The Real-World Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C in Patients Active Malignancies †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Population
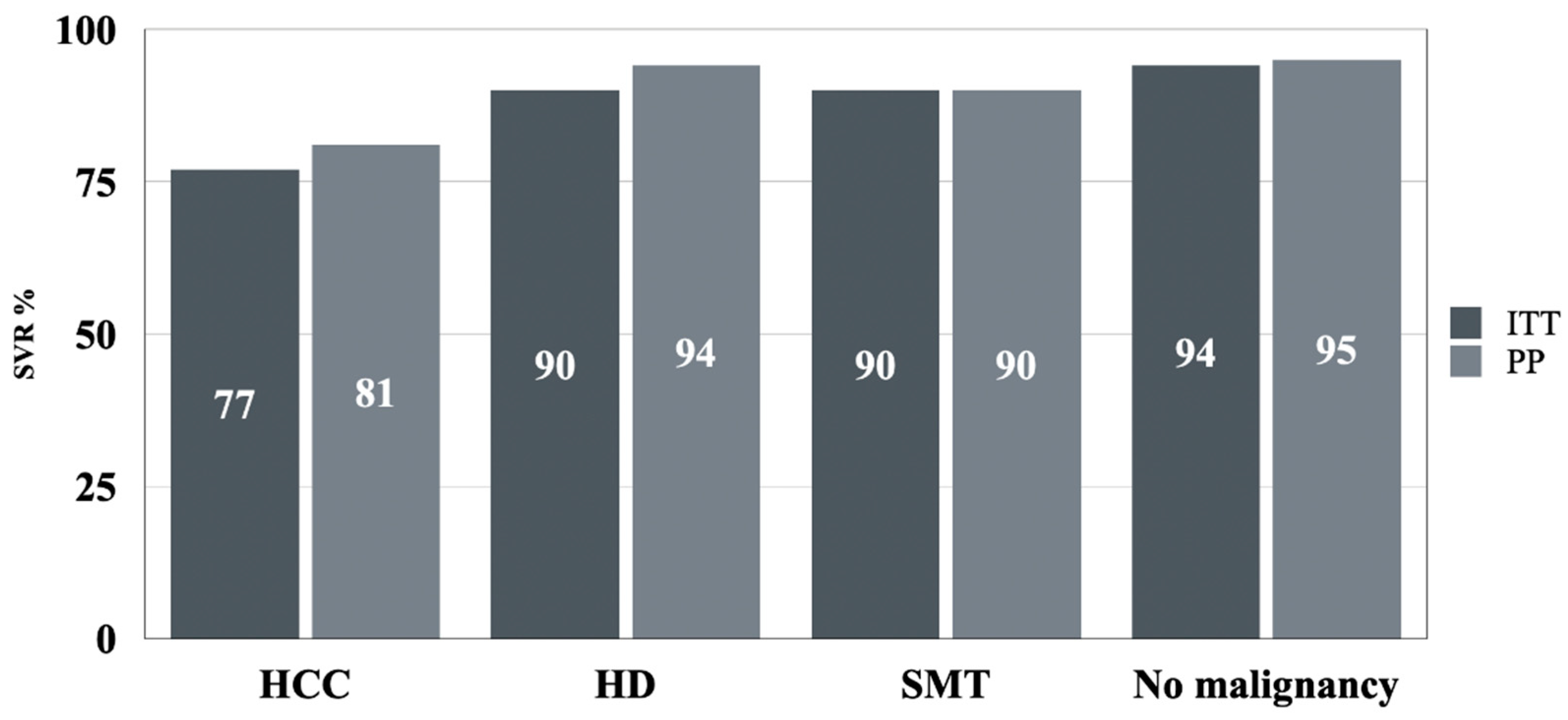
3.2. Efficacy
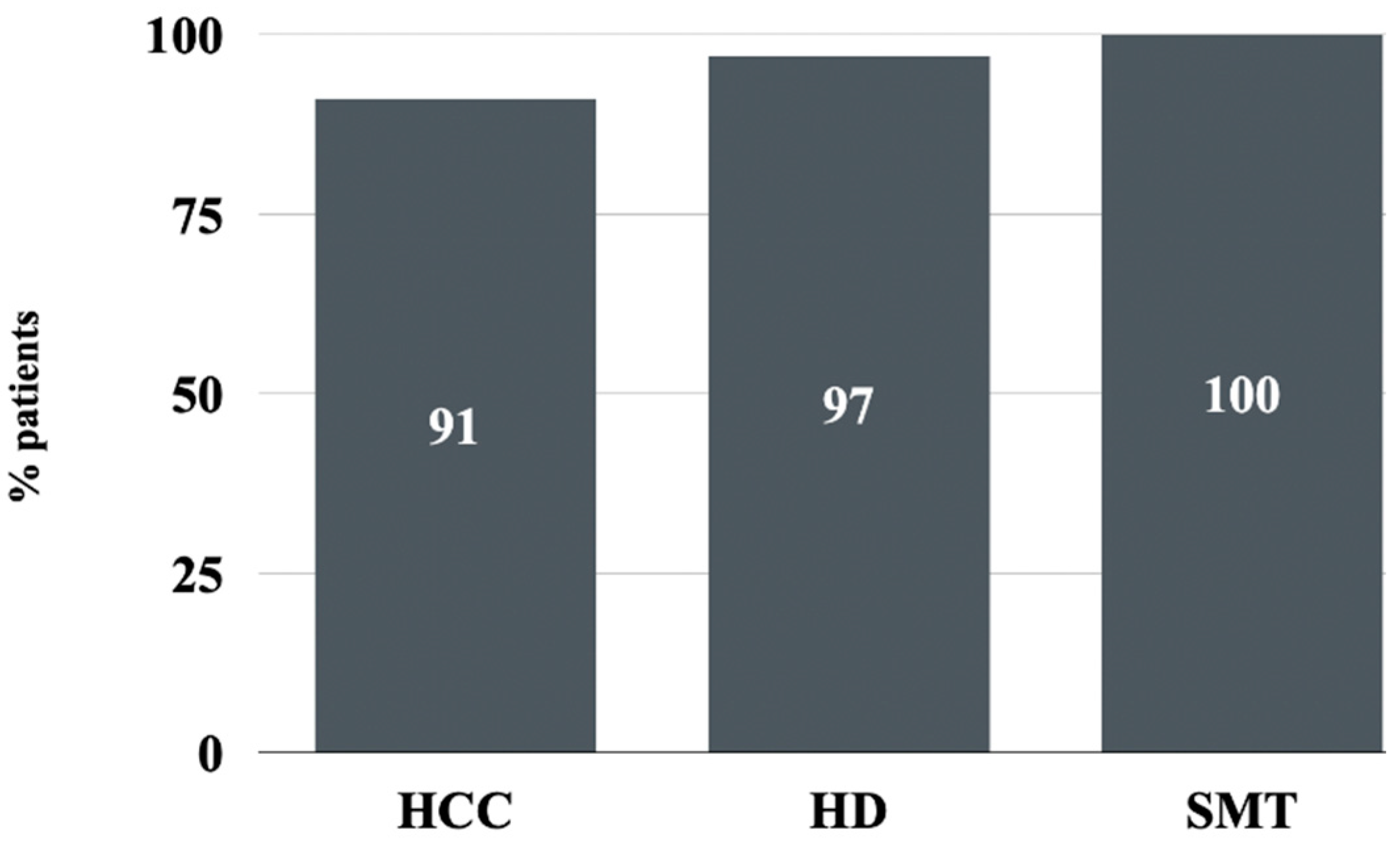
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction. J. Transl. Intern. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. 2016. Available online: https://iris.who.int/handle/10665/246177 (accessed on 24 January 2024).
