Simple Summary
The increasing production of plastics and the failure to properly manage their post use represent the primary sources of environmental and human contamination caused by plastic degradation products, namely micro- and nano-plastics (MNPLs). Accumulating evidence supports the intestinal toxicity of ingested MNPLs, and their possible role as a driver for the increased incidence of colorectal cancer (CRC) has also been proposed. Moreover, colonic mucus layer disruption may further facilitate MNPL passage into the bloodstream, thus promoting the toxic effects of MNPLs on different organ systems. We herein discuss the pathophysiological mechanisms impaired by MNPL oral exposure in healthy conditions and individuals with compromised intestinal barrier function, including inflammatory bowel disease (IBD) and CRC patients, considered high-risk populations, by highlighting the potential contribution of MNPL-induced platelet activation.
Abstract
Micro- and nano-plastics (MNPLs) can move along the food chain to higher-level organisms including humans. Three significant routes for MNPLs have been reported: ingestion, inhalation, and dermal contact. Accumulating evidence supports the intestinal toxicity of ingested MNPLs and their role as drivers for increased incidence of colorectal cancer (CRC) in high-risk populations such as inflammatory bowel disease (IBD) patients. However, the mechanisms are largely unknown. In this review, by using the leading scientific publication databases (Web of Science, Google Scholar, Scopus, PubMed, and ScienceDirect), we explored the possible effects and related mechanisms of MNPL exposure on the gut epithelium in healthy conditions and IBD patients. The summarized evidence supports the idea that oral MNPL exposure may contribute to intestinal epithelial damage, thus promoting and sustaining the chronic development of intestinal inflammation, mainly in high-risk populations such as IBD patients. Colonic mucus layer disruption may further facilitate MNPL passage into the bloodstream, thus contributing to the toxic effects of MNPLs on different organ systems and platelet activation, which may, in turn, contribute to the chronic development of inflammation and CRC development. Further exploration of this threat to human health is warranted to reduce potential adverse effects and CRC risk.
1. Introduction
A constant increase in plastic production is set to persist until 2050 [1]. Plastics can contaminate the environment due to ocean currents, winds, and atmospheric phenomena, promoting their distribution [2]. Once released, plastic degradation occurs, leading to the formation of microplastics (MPs) (smaller than 5 mm) and nanoplastics (NPs) (smaller than 1000 nm) [3,4]. It has been well recognized that micro- and nanoplastics (MNPLs) can penetrate the human body by ingestion, inhalation, and skin exposure [4,5]. They have been detected in some human tissues and biological samples, including the placenta [6], lungs [7], liver [8], breast milk [9], urine [10], and blood [11]. A large body of evidence suggests that MNPL exposure may cause a wide spectrum of adverse effects in human organ systems, including oxidative stress, inflammation, alteration in immune function and cellular and energy metabolism, suppression of cell proliferation, tissue damage and degeneration, organ development and function, biochemical parameters, and genotoxic and carcinogenic effects [12]. In addition, MNPLs bear a high toxicological risk because they incorporate harmful chemical additives including plasticizers, flame retardants, stabilizers, dyes, antistatic agents, lubricants, flow agents, foaming agents, and biocides [13]. MNPLs may adopt a fibrous shape, commonly termed “microplastic fibers” [14]. The environmental contamination by MNPL fibers is comparable to or even higher than that caused by plastic particles [15,16]. The elongated shape of microplastic fibers potentially increases their bioaccumulation and may directly damage organisms or cause adverse effects.
Worldwide, about 39,000–52,000 particles per person are ingested every year by food consumption [17]. The intestine is considered the primary line of defense against orally ingested MNPLs. Once ingested, they may impair intestinal barrier structures, alter intestinal flora, and induce mild pro-inflammatory responses and oxidative stress in healthy individuals [18,19,20,21]. However, the extent of damage to the intestine is not considered enough to determine an intestinal disease state [22,23], thus suggesting the capacity of a healthy intestine to resist the impact of MNPLs [24]. On the other hand, there is a great deal of evidence supporting the idea that MNPLs could affect the severity of diseases such as obesity, alcoholic liver injury, and enteritis [25,26,27]. Moreover, the integrity of the intestinal barrier is closely associated with developing diseases including neurodegenerative disease and inflammatory bowel disease (IBD) [28,29,30].
IBD incidence generally increases in developing industrialized countries due to severe environmental pollution [31,32,33]. In IBD patients, the intestinal microenvironment is different from that of healthy individuals, and MNPLs could significantly increase intestinal injury and exacerbate intestinal pathology development [34,35,36]. However, knowledge about the impact of MNPLs on the IBD population and related mechanisms is still limited.
Recently, the finding that the number of MNPLs in human colorectal cancer (CRC) biopsies is significantly higher than those detected in non-tumoral colon biopsies has suggested the occurrence of a connection between CRC development and MNPL exposure level [37]. This possible link is sustained by the increased CRC incidence in the under-50 population [38,39]. This recent epidemiological data may reflect the period potentially expected to see the effects associated with the fast increase of MNPLs in the environment.
Intestinal damage and inflammation caused by ingested MNPLs contribute to their entering the bloodstream and disseminating to other tissues. For example, once they reach systemic circulation, MNPLs may represent a risk to the cardiovascular system. Though knowledge about the cardiovascular effects of MNPL exposures is still limited, the presence of MNPLs in multiple human heart tissues and blood has recently been evidenced [40]. Overall, MNPLs may cause vascular endothelial damage, promoting a series of adverse cardiovascular effects via the interaction between endothelial cells and blood and immune cells [12]. In vivo studies in animal models support the capacity of MNPLs to alter heart rate and cardiac function to promote inflammatory responses, myocardial fibrosis, and endothelial dysfunction [41]. Experimental evidence also supports the idea that cardiovascular toxic effects by MNPLs may be associated with their capacity to affect platelet aggregation and blood coagulation. MNPLs may exert prothrombotic effects depending on different factors, including their surface modification, size, dose, exposure route, and protein presence. MNPL exposure may have an impact on the balance between pro- and anticoagulant pathways at molecular levels and affect blood coagulation through different mechanisms, mainly depending on particle size and surface modification [41]. However, the clinical relevance of these findings is largely unexplored. Recently, Marfella et al. [42] showed that patients with carotid artery plaque, in which MNPLs were detected, had an increased risk of a composite of myocardial infarction, stroke, or death (at 34-month follow-up) compared to patients in whom MNPLs were not evidenced. However, in the last decades, during which plastic exposure has presumably increased, the occurrence rate of cardiovascular disease has decreased [43]. This suggests that, if compared with common risk factors, the potential role of MNPLs in triggering cardiovascular disease might be limited. Nevertheless, the impact of MNPL exposure in this setting deserves further investigation.
