant.
The study implemented specific exclusion criteria as follows: incomplete data regarding residential area or household income, or lacking values in measurements or blood tests; history of concurrent liver disease including viral hepatitis (B15–B19), alcoholic liver disease (K70), or documented alcohol intake exceeding 420 g/week for males or 350 g/week for females, toxic liver disease (K71), biliary cholangitis (K74.3–K74.5), autoimmune hepatitis (K75.4), Wilson’s disease (E83.0), and hemochromatosis (E83.1); and history of any type of malignancy.
This study adhered to the ethical principles of the Declaration of Helsinki and Istanbul and was approved by the Institutional Review Board of Severance Hospital (IRB number: 4-2022-0813). The requirement for informed consent was waived owing to the retrospective nature of this study.
2.1. Main Outcomes
The primary outcome of this study was the incidence of new-onset kidney cancer, and all-cause mortality was considered the secondary outcome. The diagnosis of kidney cancer was based on the first recorded hospital encounter, coded as C64 in the International Classification of Diseases, 10th Revision (ICD-10), along with code V193. These data were derived from a registry initiative implemented by the government of the Republic of Korea in 2006, aimed at reducing copayments for rare and challenging diseases [28]. The follow-up period for the patients was extended until the development of kidney cancer, death, or December 2022, whichever occurred first.
2.2. Variables and Covariates
The MASLD classification in this study was based on the simultaneous presence of SLD and ≥1 cardiometabolic risk factors. As such, MASLD was characterized by a fatty liver index (FLI) ≥ 30, a criterion based on and aligned with methodologies used in other Asian research studies [29]. Cardiometabolic risk factors encompassed the following: body mass index (BMI) ≥ 23 kg/m2 or a waist circumference (WC) ≥ 90 cm for males and ≥80 cm for females; fasting glucose level ≥ 100 mg/dL, diagnosis of type 2 diabetes, or the use of glucose-lowering medications; blood pressure ≥ 130/85 mmHg or use of antihypertensive drugs; triglyceride concentrations ≥ 150 mg/dL or use of lipid-lowering medications; or low high-density lipoprotein cholesterol levels, defined as <40 mg/dL for males and <50 mg/dL for females, or use of lipid-lowering drugs. Among individuals diagnosed with MASLD, those who reported moderate alcohol consumption levels (weekly intake, 210–420 g for males and 140–350 g for females, in which 1 “shot” is equivalent to 10 g of alcohol) were classified as MetALD—more specifically, MASLD and increased alcohol consumption. Consequently, the individuals were primarily categorized into three groups: non-MASLD, MASLD, and MetALD.
The covariates integrated into the analysis included age, sex, residential area (capital, metropolitan, or other), household income quartile, employment status, smoking history, physical activity, and Charlson Comorbidity Index (CCI). Employment status was determined on the basis of the insurance type reported in the NHIS database for the index year. Based on lifestyle questionnaires, smoking status was categorized into non-smokers, former smokers, and current smokers. Physical activity levels were assessed by calculating the metabolic equivalent of tasks (METs)-h/week, summing the total vigorous (7 METs), moderate (4 METs), and walking (2.9 METs) activities reported [30]. Physical activity was subdivided into four categories according to METs-min/week: 0–499, 500–999, 1000–1499, and ≥1500. The updated CCI was calculated considering diagnostic codes for each disease category, based on ≥1 hospitalization(s) or ≥3 outpatient visits before the index date [31]. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, derived from national health examination results.
2.3. Statistical Analysis
Initial participant characteristics are expressed as a median (interquartile range [IQR]) or proportion (number). The cumulative incidence rates of kidney cancer and all-cause mortality in each group were calculated using the Kaplan–Meier method and compared using the log-rank test. This study applied multivariable Cox proportional hazard models to determine the adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for both primary and secondary outcomes. Model 1 included age and sex; Model 2 incorporated additional socioeconomic variables such as residential area, household income, and economic activity; and Model 3 included variables such as smoking history, physical activity, CKD, and CCI based on Model 2.