- Tronina, O.; Panczyk, M.; Zarębska-Michaluk, D.; Gotlib, J.; Małkowski, P. Global Elimination of HCV—Why Is Poland Still So Far from the Goal? Viruses 2023, 15, 2067. [Google Scholar] [CrossRef] [PubMed]
- Kamp, W.M.; Sellers, C.M.; Stein, S.; Lim, J.K.; Kim, H.S. Direct-Acting Antivirals Improve Overall Survival in Interventional Oncology Patients with Hepatitis C and Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 953–960. [Google Scholar] [CrossRef]
- Patauner, F.; Stanzione, M.; Stornaiuolo, G.; Martone, V.; Palladino, R.; Coppola, N.; Durante-Mangoni, E.; Zampino, R. Safety and Efficacy of Direct Antiviral Agents for Hepatitis C in Patients with Malignancies Other Than Liver Cancer: A Case Series. Pathogens 2022, 11, 860. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Barańska, K.; Miklewska, M.; Didkowska, J. Cancer incidence and mortality in Poland in 2020. Nowotw. J. Oncol. 2023, 73, 129–145. [Google Scholar] [CrossRef]
- Brashier, D.B.; Sharma, S.; Mathur, A.G.; Khare, P.; Gupta, S. Boceprevir: A new hope against hepatitis C virus. J. Pharmacol. Pharmacother. 2012, 3, 213–215. [Google Scholar] [CrossRef]
- Alarfaj, S.J.; Alzahrani, A.; Alotaibi, A.; Almutairi, M.; Hakami, M.; Alhomaid, N.; Alharthi, N.; Korayem, G.B.; Alghamdi, A. The effectiveness and safety of direct-acting antivirals for hepatitis C virus treatment: A single-center experience in Saudi Arabia. Saudi Pharm. J. 2022, 30, 1448–1453. [Google Scholar] [CrossRef]
- Spera, A.M. Safety of direct acting antiviral treatment for hepatitis C in oncologic setting: A clinical experience and a literature review. World J. Hepatol. 2022, 14, 525–534. [Google Scholar] [CrossRef]
- Cheung, M.C.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.; MacDonald, D.C.; Agarwal, K.; et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 65, 741–747. [Google Scholar] [CrossRef]
- Ji, F.; Yeo, Y.H.; Wei, M.T.; Ogawa, E.; Enomoto, M.; Lee, D.H.; Iio, E.; Lubel, J.; Wang, W.; Wei, B.; et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 473–485. [Google Scholar] [CrossRef]
- Barone, M.; Iannone, A.; Shahini, E.; Ippolito, A.M.; Brancaccio, G.; Morisco, F.; Milella, M.; Messina, V.; Smedile, A.; Conti, F.; et al. A different perspective on sofosbuvir-ledipasvir treatment of patients with HCV genotype 1b cirrhosis: The ital-c network study. J. Viral Hepat. 2018, 25, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019, 156, 1683–1692.e1. [Google Scholar] [CrossRef]
- Rich, N.E.; Singal, A.G. Direct-Acting Antiviral Therapy and Hepatocellular Carcinoma. Clin. Liver Dis. 2021, 17, 414–417. [Google Scholar] [CrossRef]