Several lines of evidence support the contribution of platelets to the chronic development of intestinal inflammation. Moreover, it has been shown that the chronic use of the antiplatelet agent low-dose aspirin for cardiovascular prevention reduces CRC incidence and mortality [44]. Altogether, these findings support the hypothesis that activated platelets can trigger chronic inflammation, representing a key event in intestinal tumorigenesis [44]. Once activated in response to intestinal epithelial damage, platelets contribute to acute inflammation by promoting leukocyte recruitment. This is essential to restore normal tissue function. However, uncontrolled platelet activation leads to the increased release of lipid mediators including thromboxane (TX)A2 and the prostaglandin (PG)E2 as well as growth factors, angiogenic factors, cytokines, chemokines, and medium-sized extracellular vesicles (EVs) containing genetic material (such as mRNA and microRNAs). These platelet-derived mediators promote cyclooxygenase (COX)-2 expression and PGE2 biosynthesis by tumor microenvironment cells (i.e., immune and endothelial cells and fibroblasts, as well as cancer cells). These events are considered key points for chronic development of inflammation, tumor progression, angiogenesis, and metastasis [44].
The omnipresence of plastics in our daily lives implies chronic exposure to MNPLs. However, there are still numerous knowledge gaps about the harmful effects of MNPL exposure on human health. Ethical considerations hamper clinical studies investigating the health risks of MNPL exposure in humans. Moreover, the lack of data on plastic levels in the human diet does not allow a robust scientific conclusion for humans in general and their intestinal health. It is clear that plastics contaminate water and the food chain, and a growing body of experimental evidence from in vitro and animal studies shows that MNPL ingestion disrupts essential intestinal functions such as the gut barrier function and regulation of the gut microbiota [45]. These plastic-associated effects may promote immune, inflammatory, and metabolic disorders and therefore warrant further investigation. Recently, epidemiological data suggested that CRC incidence has been increasing worldwide in individuals under 50 years of age, thus supporting the occurrence of a cohort effect since 1960. The etiology of this observation is unknown, but it is likely promoted by an environmental cause [46]. In this review, we provided a comprehensive overview of current knowledge about the potential effects of ingested MNPLs on the intestinal tract in healthy individuals. We also explored possible mechanisms of how MNPL exposure may exacerbate the chronic development of inflammation and promote intestinal tumorigenesis in IBD patients, highlighting the potential contribution of MNPL-induced platelet activation.
With this aim, the leading scientific publication databases (Web of Science, Google Scholar, Scopus, PubMed, and ScienceDirect) were used to collect and select relevant literature for clearly focused questions about the impact of MNPL on human health. They include: “microplastics”, “nanoplastics”, “detection techniques”, “oral ingestion”, “gastrointestinal tract”, “intestinal health”, “inflammatory bowel disease”, “colorectal cancer”, “animal models”, “in vitro studies”, “humans”, “oxidative stress”, “apoptosis”, and “platelets”. Furthermore, to highlight other pertinent literature in the publications we obtained, we cross-checked the sources cited. Articles were sorted by title and abstract; only full publication data were analyzed. Conferences, books, encyclopedias, editorials, letters, and non-English articles were excluded. The analysis was performed separately, one by one, and the data were visualized accordingly. The latest literature search was conducted in August 2024, and priority was given to articles published in high-impact journals during the last ten years.
2. Micro- and Nanoplastics (MNPLs): An Overview
2.1. Definition and Composition of MNPLs
Although the first use of plastics can be traced back to the 2nd millennium BC, the invention of modern natural plastic is attributed to Charles Goodyear, who patented the vulcanization process of natural rubber in 1844. Later, synthetic plastic (bakelite) was invented by Leo Baekeland in 1907. Subsequently, plasticized polyvinyl chloride (PVC) (1926), low-density polyethylene (LDPE) (1933), acrylic polymer (1936), Nylone (1939), and polyethylene terephthalate (PET) (1941) were also invented and marketed [47].
MNPLs are tiny pieces of synthetic polymers (nylon, polyethylene, polyester, Teflon, and epoxy) found in ambient air, sediments, biota, fresh, and seawater [2,47,48,49,50,51]. MNPLs can be defined and classified depending on (a) their source of origin and (b) their size.
- (a)
- There are two classes of MNPLs: the primary and the secondary. The first class includes all the MNPLs that are directly produced at micro- and nanoscale for industrial purposes (such as cosmetics, textiles, and medicine) [52,53,54]. The second category includes MNPLs created when larger plastic fragments break down through various processes, including mechanical and photochemical degradation and/or microbial actions [55,56];
- (b)
- Four groups of MNPLs can be defined starting from their sizes: macroplastics (>25 mm), mesoplastics (5–25 mm), MPs (0.001–5 mm), and NPs (1 nm–1000 nm) [57].
The environment’s most common MNPLs are composed of various types of polymers that can be produced at different rates depending on the global demand for plastic. Koelmans et al. in 2019 found that globally in freshwater and drinking water, polymers are present in the following order: polyethylene (PE), which can be of high density (HDPE) or low density (LDPE)) ≈ polypropylene (PP) > polystyrene (PS) > polyvinyl chloride (PVC) > polyethylene terephthalate (PET) [58].
About 40–60% of the synthetic polymers are constituted by styrene-butadiene rubber (SBR), which produces MNPLs known as tire-wear particles (TWP) [57]. Paint and surface coating products contain other synthetic polymers, among which are polyester (PES), alkyds, vinyl and acrylic (co)polymers, epoxy resin, and urethane resins [59]. Besides these conventional polymers, there are other types of MNPLs produced from natural bioplastics, such as poly(lactic acid) (PLA), polyhydroxyalkanoates (PHA), poly(3-hydroxybutyrate) (PHB), poly(3-hydroxyvalerate) (PHV), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) [60,61].