Multiple sensitivity analyses were also conducted. Stratified analyses were performed to determine kidney cancer risk according to age, sex, and the use of type 2 diabetes and statin medications. Age was grouped into three categories in the stratified analysis: 20–39, 40–64, and 65–79 years. Patients with a CCI score ≥ 6 were excluded. In addition, the primary analysis was replicated using different biochemical SLD models: FLI ≥ 60 (cut-off for severe SLD) [32]; FLI ≥ 31 for males and ≥18 for females [33]; and hepatic steatosis index (HSI) ≥ 36 [34].
Moreover, the cumulative relationship between the number of metabolic dysfunction components (ranging from 0 [none present] to 5 [all present]) and kidney cancer was investigated and summarized using heatmaps. This approach enabled statistical assessment of the cumulative effects of metabolic dysfunction on health risks. The risk for kidney cancer associated with the number of metabolic dysfunctions was also assessed with subgroups with FLI 30–59, or ≥60, and alcohol consumption level (either MASLD or MetALD).
All statistical analyses were performed using SAS Enterprise version 7.1 (SAS Institute, Cary, NC, USA) and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). All estimates were two-sided, and differences were considered statistically significant at p < 0.05.
3. Results
3.1. Baseline Characteristics of Patients among the Entire Population
Among the initial cohort of 9,617,980 patients, the final analysis included data from 8,829,510 individuals after excluding specific cases (Figure 1). The baseline characteristics of the patients are summarized in Table 1. Males accounted for 54.1% of the entire population, with a median age of 46.53 years (IQR: 36–56 years). Overall, 64.9% (n = 5,731,764) of the population did not have MALD, 30.3% (n = 2,679,407) had MASLD, and 4.7% (n = 418,339) had MetALD. An incremental increase was observed from non-MASLD to MASLD and then to MetALD groups in terms of the proportion of participants 40–59 years of age, male sex, and those engaged in economic activities (p < 0.001). Additionally, individuals in the MASLD and MetALD groups indicated a lower proportion of chronic kidney disease compared to the non-MASLD group (p < 0.001). Relative to the non-MASLD group, individuals in both the MASLD and MetALD groups had a higher proportion of above-average income, residing in non-metropolitan areas, CCI scores ≥ 2, smoking history, and <500 METs-min/week of physical activity (p < 0.001).
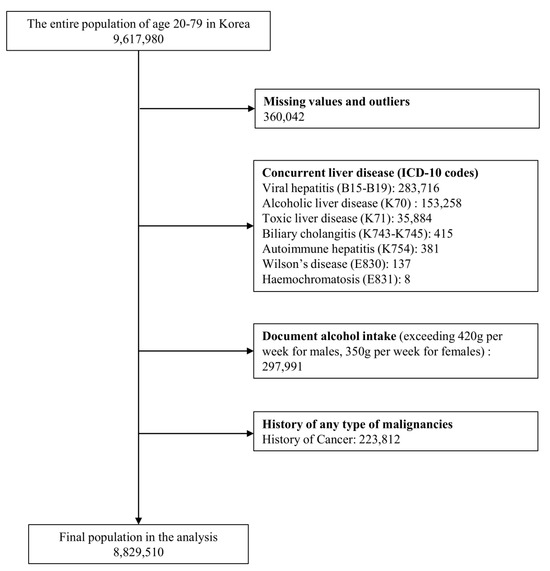
Figure 1.
Flow chart of the participant inclusion process.

Table 1.
Baseline characteristics of participants according to MASLD spectrum.