- Kanda, T.; Matsumoto, N.; Ishii, T.; Arima, S.; Shibuya, S.; Honda, M.; Sasaki-Tanaka, R.; Masuzaki, R.; Kanezawa, S.; Nishizawa, T.; et al. Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare. Viruses 2023, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Saisho, H. Acute hepatitis C virus infection, 1986–2001: A rare cause of fulminant hepatitis in Chiba, Japan. Hepatogastroenterology 2004, 51, 556–558. [Google Scholar] [PubMed]
- Theilmann, L.; Solbach, C.; Toex, U.; Müller, H.M.; Pfaff, E.; Otto, G.; Goeser, T. Role of hepatitis C virus infection in German patients with fulminant and subacute hepatic failure. Eur. J. Clin. Investig. 1992, 22, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Rule, J.A.; Cerro-Chiang, G.; Stravitz, R.T.; McGuire, B.M.; Lee, G.; Fontana, R.J.; Lee, W.M. Role of Hepatitis C Infection in Acute Liver Injury/Acute Liver Failure in North America. Dig. Dis. Sci. 2023, 68, 304–311. [Google Scholar] [CrossRef]
- Torres, H.A.; Hosry, J.; Mahale, P.; Economides, M.P.; Jiang, Y.; Lok, A.S. Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study. Hepatology 2018, 67, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, N.; Suzuki, F.; Akuta, N.; Suzuki, Y.; Sezaki, H.; Hosaka, T.; Someya, T.; Kobayashi, M.; Saitoh, S.; Arase, Y.; et al. Clinical and virological characteristics of untreated patients with chronic hepatitis C who develop serum alanine aminotransferase flare-up. J. Med. Virol. 2005, 75, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.G.; De Filippi, F.; La Vecchia, C.; Donato, M.F.; Gallus, S.; Del Ninno, E.; Colombo, M. Hepatitis C reactivation in patients with chronic infection with genotypes 1b and 2c: A retrospective cohort study of 206 untreated patients. Gut 2005, 54, 402–406. [Google Scholar] [CrossRef]
- Jennings, J.J.; Mandaliya, R.; Nakshabandi, A.; Lewis, J.H. Hepatotoxicity induced by immune checkpoint inhibitors: A comprehensive review including current and alternative management strategies. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 231–244. [Google Scholar] [CrossRef]
- Vidal-Robau, N.; Caballero, G.; Archilla, I.; Ladino, A.; Fernández, S.; Ortiz-Maldonado, V.; Rovira, M.; Gómez-Hernando, M.; Delgado, J.; Suárez-Lledó, M. Post-mortem neuropathologic examination of a 6-case series of CAR T-cell treated patients. Free Neuropathol. 2022, 27, 3–23. [Google Scholar] [CrossRef]