Plastic additives aim to improve the physical features of plastic products since they act as softeners, flame retardants, UV and heat stabilizers, and production chemical agents [45]. Over 400 additives are listed by the European Chemicals Agency (ECHA) [62]. Plastic additives potentially affecting human health include phthalates and bisphenols [45]. These chemicals can pass from the polymer surface to the surrounding environment. Leaching and degradation of plasticizers and polymers are complex processes influenced by environmental conditions and the chemical properties of each additive. For example, long-lasting sunlight exposure may affect the polymer structure and make additive migration more likely. This may, on the one hand, promote additives’ release into the environment and, on the other hand, allow chemicals to adsorb on the surface [45].
Existing data indicate that bisphenol (BP) A/S/F concentrations adsorbed on MNPLs are approximately 9.52–4961 ng/g [63]; however, more investigations are required due to the small sample size. Moreover, hydrophobic interaction forces are mainly involved in maintaining the adsorption of MNPLs and BPs, and hydrogen and halogen bonds promote the adsorption. The simultaneous exposure to MNPLs and BPs can result in immune responses, neurotoxicity, inhibition of reproduction, and even reduced survival in experimental organisms [63]. However, the toxicological information on the effects of co-exposure to MNPLs and BPs is still lacking. Further research should address the ecological risks of MNPLs and BPs at environmental concentrations and promote the reduction in their flow to the natural environment and the development of non-toxic alternative materials.
Di-(2-ethylhexyl) phthalate (DEHP) is a widely used plasticizer, and it is also a known environmental endocrine disruptor that causes harm to male reproduction [64]. Due to its unstable binding to plastic compounds, it is easily released into the environment [65]. The adsorption of DEHP on MPs is higher than that of other phthalate esters (PAEs), such as dibutyl phthalate (DBP), dimethyl phthalate (DMP), or diethyl phthalate (DEP) [66], and studies in mice showed more significant disruption of spermatogenesis and more serious sperm physiological alternations after DEHP-contaminated MPs exposure than DEHP alone [66]. However, whether plastic particles could enhance the toxicity of this type of co-contaminants is still unknown.
2.2. Detection Techniques of MNPLs
Although MNPL investigation and detection methodologies have recently been widely investigated, the development of reliable analytical methods to measure them is still lacking, especially considering NPs [67]. The primary steps typically needed to analyze MNPLs are the MNPL sampling, sampling processing, and sample detection analysis [61].
The sampling process needs, firstly, the identification of a sampling site with adequate MNPL sources for research use, the evaluation of the biotic or abiotic factors potentially influencing MNPL properties, and, finally, the use of non-contaminated sampling tools [61]. The MNPL samplings can be performed in soil, water, and air. Soil is a significant sink for plastic debris through different mechanisms such as water runoff or atmospheric deposition, showing an uneven vertical distribution because of MNPL sources, soil type, land use, and climate [68]. In water and sediments, the sampling of MNPL is very complex, and possible factors potentially affecting the measurements should be considered, including MNPL density or shape and the environmental conditions such as currents, flows, wind, or water density [69]. Tools typically used to sample MNPLs are fine nests, manta trawls, AVANI trawls, or neuston nets [70,71]. The airborne MNPLs can be sampled passively using, for example, a stainless-steel funnel or actively by vacuum/sampling pumps or by post-deposition collection using brushes or spoons [72].
Due to the different MNPL sizes and compositions and the characteristics of the different matrices (soil, water, or air) in which they can be accumulated, the processing of the samples is very multifaceted. As an example, typically for soil samples, density flotation, filtration, and sieving are commonly used methodologies to process extrapolated MNPLs [73]. Differently, the flotation of MNPLs with lower density in more dense liquid [74,75], elutriation [76], and the Munich Plastic Sediment Separator [77] are some of the technologies used for sediment samples.
The detection analysis approaches can be different based on the MNPL properties. Generally, the MNPL analysis can be differentiated between physical imaging, which investigates their morphology or topology, and chemical analysis, which can provide their chemical composition. Different techniques need to be used depending on the nature of the analysis. Figure 1 summarizes the most common methods used to analyze the MNPLs.
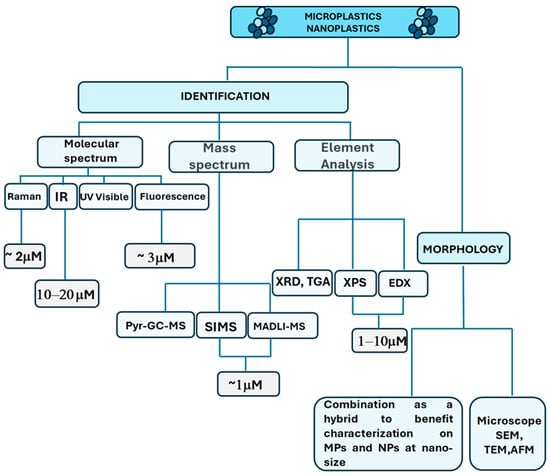
Figure 1.
Different approaches and techniques employed for MNPL identification and characterization [78].
Physical imaging includes microscopy (such as stereomicroscopy), scanning electron microscopy (SEM), transmission electron microscope (TEM), and atomic force microscopy (AFM). The microscopy techniques are usually based on fluorescence microscopy, binocular microscopy, and polarized light microscopy (PLM) [77,78,79,80]. The techniques use physical characterization (i.e., color, glossiness, cross-sectional properties, surface structure, thickness, and hardness) to identify MNPLs. SEM has always been associated with high-resolution images to obtain morphological features through surface analysis, thus uncovering the information that the particles can provide [81].
The methods used for the chemical composition of element analysis include mass spectrum and molecular spectrum. The elemental analysis consists of X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS) [82], energy-dispersive X-ray spectroscopy (EDX), and thermogravimetry (TGA) [83]. Elemental analysis is often used in combination with mass spectrometry and SEM; for example, thermogravimetry coupled to mass spectrometry (TGA-MS) was developed to quantify PS-MPs in the atmosphere near an agricultural area [84]. EDX is often used in tandem with SEM [79,85]. Scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM-EDX) can analyze the morphology and elemental composition of MNPLs [79].
Fourier-transform infrared spectroscopy (FTIR) is used to identify MNPLs at the molecular level through the exposure of MNPLs to infrared radiation at different wavelengths and reference spectral libraries [86]. Micro-Fourier-transform infrared spectroscopy (μ-FTIR) and micro-attenuated total reflectance Fourier-transform infrared spectroscopy (μATR FTIR) can analyze some specific polymer types [87,88]. These represent relatively mature methods for measuring MNPLs [89]. Micro-Raman spectroscopy (μ-Raman) with Nile red can detect MNPLs with smaller size (1 μm), but the results of this analysis can be affected by additive interference [90,91].