3.2. Cumulative Risk for Primary and Secondary Outcomes According to MASLD or MetALD Compared with Non-MASLD
Over a median follow-up period of 13.25 years, 17,555 participants (0.20% of the total) developed kidney cancer. Among them, 8179 (0.15%), 8105 (0.19%), and 822 (0.009%) patients were classified into the non-MASLD, MASLD, and MetALD groups, respectively. The age-standardized 5-year cumulative incidence rates of kidney cancer were 0.04% for the non-MASLD group and 0.07% for both the MASLD and MetALD groups. Statistical analysis showed significant differences, with p < 0.001 between the non-MASLD vs. MASLD groups and non-MASLD vs. MetALD groups, while there was no significant difference between MASLD and MetALD (p = 0.97) (Figure 2A).
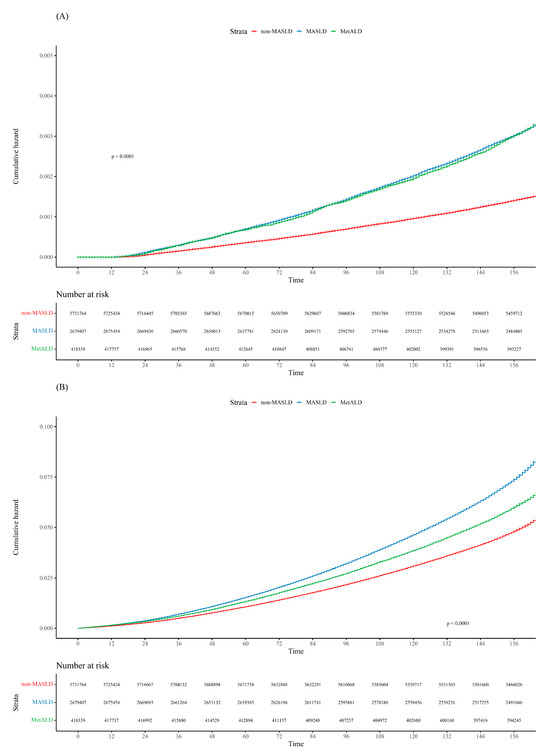
Figure 2.
Cumulative incidence plot of (A) kidney cancer and (B) all-cause mortality according to the MASLD spectrum.
During the follow-up period, 539,834 patients died. The all-cause mortality rates in the non-MASLD, MASLD, and MetALD groups were 5.09%, 5.73%, and 5.80%, respectively (p < 0.001). The age-standardized 5-year cumulative incidence rates of all-cause mortality were 1.07% in the non-MASLD group, 1.53% in the MASLD group, and 1.33% in the MetALD group. Statistical analysis revealed significant differences among the groups, with p-values of <0.001 for non-MASLD vs. MASLD, non-MASLD vs. MetALD, and MASLD vs. MetALD (Figure 2B).
The data reported in Table 2 show that both MASLD and MetALD were significantly associated with a higher risk of kidney cancer through Model 3 using Cox proportional hazard models: adjusted HR 1.51 (95% CI, 1.46–1.56) for the MASLD group and 1.51 (95% CI, 1.42–1.61) for the MetALD group. The analysis indicated a similar risk of kidney cancer between the MASLD and MetALD groups (p = 0.97).

Table 2.
Adjusted HR (95% CI) of KC associated with metabolic SLDs.
Similarly, both MASLD and MetALD, compared with non-MASLD, were significantly associated with higher all-cause mortality: adjusted HR 1.09 (95% CI, 1.09–1.11) for the MASLD group and 1.19 (95% CI, 1.17–1.20) for the MetALD group. In contrast, the MetALD group exhibited a slightly higher all-cause mortality than the MASLD group.
3.3. Stratification Analyses of the Risk for Kidney Cancer According to MASLD or MetALD Compared with Non-MASLD
Stratification analyses according to age and sex for the risk of kidney cancer according to MASLD or MetALD compared with non-MASLD are summarized in Table 3. The risk of kidney cancer in the MASLD and MetALD groups remained significantly higher than that in the non-MASLD group, consistently among all age and sex subgroups (p < 0.001). Furthermore, similar to the main analyses, a comparable risk of kidney cancer between the MASLD and MetALD groups was observed in all age and sex subgroups (p > 0.001).