- Dang, H.; Yeo, Y.H.; Yasuda, S.; Huang, C.F.; Iio, E.; Landis, C.; Jun, D.W.; Enomoto, M.; Ogawa, E.; Tsai, P.C.; et al. Cure With Interferon-Free Direct-Acting Antiviral Is Associated With Increased Survival in Patients With Hepatitis C Virus-Related Hepatocellular Carcinoma From Both East and West. Hepatology 2020, 71, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Zou, Y.; Yue, M.; Zhang, M.; Yu, R.; Chen, H.; Huang, P. Evaluating short-term and long-term liver fibrosis improvement in hepatitis C patients post-DAA treatment. J. Biomed. Res. 2024, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.A.; Adam, J.P.; Martel, D.; Doucet, S.; Martel-Laferrière, V. Risks of hepatitis C virus reactivation in a real-life population of oncology patients treated in an academic center. J. Oncol. Pharm. Pract. 2021, 27, 1815–1820. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Turco, A.; Buccino, R.V.; Nacchiero, M.C.; Muscatiello, N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J. Gastroenterol. Hepatol. 2018, 33, 942–949. [Google Scholar] [CrossRef]
- Badia Aranda, E.; Fernández Marcos, C.; Puebla Maestu, A.; Gozalo Marín, V.; Vinuesa Campo, R.; Calvo Simal, S.; Gómez Camarero, J. Evolution of patients with chronic hepatitis C infection with advanced fibrosis or cirrhosis cured with direct-acting antivirals. Long-term follow-up. Gastroenterol. Hepatol. 2022, 45, 767–779. [Google Scholar] [CrossRef]
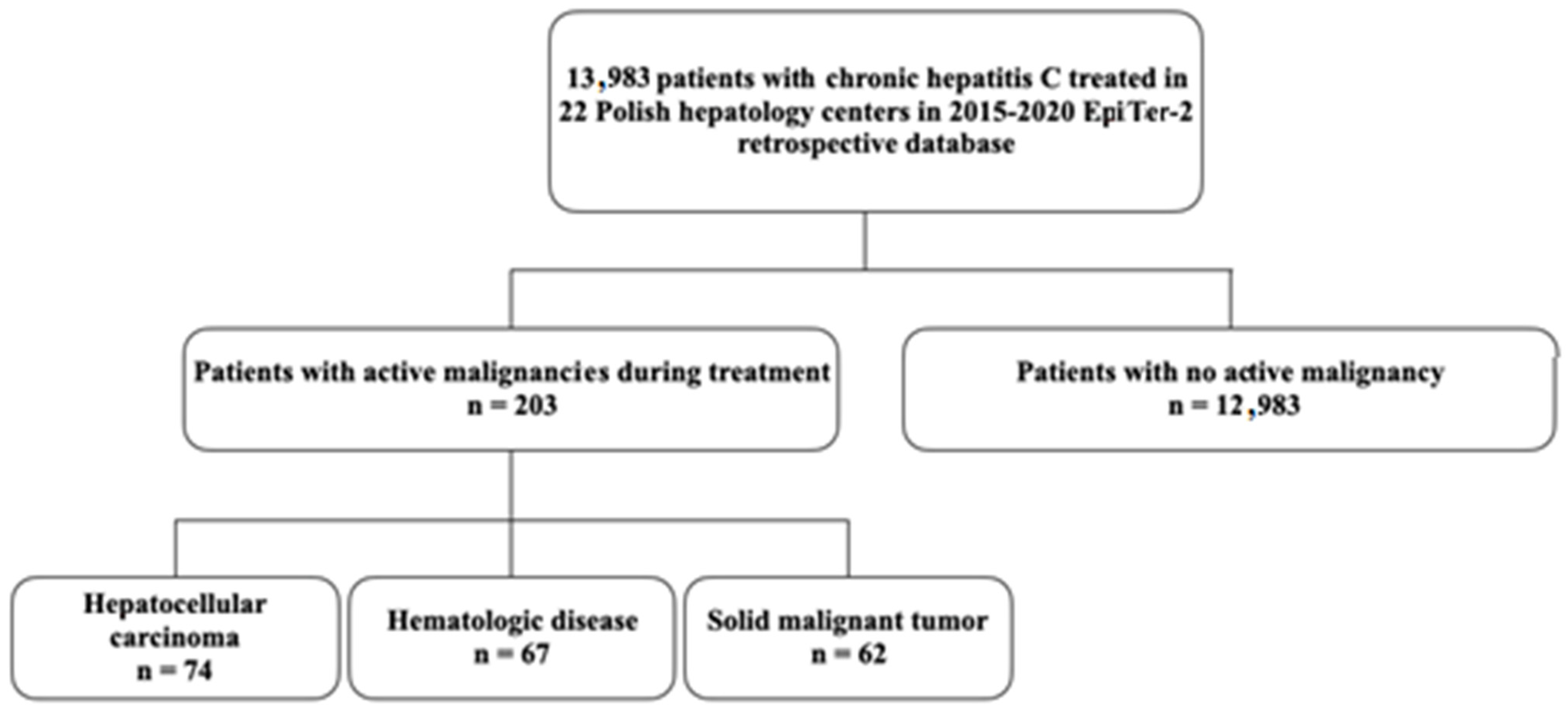
| Parameter | HCC n = 74 | HD n = 67 | SMT n = 62 | NAM n = 12,983 | p= |
|---|---|---|---|---|---|
| Gender, males/females, n (%) | 47 (63.5)/27 (36.5) | 44 (65.7)/23 (34.3) | 29 (46.8)/35 (53.2) | 6323 (48.7)/6656 (51.3) | 0.002 |
| Age (years) mean, SD Median (Q1–Q3) | 62, 10.1 62 (57–68) | 59.3, 14.6 59 (49.5–70) | 60.2, 12.2 62 (52–68) | 51.5, 25.1 52 (39–62) | <0.001 |
| BMI median (Q1–Q3) | 25.9 (22.1–27) | 25 (23.1–29) | 25.7 (22.5–30) | 25.8 (23.1–29) | 0.48 |
| Any comorbidity, n (%) | 49 (66.22) | 31 (36.27) | 28 (43.75) | 5054 (38.94) | <0.001 |
| Arterial hypertension, n (%) | 40 (54.05) | 23 (34.33) | 27 (42.19) | 4165 (32.09) | <0.001 |
| Diabetes, n (%) | 20 (27.03) | 11 (16.42) | 8 (12.5) | 1491 (11.49) | <0.001 |
| Autoimmune disease, n (%) | 4 (5.41) | 2 (2.99) | 2 (3.13) | 619 (4.77) | 0.82 |
| Kidney disease, n (%) | 5 (6.76) | 5 (7.46) | 5 (7.81) | 501 (3.86) | 0.09 |
| Depression, n (%) | 3 (4.05) | 1 (1.49) | 4 (6.26) | 487 (3.75) | 0.56 |
| ALT IU/L median (Q1–Q3) | 81 (51–134) | 52 (38–101) | 59.5 (37.5–95) | 58.0 (37–97) | <0.001 |
| Bilirubin mg/dL median (Q1–Q3) | 1 (0.72–1.4) | 1 (0.52–1.15) | 0.6 (0.46–0.8) | 0.6 (0.47–0.91) | <0.001 |