Pyrolysis–gas chromatography–mass spectrometry (Pyr-GC-MS) [92] and thermal desorption–gas chromatography–mass spectrometry (TD-GC-MS) [93] can quantify the mass concentration of specific MNPLs, but the sample can be easily damaged, thus affecting the accuracy of the analysis results during the heating process [85,88]. In the latest research, a high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS) was used to measure PS online in laboratory-generated and environmental aerosols [94]. Although this method has a high temporal resolution, it does not allow for particle mixing, and there is some uncertainty about the elucidation of the mixture. The principles, advantages, and limitations of the currently used microscopic and analytical techniques for MNPL identification and quantification are summarized in Table 1.

Table 1.
Analytical methods mainly used for MNPL identification and quantification.
3. Oral Exposure to MNLPs: Implications for Human Health
Although the dispersion of MNPLs in the environment is considered a global issue, knowledge concerning their impact on human health is still lacking. The literature identified three major routes for MNPL exposure: ingestion [99], inhalation [100], and dermal contacts [52]. MNPLs have been found in different foodstuff classes, such as seafood, beverages, drinking water, salt, sugar, honey, terrestrial meat, and crops and livestock [101,102]. Different classes of polymers, e.g., PP, PE, PVC, and PET, have been measured in mussels, with contamination percentages ranging between 61% and 97% of the mussels containing MNPLs and concentrations from about 0.040 ± 0.003 MPs/g wet weight (ww) in fresh mussels and up to 0.9 ± 0.1 MPs/g ww in processed mussels [89]. Polyamide (PA), PP, PVC, PS, and PET have also been identified in prawns, crabs, squid, and shrimps with MNPL diameters from 20 µm to 5000 µm [101,102]. Pyr-GC-MS detected polyvinyl chloride in oysters, prawns, squid, crabs, and sardines and a total concentration of PE between 0.04 and 2.4 mg g−1 of tissue [103]. Similarly, MNPLs of different chemical compositions in fish meat have been found with concentrations ranging between 0–100 µg/g and 0–2400 µg/g for PS and PE, respectively [103]. Extruded polystyrene (XPS), widely used to pack meat, contaminated chicken meat at a level ranging from 4.0 to 18.7 MP-XPS/kg of packaged meat [104]. Although the study of MNPLs in cereals, which account for 55–70% of the total diets of developing countries, is still lacking, the possible transfer of plastics to cereals could occur through MNPL presence in agricultural soils and the use of contaminated waters for irrigation or of fertilizer [101].
Human oral exposure to MNLPs represents a pressing concern in environmental health research, underscored by mounting evidence of plastic’s pervasive presence in food and beverages. Once ingested, these plastic particles encounter the gastrointestinal tract and undergo a complex array of physical and chemical transformations.
Before entering the gastrointestinal system, MNLPs first encounter saliva in the mouth. Different proteins attach to plastic particles at this level, inducing a corona formation. Dorsch et al. [105] suggested that NP size and surface chemistry dictate the interaction with human saliva proteins, which has significant implications for human plastic uptake. They used PS-MNLPs of two different sizes and three different charges, incubating them individually with saliva samples from healthy volunteers [105]. This study showed that, in saliva samples, protein profiles and relative quantities were determined by the plastic particle’s size and charge, which affect their hydrodynamic size, polydispersity, and zeta potential. These processes are dynamic and influenced by exposure times [105]. Smaller particles appeared more reactive with surrounding proteins, and when cultured with different cell lines, they affected cellular metabolic activity and genotoxicity [105].
Different studies have elucidated the ability of MNPLs to accumulate within the gastrointestinal tract, with some particles translocating across intestinal epithelial barriers and entering systemic circulation [106]. This phenomenon raises profound concerns regarding their potential to induce adverse health effects, including inflammation, oxidative stress, and dysregulation of gut microbiota composition, all of which could have significant implications for overall health and well-being [107]. The persistent nature of MNPLs exacerbates these concerns, as their prolonged permanence time in the gastrointestinal tract increases the likelihood of interactions with intestinal cells and absorption into the systemic circulation, amplifying the potential health risks for human health.
Furthermore, the physicochemical properties of MNPLs influence their capacity to adsorb and transport a wide array of organic pollutants, heavy metals, and microbial pathogens, thus serving as vectors for environmental contaminants within the human body [108]. This characteristic adsorption capability facilitates the binding of toxic chemicals onto plastic surfaces, consequently enhancing their bioavailability and prolonging their persistence in biological tissues and potentially exacerbating their toxic effects [109]. Moreover, the diminutive size of NPs raises additional concerns regarding their ability to traverse biological barriers, including cellular membranes and the blood–brain barrier, and to engage with subcellular structures such as organelles and DNA [106,110]. This enhanced capability for cellular and subcellular interaction amplifies the potential adverse effects of NPs, as it augments their likelihood of inducing cellular dysfunction, genetic mutations, and cytotoxicity within biological systems [106]. Various studies have reported MNPL accumulation in lysosomes, affecting lysosomal-associated cellular activities following cellular internalization [111,112]. The subcellular localization of NPs in mitochondria and their effects on mitochondrial function were also described [113]. However, MPs have not yet been reported to accumulate in mitochondria, but their interaction was described [114]. Altogether, data from the literature suggest that MNPLs target mitochondria, affecting all biochemical energy pathways of the cell [106]. MNPLs were also reported to damage DNA inside the nucleus [115] Finally, the abundance of ribosomal protection protein genes linked to the risk of antibiotic resistance gene propagation was reported [116]. In a recent review, Poma et al. [117] collected published studies on the epigenetics effects and gene expression modulation induced by MNPLs. Although these data are still scarce, it is becoming evident that MNPLs, whether acutely or chronically administered, can induce such changes in various model organisms.
The burgeoning field of research concerning the potential health impacts of oral exposure to MNPLs has seen significant advancements in recent years, offering valuable insights into the intricate mechanisms underlying their toxicity. For instance, the groundbreaking study by Schwabl et al. [118] represented a pivotal milestone in this field, as it revealed the presence of MPs in human stool samples. This finding indicated the remarkable resilience of plastic particles through the gastrointestinal tract and raised concerns about the potential long-term accumulation of plastic debris within the human body. Building upon this discovery, Garcia and colleagues [119] further elucidated the systemic distribution of ingested MPs by detecting their presence in various human tissues, including the liver, spleen, and kidneys.