Table 3.
Stratified analyses on the association of KC risk with metabolic SLD by age and sex.
Notably, the impact of both MASLD and MetALD compared with non-MASLD for the risk for kidney cancer was the most prominent among the young age subgroup than among the middle and older subgroups (adjusted HR 1.91–1.93 vs. 1.32–1.51, respectively). In the stratification analyses based on the use of type 2 diabetes and statin medications (Table S1), the results indicated that individuals not taking diabetes or statin medications had a slightly higher risk of kidney cancer associated with MASLD/MetALD compared to those without MASLD.
Results of the sensitivity analysis, which included patients after excluding those with a CCI score ≥ 6 and those with various cut-off values to define SLD, are summarized in Table S2. Similar results were consistently reproduced; both the MASLD and MetALD groups exhibited a higher risk of kidney cancer than the non-MASLD group, and there was no significant difference between the MASLD and MetALD groups.
3.4. Analysis of the Risk for Kidney Cancer in Relation to Metabolic Burden Defined as the Number of Metabolic Components
The correlation between kidney cancer risk and metabolic burden, defined as the number of metabolic components (ranging from 0 [none present] to 5 [all present]), was further assessed across the MASLD spectrum (the number of metabolic components starting from 1 to 5 in the MASLD and MetALD groups). A cumulative relationship between the risk of kidney cancer and the number of metabolic components was observed in all MASLD categories, more specifically, non-MASLD, MASLD, and MetALD (Table 4). This relationship is also depicted in a heatmap illustrating the age-standardized incidence rate of kidney cancer (Figure 3).

Table 4.
Risk of KC associated with metabolic SLDs according to the number of metabolic components.
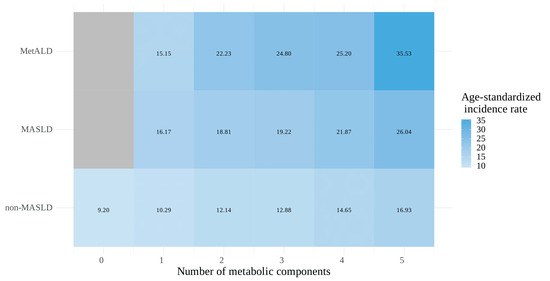
Figure 3.
Heatmap of the age-standardized incidence rate (per 100,000 person-years) for kidney cancer associated with the number of metabolic components across the MASLD spectrum.
In additional sensitivity analyses, the cumulative relationship between the risk of kidney cancer and the number of metabolic components persisted among various subgroups defined using alcohol consumption and/or different FLI cut-off values (Figure S1).
4. Discussion
In this comprehensive study based on a nationwide representative cohort in the Republic of Korea, we investigated the association between kidney cancer risk and MASLD or MetALD. We found that both MASLD and MetALD were significantly associated with an increased risk of kidney cancer compared with non-MASLD. The effect of increased alcohol intake within 420 g/week for males and 350 g/week for females, in addition to MASLD (the so-called MetALD), was negligible in increasing the risk of kidney cancer. Similar trends were observed in stratification analyses according to age and sex. To date, research investigating the risk factors of kidney cancer has been constrained by the relatively low incidence of the disease, which limits the statistical power to detect associations. However, primarily because the incidence of kidney cancer has been gradually increasing (to 431,288 new cases in 2020) [2], the need for comprehensive research investigating the risk factors for kidney cancer has become an important health issue.