| Albumin g/dL median (Q1–Q3) | 3.7 (3.2–4.1) | 4 (3.4–4.18) | 4.0 (3.7–4.18) | 4.1 (3.8–4.4) | <0.001 |
| Hemoglobin g/dL Median (Q1–Q3) | 13.7 (12.4–14.7) | 13 (12.4–14.3) | 13.9 (12.7–14.7) | 14.5 (13.4–15.5) | <0.001 |
| Platelets ×1000/μL, median (Q1–Q3) | 125 (76–165) | 125 (87–186) | 187.5 (153–249) | 194.0 (143–240) | <0.001 |
| HCV RNA ×106 IU/mL, median (Q1–Q3) | 664,500 (160,500–1,518,281.5) | 1,680,000 (337,376–4,900,000) | 541,000 (239,000–1,820,000) | 949,118 (317,000–2,463,982.5) | <0.001 |
| Parameter | HCC n = 74 | HD n = 67 | SMT n = 62 | NAM n = 12,983 | p= |
|---|---|---|---|---|---|
| Liver fibrosis, n (%) | <0.001 | ||||
| F0 | 0 (0.0) | 1 (1.5) | 0 (0.0) | 270 (2.1) | |
| F1 | 3 (4.0) | 14 (21.0) | 16 (25.8) | 5107 (39.3) | |
| F2 | 4 (5.4) | 16 (23.9) | 16 (25.8) | 2444 (18.8) | |
| F3 | 3 (4.0) | 16 (23.9) | 14 (22.6) | 1812 (13.9) | |
| F4 | 62 (83.8) | 19 (28.3) | 14 (22.6) | 3115 (24.0) | |
| no data | 2 (2.7) | 1 (1.5) | 2 (3.2) | 235 (1.8) | |
| Documented esophageal varices, n (%) | 30 (40.5) | 5 (7.5) | 5 (8.1) | 974 (7.5) | <0.001 |
| No data | 11 (14.9) | 12 (17.9) | 14 (22.6) | 2907 (22.4) | |
| Ascites at baseline, n (%) | 6 (8.1) | 2 (3.0) | 1 (1.6) | 151 (1.2) | <0.001 |
| Encephalopathy at baseline, n (%) | 3 (4.0) | 1 (1.5) | 1 (1.6) | 70 (0.5) | 0.2 |
| Child–Pugh, n (%) | <0.001 | ||||
| B | 11 (14.9) | 9 (13.4) | 2 (3.2) | 354 (2.7) | |
| C | 1 (1.3) | 0 (0.0) | 0 (0.0) | 17 (0.1) | |
| HBV-coinfection, n (%) | 13 (17.6) | 10 (14.9) | 10 (16.1) | 1689 (13.0) | 0.6 |
| HIV-coinfection n (%) | 2 (2.7) | 2 (3.0) | 1 (1.6) | 729 (5.6) | 0.17 |
| HCC | HD | SMT | NAM | p= | |
|---|---|---|---|---|---|
| ITT, n (%) | 57/74 (77) | 60/67 (89.6) | 56/62 (90) | 12,242/12,919 (94.8) | <0.001 |
| PP, n (%) | 54/67 (80.6) | 59/63 (93.6) | 55/61 (90.2) | 12,100/12,684 (95.4) | <0.001 |
| Patient | Age | F, CP | Regimen | History of Previous Therapy | Baseline HCV RNA | Treatment Course Discontinued in | Reason for Discontinuation | Comorbidities | Anticancer Concomitant Medications |
|---|---|---|---|---|---|---|---|---|---|
| Male 1 | 76 | 4, no data | LDV/SOF + RBV, 12 weeks | relapser | 708150 | week 4 | performance status deterioration | hypertension, diabetes | sorafenib |
| Male 2 | 63 | 2, A | OBV/PTV/r + DSV + RBV, 24 weeks | treatment-naive | 1410000 | week 12 | HCC progression | hypertension, diabetes | no |
| Male 3 | 57 | 3, A | LDV/SOF + RBV, 24 weeks | Non-responder | 615915 | week 20 | decompensation | kidney disease | no |
| Male 4 | 53 | 4, C | LDV/SOF + RBV, 24 weeks | treatment-naive | 50412 | week 20 | HCC diagnosed during treatment | no data | no |