As emerging evidence continues to underscore the potential health risks related to human exposure to MNPLs via the oral route, urgent action is required to address these concerns through comprehensive risk assessments and regulatory measures. The imperative for such initiatives is underscored by the need for interdisciplinary collaboration among researchers, policymakers, and stakeholders to develop robust methodologies for detecting and quantifying plastic contaminants in food and beverages. Moreover, elucidating the intricate mechanisms of plastic particle toxicity and evaluating their long-term health effects are critical components of these efforts [120]. Additionally, proactive measures aimed at curbing plastic pollution at its source are indispensable, necessitating improved waste management practices and advancing sustainable alternatives to conventional plastics. By undertaking these concerted efforts, society can take decisive steps toward safeguarding human health and the environment from the pervasive threat posed by plastic pollution [121].
3.1. The Role of MNPLs in Cell Membrane Disruption
The potential of MNPLs to disrupt cell membranes is an increasing concern in environmental health research, primarily due to their pervasive presence and possible harmful impacts on cellular integrity and function. These plastic particles have been detected across various environmental compartments, underscoring their extensive distribution [122]. Their interactions with cell membranes occur through both physical and chemical mechanisms, leading to significant structural and functional alterations [123]. Physically, MNPLs can adhere to the membrane surface, integrate into lipid bilayers, or induce mechanical stress that disrupts membrane integrity. NPs exhibit enhanced capabilities for penetrating membranes due to their high surface area-to-volume ratio, which may facilitate their internalization into cells [100]. This increased membrane interaction can compromise cell function and viability.
Chemically, these particles can release additives such as plasticizers and flame retardants, which can integrate into lipid bilayers and modify membrane fluidity, thereby affecting cell function [122]. Additionally, the hydrophobic nature of plastic surfaces encourages the adsorption of hydrophobic organic pollutants (HOPs) such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). These pollutants can accumulate on plastic particles and subsequently desorb onto cell membranes upon ingestion [124]. The adsorbed pollutants can further disrupt cell membranes by altering lipid composition, changing membrane protein conformation, and affecting ion channel activity, thus distorting membrane permeability and cellular homeostasis [125].
Kihara and colleagues [126] found mild cytotoxicity and cellular uptake of PS-NPs, with no clear trend based on NP size (20 and 200 nm) or surface charge. In contrast, they observed an NP size dependency on bilayer disruption, suggesting that membrane disruption, resulting from direct interaction with PS-NPs, has little correlation with cytotoxicity. Furthermore, they reported that the level of bilayer disruption was limited to the hydrophilic headgroup, thus suggesting that transmembrane diffusion was an unlikely pathway and that cellular uptake–endocytosis is the viable mechanism. They reported that small PS-NPs (20 nm) were rarely found near chromosomes without a nuclear membrane surrounding them; however, this was not detected for larger PS-NPs (200 nm). Their findings suggest NPs can interact with chromosomes before nuclear membrane formation. Overall, PS particles’ precoating with protein coronae have been shown to reduce cytotoxicity irrespective of the corona type [126] (Table 2).
In accordance, Milillo and colleagues [127] showed that PS-NPs (800 nm) exhibited no permeation capability across membranes. They also reported internalization by target cells (human adenocarcinoma alveolar epithelial cells A549) characterized by electron-dense particles and autophagic vacuoles in a concentration-dependent manner. At higher concentrations, NPs appear dispersed inside cellular endosomes, and cells start showing signs of toxicity [127] (Table 2).
Liu et al. recently reported a deep analysis of the cellular internalization and release of MNPLs as a key finding in predicting their cytotoxicity [128]. In basophilic leukemia RBL-2H3 cells exposed to PS particles, they found that PS particles (50 and 500 nm) interact with negatively charged cell membranes through the hydrophobic interaction and Van der Waals’ forces, while 5000 nm particles were not able to absorb on the cell membrane. PS particles, sizing 50 nm and 500 nm, were internalized by cells via both passive membrane penetration and active endocytosis via clathrin-mediated and caveolin-mediated pathways (for 50 nm particles) or micropinocytosis (for 500 nm particles). Moreover, cells were able to release the accumulated intracellular particles through energy-dependent lysosomal exocytosis and energy-independent membrane penetration [128].
The disruption of cell membranes by MNPLs was linked to several adverse effects, including cytotoxicity, genotoxicity, and inflammation. The internalization of plastic particles can trigger immune responses and inflammatory cascades, releasing proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which further exacerbate cellular damage and tissue inflammation [129] (Table 2).
Understanding the mechanisms by which MNPLs disrupt cell membranes is crucial for assessing their potential health risks. By identifying these mechanisms, researchers can develop strategies to mitigate the adverse effects of these particles on human health and the environment. Continuous investigation of their interactions with cellular structures will provide information for regulatory policies and environmental protection measures.
3.2. MNPL Activation of Oxidative Stress and Apoptosis Pathways
The intertwined nature of oxidative stress, apoptosis, and the toxicity of MNPLs constitutes a critical area of environmental health research, drawing considerable attention to their impact on cellular homeostasis and organismal well-being. Oxidative stress derives from an altered balance between reactive oxygen species (ROS) production and the body’s ability to counteract their harmful effects through antioxidant defenses. This imbalance is a central mechanism by which various environmental stressors, including MNPLs, induce cellular damage [130]. These plastic particles can initiate ROS production upon exposure via multiple pathways, such as mitochondrial dysfunction, NADPH oxidases’ activation, and redox-sensitive signaling cascades’ disruption [87]. The elevated ROS levels can inflict oxidative damage on critical cellular macromolecules, including lipids, proteins, and DNA, impairing cellular functions and precipitating cell death.