Our study has several strengths. Using a large-scale nationwide dataset from the Republic of Korea, we drew robust conclusions regarding the positive association between kidney cancer risk and MASLD or MetALD. Approximately 25% of kidney cancers can be attributed to overweight or obesity [35]. Our study underscores the importance of controlling other metabolic dysfunctions such as hepatic steatosis, dyslipidemia, insulin resistance, and hypertension, in addition to obesity, for the primary prevention of kidney cancer. Second, by analyzing different age groups, we highlighted that the detrimental effect of metabolically unhealthy conditions on the risk for kidney cancer was the most prominent in the young age group compared with the middle and older subgroups (adjusted HR 1.91–1.93 vs. 1.32–1.51, respectively). This is a noteworthy finding, given that the incidence of kidney cancer among younger patients has steeply increased for several decades, possibly resulting from shifts in dietary habits and an increase in metabolic dysfunction [36,37,38]. Although the overall incidence of kidney cancer in the older patients remains higher than that in the young(er) patients, the carcinogenic effect of metabolic dysfunction is strongest in the young age group. This could be attributed to the generally lower baseline risk for kidney cancer in younger age groups, making the relative increase in the detrimental effects of metabolic dysfunction more pronounced. According to the baseline characteristics of metabolic components and lifestyle factors in the participation from each age group (Table S3), younger patients (age < 40) showed the biggest difference in BMI, the proportion of current smokers, blood pressure, and fasting blood sugar level between non-MASLD and MASLD/MetALD populations. Moreover, several murine studies specifically show that obesity and obesogenic diets not only raise the likelihood of developing malignancies but also speed up their progression and lead to their onset at younger ages [36,39,40]. This also emphasizes that younger patients are more susceptible to the oncogenic effects of metabolic dysfunction, indicating the importance of controlling metabolic dysfunction for effective prevention in specific populations. Finally, we confirmed consistent results using various stratification and sensitivity analyses.
However, the pathogenesis underlying this association remains unclear. The most likely explanation is that the accumulation and dysfunction of excess adipose tissue create an ideal environment for the initiation and progression of kidney cancer. This may be linked to factors such as hormonal imbalances, chronic tissue hypoxia, heightened inflammation, altered cellular energy metabolism, increased angiogenesis, epithelial-to-mesenchymal transition, and genomic instability. These changes connect unhealthy metabolic conditions with an increased risk of kidney cancer [41]. Interleukin-6 (IL-6), a significant marker in the pathogenesis of metabolic syndrome and its cardiovascular complications [42], also induces 5′ AMP-activated protein kinase phosphorylation, a critical process for IL-6-mediated glucose uptake and lipid oxidation [43], which is implicated in obesity-related cancers [44]. Moreover, a recent review highlighted visceral obesity’s involvement in kidney cancer, further emphasizing the metabolic impact on tumorigenesis [45]. In obesity, the expansion of adipose tissue leads to hypoxia, which triggers compensatory mechanisms like angiogenesis to restore oxygen supply. Hypoxia activates hypoxia-inducible factors (HIFs), specifically, HIF-1α and HIF-2α, which regulate several pathways related to metabolism, angiogenesis, and tumor growth [46]. In addition, the role of adipokines, such as leptin and adiponectin, in modulating inflammatory responses and influencing cancer progression has gained attention [47]. Leptin, often elevated in obese individuals, promotes cell proliferation and angiogenesis [48], while adiponectin has been linked to increased antitumor properties through suppressing mTOR and Stat3 pathways and stimulating the activity of 5′AMP-activated protein kinase (AMPK) [49]. These adipokines, in conjunction with HIF-mediated responses, further create a microenvironment conducive to tumor growth. Understanding the interplay among these factors may reveal novel therapeutic targets for obesity-associated kidney cancer. Further studies investigating the biological and epidemiological aspects are required to address these issues.