| Male 5 | 60 | 4, B | OBV/PTV/r + DSV, 12 weeks | treatment-naive | 103000 | week 8 | decompensation | hypertension, porphyria | no |
| Male 6 | 65 | 4, A | OBV/PTV/r + RBV, 24 weeks | Non-responder | 3310000 | week 12 | liver transplantation | hypertension | no |
| Female 1 | 60 | 4, B | OBV/PTV/r + DSV, 12 weeks | treatment-naive | 81200 | week 5 | decompensation | diabetes, atherosclerosis | sorafenib |
| Patient | Age | Malignancy | F, CP | Regimen | History of Previous Therapy | Baseline HCV RNA | Treatment Course Discontinued in | Reason for Discontinuation | Comorbidities | Anticancer Concomitant Medications |
|---|---|---|---|---|---|---|---|---|---|---|
| Female 1 | 40 | multiple myeloma | 4, A | LDV/SOF, 12 weeks | treatment-naive | 5167178 | week 20 | myeloma exacerbation | no | Bortezomib |
| Male 1 | 80 | Non-Hodgkin Lymphoma | 3, B | VEL/SOF, 12 weeks | treatment-naive | 14900000 | week 2 | death | hypertension, diabetes | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, M.; Jaroszewicz, J.; Sitko, M.; Janocha-Litwin, J.; Zarębska-Michaluk, D.; Janczewska, E.; Lorenc, B.; Tudrujek-Zdunek, M.; Parfieniuk-Kowerda, A.; Klapaczyński, J.; et al. The Real-World Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C in Patients Active Malignancies. Cancers 2024, 16, 3114. https://doi.org/10.3390/cancers16173114
Dąbrowska M, Jaroszewicz J, Sitko M, Janocha-Litwin J, Zarębska-Michaluk D, Janczewska E, Lorenc B, Tudrujek-Zdunek M, Parfieniuk-Kowerda A, Klapaczyński J, et al. The Real-World Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C in Patients Active Malignancies. Cancers. 2024; 16(17):3114. https://doi.org/10.3390/cancers16173114
Chicago/Turabian StyleDąbrowska, Maria, Jerzy Jaroszewicz, Marek Sitko, Justyna Janocha-Litwin, Dorota Zarębska-Michaluk, Ewa Janczewska, Beata Lorenc, Magdalena Tudrujek-Zdunek, Anna Parfieniuk-Kowerda, Jakub Klapaczyński, and et al. 2024. "The Real-World Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C in Patients Active Malignancies" Cancers 16, no. 17: 3114. https://doi.org/10.3390/cancers16173114
APA StyleDąbrowska, M., Jaroszewicz, J., Sitko, M., Janocha-Litwin, J., Zarębska-Michaluk, D., Janczewska, E., Lorenc, B., Tudrujek-Zdunek, M., Parfieniuk-Kowerda, A., Klapaczyński, J., Berak, H., Socha, Ł., Dobracka, B., Dybowska, D., Mazur, W., Ważny, Ł., & Flisiak, R. (2024). The Real-World Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C in Patients Active Malignancies. Cancers, 16(17), 3114. https://doi.org/10.3390/cancers16173114