After the mouth, the stomach is the primary organ that MPs-contaminated food or water reach along the digestive tract. Recently, Sun et al. [131] indicated that PS-MPs (50 nm and 250 nm) at environmental-related doses significantly decreased stomach organ coefficient, inhibited gastric juice and mucus secretion, disrupted gastric barrier function, and suppressed antioxidant ability in mice. They also showed that PS-MPs inhibited cell viability, increased ROS generation, and induced apoptosis through a mitochondria-dependent pathway in human gastric epithelium cells (GES-1). Simultaneously, PS-MPs decreased mitochondrial membrane potential and ATP levels, disrupted mitochondrial kinetic homeostasis, and activated the P62/Nrf2/Keap1 pathway [131] (Table 2). Another study showed that in mice receiving drinking water containing PS-NPs for 32 consecutive weeks, plastics exposure led to a significant time- and dose-dependent increase in ROS and malonaldehyde (MDA) levels and concurrently decreased glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) contents in intestinal tissues of mice [132] (Table 2). Using PS-NPs (800 nm) on the A549 cell line, Milillo and coworkers [127] found that increased production of H2O2 downstream reduced expression of cellular antioxidant network glutathione peroxidase (Gpx)1, catalase (CAT), SOD1, and SOD2. Additionally, they reported that the gene expression of Heme Oxygenase 1 (HMOX1), whose product is a ubiquitous stress-response protein, significantly increased following PS-NP treatment. This collective analysis strongly suggests that PS-NPs induce oxidative stress [127] (Table 2).
Apoptosis, also known as programmed cell death, is a tightly regulated process that eliminates damaged or stressed cells. Exposure to MNPLs can prompt apoptotic pathways through several mechanisms, including mitochondrial dysfunction, death receptor activation, and modulation of pro- and anti-apoptotic protein expression [133]. The misregulation of apoptosis due to these particles can cause tissue damage and organ dysfunction, illustrating the complex interaction between oxidative stress and apoptotic signaling in cellular responses to environmental contaminants. Previous studies have confirmed the PS microplastics’ capacity to induce apoptosis across various tissues and organs, including reproductive [134,135], cardiovascular [136], and nervous systems [137], and in a mixed form instead of a singular mode [138]. In the A549 cell line, the expression levels of the pro-apoptotic protein Bax and the effector Caspase-3 increased after just 2 h of exposure to PS-NPs (Table 2). On the other hand, the anti-apoptotic protein Bcl-2 expression decreased at the lowest concentrations, namely 10 and 100 μg/mL, and showed a slight increase at the highest concentration, i.e., 500 μg/mL [137].
The toxicity of MNPLs is complex and involves their interactions with various cellular components and organelles, resulting in significant health implications. Examining the molecular mechanisms that underlie these plastic particles’ induction of oxidative stress and apoptosis is crucial for comprehending their toxicological impacts. This understanding will allow the development of strategies to reduce their detrimental effects on the environment and human health. Moreover, comprehensive assessments of MNPL toxicity need an integrative approach combining in vitro cell culture models, animal studies, and epidemiological research.


Table 2.
PS particle effects on cell membrane disruption, activation of oxidative stress, and apoptosis pathways.
Table 2.
PS particle effects on cell membrane disruption, activation of oxidative stress, and apoptosis pathways.
| Particle Type | Particle Size | Experimental Models | Mechanisms of Membrane Interaction/Internalization | Findings | Ref. |
|---|---|---|---|---|---|
| PS | 20 nm 200 nm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [126] |
| PS | 800 nm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [127] |
| PS | 50 nm 500 nm 5 μm |
|
| In in vitro model, the exposure to PS particles was associated with the following:
| [128] |
| PS | 50 nm 250 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [131] |
| PS | 50 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [132] |
| PS | 50 nm 500 nm 5000 nm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [133] |
| PS | 0.5 μm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [134] |
| PS | 0.5 µm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [135] |
| PS | 0.5 µm |
|
| In in vivo model, the exposure to PS particles was associated with the following:
| [136] |
| PS | 100 nm |
|
| In in vitro models, the exposure to PS particles was associated with the following:
| [137] |
4. Intestinal Effects of Oral Exposure to MNPLs
Currently, there is no legislation for MNPLs as food contaminants. Consistent data gaps concerning exposure and toxicity of such particles limit the risk assessment [139]. Information about the fate of MNPLs in the gastrointestinal tract still needs to be included. Based on in vitro and in vivo data from 2016, the European Food Safety Authority (EFSA) reviewed knowledge about the uptake along the gastrointestinal tract of MNPLs [5]. The available data on toxicokinetics are only related to absorption and distribution, while there is no information about metabolism and excretion.
Moreover, it is not known whether orally ingested MPs are degraded to NPs in the gastrointestinal tract [5]. MPs over 150 μm are not absorbed; they can bind to the intestinal mucus layer and realize direct contact with the apical part of intestinal epithelial cells, thus promoting gut inflammation and local effects on the immune system. Differently, smaller particles (<150 μm) can cross the mucus barrier. Size-dependent uptake of MNPLs may involve several mechanisms, including (i) endocytosis through enterocytes; (ii) transcytosis through microfold cells (also named M-cells), a subgroup of intestinal epithelial cells in gut lymphoid tissue; (iii) persorption (i.e., the passage through gaps formed at the villous tip due to the loss of enterocytes); and (iv) paracellular uptake [140].
Although the intestinal uptake of MPs is not very efficient [141], intestinal absorption of MPs may be associated with systemic exposure levels with a toxicological relevance. The reduced size of NPs promotes their penetration deeply into organs. Results from animal studies demonstrated that once absorbed, NPs distribute to the liver, spleen, heart, lungs, thymus, reproductive organs, kidney, and brain (i.e., they cross the blood–brain barrier) [5,142].
Moreover, airborne MPs may also impact the immune system. In particular, the smallest particles (i.e., the inhalable fraction) can be absorbed through the pulmonary epithelium [143,144], reach the systemic circulation, and exert immune effects within the so-called gut–lung axis [145]. The larger particles (the extrathoracic fraction) can be transported, at least partially, to the gastrointestinal tract by mucociliary clearance. Here, they have the same fate as ingested particles. Thus, based on their size, both ingested and inhaled plastics interact with intestinal tissues, reach the circulation, and may potentially affect the immune response.