To address this unmet need, we assessed the correlation between the number of metabolic dysfunctions and the risk of kidney cancer and demonstrated that the risk increased incrementally with the aggregation of metabolic risk factors. Such a cumulative relationship shown in the heatmaps was reproduced not only in the main analyses but also in the sensitivity analyses according to FLI cut-offs and/or alcohol intake. Conversely, those with higher FLI (≥60) consistently exhibited an overall higher risk for kidney cancer than those with lower FLI (30–59) at every stratum according to the number of metabolic components, suggesting that significant intrahepatic fat accumulation may be regarded as another component defining the so-called “metabolic syndrome” in addition to the five existing criteria. Indeed, numerous studies have demonstrated a strong link between liver triacylglycerol accumulation—specifically, when exceeding 55 mg/g liver (5.5%), a threshold indicative of NAFLD—and visceral adipose tissue and insulin resistance, suggesting that liver triacylglycerol plays a crucial role in metabolic dysregulation [50,51,52].
The present study has several limitations. First, we defined SLD based on biochemical markers rather than imaging or histopathology, potentially leading to misclassification. However, to overcome this drawback, we performed sensitivity analyses using various biochemical scoring cutoffs, striving to ensure the accuracy of the SLD classification despite the absence of imaging or histopathological data. Second, another issue is that the NHIS database lacks data regarding specific variables such as dietary habits and genetic factors, which could act as unmeasured confounders, potentially introducing bias into our results. Third, different classes of therapies may have varying effects on the disease course of MASLD [53]. While data on therapies influencing metabolic status, such as diabetes or lipid-lowering medications, were available, their potential impact on the association between liver steatotic disease and kidney cancer was not fully explored in this study. Further analysis is needed to understand how these treatments may have influenced the findings. Finally, because this study focused on the population of the Republic of Korea, the findings may not apply to other ethnic or racial groups. Further studies are required to confirm these findings, as the impact of MASLD on kidney cancer may vary by race or ethnicity [54].
5. Conclusions
Our study delineates a positive association between MASLD and the risk of kidney cancer, emphasizing the importance of a comprehensive approach to metabolic health. As the demographic profile of kidney cancer has shifted toward younger ages, our findings emphasize the urgent need for preventive strategies to address the multifactorial nature of this disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16183161/s1, Table S1. Stratified analyses based on the use of medications regarding the association of KC risk with metabolic SLD; Table S2. Sensitivity analyses on the risk of KC associated with metabolic SLD with varying cut-off and scoring system; Table S3. The proportion of metabolic components and lifestyle factors among participants stratified by age; Figure S1: A heatmap for the risk of kidney cancer associated with the combination of fatty liver disease, alcohol intake, and the number of metabolic components.
Author Contributions
J.O.: Data curation, Formal analysis, Investigation, Writing—original draft. B.K.K.: Conceptualization, Investigation, Software, Resources, Writing—review and editing, Funding acquisition. J.-H.Y.: Conceptualization, Methodology, Validation, Writing—review and editing. H.H.L.: Investigation, Software, Validation. H.P.: Formal analysis, Validation, Visualization. J.L.: Visualization, Investigation. Y.P.: Investigation, Validation. B.Y.: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing—original draft. J.C.: Conceptualization, Investigation, Project administration, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korean Cancer Survivors Healthcare R&D Project through the National Cancer Center, funded by the Ministry of Health & Welfare, Republic of Korea (RS-2023-CC139789).
Institutional Review Board Statement
This study adhered to the ethical principles of the Declaration of Helsinki and Istanbul and was approved by the Institutional Review Board of Severance Hospital (IRB number: 4-2022-0813, approval date: 5 August 2022).
Informed Consent Statement
Requirements for informed consent were waived due to the retrospective nature of this study.