Most publications focus on plastic particles’ environmental degradation [146,147]. However, before reaching the intestinal epithelium, MNPL passage through different gastrointestinal compartments may affect their physicochemical properties and surface parameters. Nowadays, knowledge of MNPL fate along the mammalian gastrointestinal tract is still limited. Nonetheless, one of the most relevant aspects to consider is the stability of plastic materials. Low gastric pH represents the most complex chemical condition, but specialized enzymes for plastic degradation could also contribute; however, they are lacking in the mammalian intestine [45]. This suggests that, most likely, no major degradation of plastic particles occurs during digestion. Some bacteria could contribute to plastic degradation through the action of oxygenases by adding oxygen to long carbon chains, thus destabilizing the local electric charge and provoking plastic degradation [45]. However, the distribution of these enzymes is restrained because they could also degrade bacterial molecules [148,149]. It is plausible that the most critical parameter affected by digestion is the corona formation, consisting of proteins and other surrounding molecules attached to the surface in the environment and during the first contact with digestive fluids: the digestion of protein corona composition may induce a shift towards low-molecular-weight proteins [149]. Most in vitro studies ignore the phenomenon of protein corona formation, which consists of proteins from the cell culture media and may be different from environmental and digestive fluid samples.
The uptake and transport of particles up to a maximum of 5 to 10 μm in size into intestinal cells appears plausible. However, it should be considered that the previous contact with intestinal fluids can promote particle agglomeration, thus influencing the uptake compared to single particles [45]. Intracellular uptake of larger particles seems incompatible with the size of intestinal epithelial cells of about 10 μm. According to the European Food Safety Authority (EFSA), MPs have a limited bioavailability (<0.3%), and only plastics smaller in size (<150 μm) may cross the intestinal epithelium. They can also be found in tissue but are plausibly unreactive and deposited and thus not systemically bioavailable. Only particles up to 1.5 μm in size might be distributed systemically. The highest uptake and transport were found possible for the smallest particles (with size in the submicron range). A systemic distribution of plastic particles (in the nano size range) has been proposed [150,151,152]. Cellular uptake of particles > 10 μm in size could be possible in specialized cells, such as macrophages [153,154]. A paracellular transport of particles could also occur through gut leakages in the intestinal cell monolayer. Due to this phenomenon (called persorption), bigger particles could reach portal circulation [155].
Most of the studies using in vitro models to investigate plastic particle uptake are performed with the cell line Caco-2, a widely used model for human enterocytes [156]. Caco-2 cells spontaneously differentiate, and if cultured on porous membranes, they acquire a monolayer organization suitable for transport assessments [157,158,159]. Cellular uptake by Caco-2 has been shown for fluorescent plastics from 25 to 500 nm, with the highest efficiency observed for 100 nm particles [160]. Results from other studies have indicated a small translocation of PET NPs through the intestinal barrier [161].
In vivo animal studies support a low oral bioavailability of MP particles, thus suggesting a substantial fecal excretion estimated to be more than 90% through feces (depending on different features, including size, shape, and chemical composition) [5,106]. Recently, MPs have been found in human feces [118]. At least nine different types of plastics, ranging from 50 to 500 μm, mainly PP and PET, have been detected. It is noteworthy that plastic particle detection in feces indicates their oral uptake, but it cannot sustain conclusions about oral bioavailability.
4.1. Impact of MNPLs on Healthy Intestine
The intestinal effects of MNPLs have emerged as a complex and multifaceted area of concern within environmental health research, highlighting the potential ramifications for gut health and, in general, human well-being. Once in the gut, these particles interact with many cellular and molecular components, creating a cascade of immunological and inflammatory responses that significantly impact intestinal homeostasis.
The intricate structure of the intestinal barrier, primarily consisting of epithelial cells held together by tight junctions, plays a crucial role in regulating the passage of nutrients and other substances while preventing the entry of harmful pathogens and toxins. However, exposure to MNPLs has been shown to compromise the integrity of this barrier. These particles disrupt tight junction proteins and increase intestinal permeability [162]. Consequently, this breach allows for the translocation of MNPLs and associated toxicants across the epithelial barrier into the systemic circulation, potentially eliciting effects beyond the gut [163].
In addition, ingesting MNPLs is associated with the induction of oxidative stress within the gastrointestinal tract. These plastic particles can act as sources of ROS or induce ROS production in surrounding cells, overwhelming antioxidant defenses and provoking oxidative damage to cellular macromolecules [99]. This oxidative stress contributes to local tissue injury and inflammation and exacerbates intestinal barrier dysfunction, perpetuating a cycle of gut dysbiosis and inflammation. Moreover, the immunomodulatory properties of MNPLs have garnered significant attention due to their capacity to modulate immune responses within the gut. These plastic particles can activate immune cells such as macrophages and dendritic cells, triggering the release of pro-inflammatory cytokines and chemokines [164].
Clinical studies designed to analyze the health risks of MNPLs are not feasible in humans due to ethical concerns. Consequently, a clear understanding of the health impact of MNPLs on humans is difficult to achieve. There needs to be more awareness of the extent of MNPL absorption and accumulation in humans and the pharmacokinetic and pharmacodynamic mechanisms associated with these processes [164].
Studies assessing the effects of MNPL exposure on the gut epithelium in vivo have been performed in invertebrates, aquatic vertebrates, and zebrafish. Evidence of MNPL intestinal toxicity in mammals is emerging [165].
Table 3 summarizes studies assessing the effects of MNPLs in murine models. The main results from these studies include the capacity of PS-MPs to decrease mucus secretion significantly and the transcript levels of genes associated with mucus secretion and ion transport in the gut [18]. Evidence about the role of PS-MP exposure on intestinal inflammation in mice is controversial: while no evidence of intestinal inflammation was found in a study in which mice were exposed to PS-MPs [66], in two other studies with polyethylene MPs, mice developed histological signs of colon and duodenum inflammation and showed higher protein amounts of the innate immune receptor toll-like receptor 4 (TLR4), the pro-inflammatory transcription factor activator protein 1 (AP-1), and interferon regulatory factor 5 (IRF5) [20]. Impairments of intestinal permeability were also observed in another study with polyethylene MPs in mice [166]. These discrepancies may be explained by differences in the mouse’s genetic background, exposure schedule, and dosage.
More recently, Huang et al. [167] performed a study in C57BL/6 mice orally administered with five different sizes of PS-NPs (20, 50, 100, 200, and 500 nm). They found that after long-term oral exposure to PS-NPs 75 mg/kg (200 nm and 500 nm), significant hepatotoxicity was induced, as revealed by increased oxidative stress, liver dysfunction, and lipid metabolism disorders. Most importantly, NPs (200 nm and 500 nm) generated local immunotoxic effects by recruiting and activating inflammatory cells, i.e., neutrophils and monocytes in the liver or intestine, potentially resulting in increased pro-inflammatory cytokine secretion and tissue damage [168]. In mice receiving drinking water containing PS-NPs (0.1, 1, and 10 mg/L) for 8 consecutive months, the levels of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β were markedly elevated [133]. In another study, exposure to MNPLs decreased the levels of secretory immunoglobulin A in the intestine and the differentiation of CD4+ and CD8+ T cells in mesenteric lymph nodes.