Data Availability Statement
Data sharing is not applicable due to the policy of NHIS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. Mafld: How is it different from nafld? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.; Shin, H.R.; Lee, B.; Shin, A.; Jung, K.W.; Jee, S.H.; Kim, D.H.; Yun, Y.H.; Park, S.K.; et al. Population-attributable causes of cancer in korea: Obesity and physical inactivity. PLoS ONE 2014, 9, e90871. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Chadid, S.; Singer, M.R.; Kreger, B.E.; Denis, G.V. Metabolic health reduces risk of obesity-related cancer in framingham study adults. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.M.; Summers, S.A.; Gunter, M.; et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity (Silver Spring) 2022, 30, 1323–1334. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (nafld) and nonalcoholic steatohepatitis (nash): A systematic review. Hepatology (Baltim. Md.) 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Golabi, P.; Paik, J.M.; AlQahtani, S.; Younossi, Y.; Tuncer, G.; Younossi, Z.M. Burden of non-alcoholic fatty liver disease in asia, the middle east and north africa: Data from global burden of disease 2009–2019. J. Hepatol. 2021, 75, 795–809. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of mafld and nafld diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef]
- The Lancet Gastroenterology Hepatology. Redefining non-alcoholic fatty liver disease: What’s in a name? Lancet. Gastroenterol. Hepatol. 2020, 5, 419. [Google Scholar] [CrossRef]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. Mafld: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Bhagat, N.; Mehta, M.; Taneja, S.; Duseja, A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J. Hepatol. 2024, 80, e61–e62. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety delphi consensus statement on new fatty liver disease nomenclature. Hepatology (Baltim. Md.) 2023, 78, 1966–1986. [Google Scholar]
- Staufer, K.; Stauber, R.E. Steatotic liver disease: Metabolic dysfunction, alcohol, or both? Biomedicines 2023, 11, 2108. [Google Scholar] [CrossRef]
- Kim, G.A.; Moon, J.H.; Kim, W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of janus-faced modernity. Clin. Mol. Hepatol. 2023, 29, 831–843. [Google Scholar] [CrossRef]
- Yoon, E.L.; Jun, D.W. Waiting for the changes after the adoption of steatotic liver disease. Clin. Mol. Hepatol. 2023, 29, 844–850. [Google Scholar] [CrossRef]
- Mantovani, A.; Morieri, M.L.; Aldigeri, R.; Palmisano, L.; Masulli, M.; Bonomo, K.; Baroni, M.G.; Cossu, E.; Cimini, F.A.; Cavallo, G.; et al. Masld, hepatic steatosis and fibrosis are associated with the prevalence of chronic kidney disease and retinopathy in adults with type 1 diabetes mellitus. Diabetes Metab. 2023, 50, 101497. [Google Scholar] [CrossRef]
- Sun, Y.; Hong, L.; Huang, Z.; Wang, L.; Xiong, Y.; Zong, S.; Zhang, R.; Liu, J.; Zang, S. Fibrosis risk in nonalcoholic fatty liver disease is related to chronic kidney disease in older type 2 diabetes patients. J. Clin. Endocrinol. Metab. 2022, 107, e3661–e3669. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, H.; Liu, Y.; Hou, X.; Wei, L.; Bao, Y.; Yang, C.; Zong, G.; Wu, J.; Jia, W. Association of mafld with diabetes, chronic kidney disease, and cardiovascular disease: A 4.6-year cohort study in china. J. Clin. Endocrinol. Metab. 2022, 107, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Bilson, J.; Mantovani, A.; Byrne, C.D.; Targher, G. Steatotic liver disease, masld and risk of chronic kidney disease. Diabetes Metab. 2023, 50, 101506. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Ma, X.F.; Zhao, J.; Du, S.X.; Zhang, J.; Dong, M.Z.; Xin, Y.N. Association between nonalcoholic fatty liver disease and extrahepatic cancers: A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, C.; Suo, C.; Zhao, R.; Jin, L.; Zhang, T.; Chen, X. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metab. Clin. Exp. 2022, 127, 154955. [Google Scholar] [CrossRef]
- Konyn, P.; Ahmed, A.; Kim, D. Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S43–S57. [Google Scholar] [CrossRef]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.Y.; Kwon, H.S.; Cha, B.S.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Lee, B.W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.Y.; Han, K.; Shen, J.J. Association between glycemic status and the risk of kidney cancer in men and women: A nationwide cohort study. Diabetes Care 2023, 46, 38–45. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.H.; Kim, S.U.; Kim, H.C. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin. Gastroenterol. Hepatol. 2021, 19, 2138–2147.e10. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, S.H.; Kang, S.H.; Kim, H.J.; Yoon, C.H.; Youn, T.J.; Chae, I.H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Han, K.D.; Lee, Y.H.; Kim, K.S.; Hong, S.; Park, J.H.; Park, C.Y. Fatty liver & diabetes statistics in korea: Nationwide data 2009 to 2017. Diabetes Metab. J. 2023, 47, 347–355. [Google Scholar] [PubMed]
- Oh, J.; Lee, S.; Sim, J.; Kim, S.; Cho, A.; Yun, B.; Yoon, J.-H. Association between self-perceived social support in the workplace and the presence of depressive/anxiety symptoms. Int. J. Environ. Res. Public Health 2021, 18, 10330. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Larose, T.L. Epidemiology and risk factors for kidney cancer. J. Clin. Oncol. 2018, 36, Jco2018791905. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.A. Young adult cancer: Influence of the obesity pandemic. Obesity (Silver Spring Md.) 2018, 26, 641–650. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331. [Google Scholar] [CrossRef]
- Berger, N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef]
- Doerner, S.K.; Reis, E.S.; Leung, E.S.; Ko, J.S.; Heaney, J.D.; Berger, N.A.; Lambris, J.D.; Nadeau, J.H. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol. Cancer Res. MCR 2016, 14, 953–965. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Rysz, J.; Ławiński, J.; Franczyk, B. Renal cell cancer and obesity. Int. J. Mol. Sci. 2022, 23, 3404. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Liu, G.; Wei, Y. Association between il-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. Int. J. Clin. Exp. Med. 2015, 8, 13491–13499. [Google Scholar] [PubMed]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via amp-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ashcraft, K. An il-6 link between obesity and cancer. Front. Biosci. (Elite Ed.) 2013, 5, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef]
- van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef]
- Chen, C.; Chang, Y.C.; Lan, M.S.; Breslin, M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin d1 and mcl-1 expression via the activation of the mek/erk1/2 and pi3k/akt signaling pathways. Int. J. Oncol. 2013, 42, 1113–1119. [Google Scholar] [CrossRef]
- Sugiyama, M.; Takahashi, H.; Hosono, K.; Endo, H.; Kato, S.; Yoneda, K.; Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; et al. Adiponectin inhibits colorectal cancer cell growth through the ampk/mtor pathway. Int. J. Oncol. 2009, 34, 339–344. [Google Scholar]
- London, A.; Lundsgaard, A.M.; Kiens, B.; Bojsen-Møller, K.N. The role of hepatic fat accumulation in glucose and insulin homeostasis-dysregulation by the liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef]
- Hsiao, T.-J.; Chen, J.-C.; Wang, J.-D. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int. J. Obes. 2004, 28, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Bae, S.J.; Lee, M.J.; Kim, E.H.; Park, H.; Kim, H.S.; Cho, Y.K.; Jung, C.H.; Lee, W.J.; Choe, J. Association of visceral fat obesity, sarcopenia, and myosteatosis with non-alcoholic fatty liver disease without obesity. Clin. Mol. Hepatol. 2023, 29, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, H.; Kim, M.G.; Kim, K. Comparison of glucagon-like peptide-1 receptor agonists and thiazolidinediones on treating nonalcoholic fatty liver disease: A network meta-analysis. Clin. Mol. Hepatol. 2023, 29, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Le, I.; Ha, A.; Le, R.H.; Rouillard, N.A.; Fong, A.; Gudapati, S.; Park, J.E.; Maeda, M.; Barnett, S.; et al. Differences in liver and mortality outcomes of non-alcoholic fatty liver disease by race and ethnicity: A longitudinal real-world study. Clin. Mol. Hepatol. 2023, 29, 1002–1012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).