Moreover, the impact of exposure on mammalian intestinal health is influenced by the duration and particle size rather than the concentration [168]. Hirt and Body-Malapel also reviewed the studies assessing the impact of MPs on intestinal microbiota in several contests [165]. All reports showed that MP exposure causes dysbiosis in animals. The induced variations in microflora diversity and composition are likely involved in functional immune system impairments caused by MP exposure.
The intestinal immune system continuously interacts with non-pathogenic commensal organisms and innocuous food antigens, rapidly responding to infectious threats and toxins. This capacity involves several mechanisms mediated by myeloid cells, innate lymphoid cells, and T cells. These cells are resident in the intestinal lamina propria and the draining mesenteric lymph node and are critical players of the immune system. The immunotoxicity of plastics has not been directly evaluated on the intestinal immune system. However, immune cells and also those of the intestinal immune system can be a target for plastics-induced intestinal damage, as demonstrated by studies conducted mainly in invertebrates and vertebrates, showing that the immune system is compromised by exposure to MNPLs [165].
In mice, exposure to PE-MPs affects serum levels of IL1α and granulocyte colony-stimulating factor G-CSF, decreases the regulatory T-cell count, and increases the proportion of Th17 cells in splenocytes [20]. In cross-generational studies in mice, PE exposure increased blood neutrophil counts and IgA levels in dams and altered spleen lymphocytes in both dams and offspring [169]. Altogether, these results strongly support the alteration of the immune system by plastics, highlighting the need for further immunotoxicity studies.
Although the tested exposure doses of MNPLs are generally higher than the actual dose intake by humans, the damage to the intestine is not adequately considered a disease state [22,23]. Most detected changes in intestinal functions are only at the molecular transcriptional level and are not associated with significant pathological damage in the intestine [18,170,171]. This suggests that, in healthy organisms, the intestine resists the impact of MNPLs [172,173]. However, introducing MNPLs into the intestine may alter the balance of the intestinal immune system. Consequently, non-specific immune stress affecting the integrity of the intestinal barrier may occur [174,175].
Studies in mice on the safety assessment of MNPLs show great particle size and dose variations, which may explain different experimental results. Therefore, clear evidence that MNPLs can cross the intestinal barrier of healthy mammals still needs to be sought [133,134,176,177]. As summarized in Table 3, in vivo studies conspicuously support the idea that MNPL exposure causes alteration of the intestinal microbiota structure of healthy individuals and induces immune stress in the intestinal mucosa. These events may induce mild intestinal inflammation at higher doses; however, severe damage to the intestine in healthy individuals has not been demonstrated [178]. Moreover, while there is evidence of the impact of MNPLs on the immune system, a large part of the studies mainly focused on the innate immune response, and the effects of MNPLs on the adaptive immune response still need to be explored. A scheme of the potential impacts of MNPL contaminations on intestinal health and immune response is reported in Figure 2.
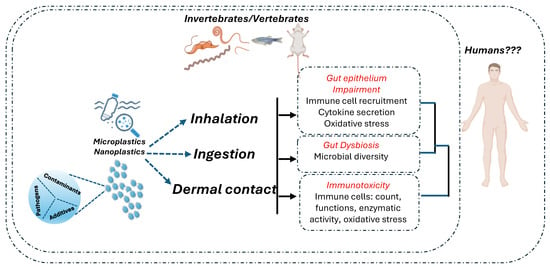
Figure 2.
The literature identified three significant routes for MNPL exposure: ingestion, inhalation, and dermal contact. MNPL exposure leads to the impairment of oxidative and inflammatory intestinal balance and disruption of the gut’s epithelial permeability. Moreover, the effects of MNPL exposure include dysbiosis (changes in the gut microbiota) and immune cell toxicity. MNPLs contain additives and adsorb contaminants and may promote the growth of bacterial pathogens on their surfaces. Thus, they act as potential carriers of intestinal toxicants and pathogens, which can potentially increase their adverse effects. Despite the possible impact of MNPL exposure on intestinal health and immune response that has been evidenced in vertebrates and invertebrates, there is a scarcity of studies demonstrating that these effects are relevant to humans [165]. Created with biorender.com.
The intestinal epithelium interacts with a wide of plastics. However, we still need more data about the amounts and features of ingested plastics. The analytical methods used to detect plastics are still limited to micro-sized particles. Moreover, these data are controversial due to the limited number of studies and poor data quality (mainly for contamination) [179]. Importantly, the lack of standardized technical methods for collection and analysis does not allow reliable interstudy. Up to now, the available data have provided only a few clues about the plastic pollutants in drinking water and in a small number of food products. Larger studies of plastics in general diets are necessary to provide a realistic estimate of oral exposure to plastics, but they still need to be performed. In this regard, it would be advisable to focus on plastics in human stools, which allow us to identify plastics with harmful effects via direct contact with the intestinal mucosa.
Although the intestinal and immunotoxic effects of NP ingestion by mammals have never been studied, it is well known that ingestion of non-plastic nanoparticles such as TiO2 nanoparticles has harmful effects, including impaired intestinal and systemic immune homeostasis and variations in the gut microbiota and metabolism [180]. This means that the presence of some NPs in the diet is likely to cause harmful effects on the intestinal and immune systems.
The toxicological effects of some types and shapes of MPs are now starting to be assessed. Moreover, the MPs most frequently studied for their in vivo effects are PS-MPs, while PP-MPs, the most abundant MPs in human feces, have been studied only to a lesser extent. The evaluation of MNPL effects on intestinal human health is further complicated by chronic exposure to MPs, which is associated with very variable effects. It is also likely that the effects of the exposure to a “mixture” of MPs (as in real life) are different from those of individual components.


Table 3.
Effects of oral exposure to MNPLs on the healthy intestine in murine models.
Table 3.
Effects of oral exposure to MNPLs on the healthy intestine in murine models.
| Mouse Model | MNPLs | MNPLs Dose | Routes of Exposure | Time of Exposure | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| ICR | PS 0.5 and 50 µm | 100 and 1000 μg/L | Drinking water | 5 weeks |
| [173] |
| ICR | PS 5 µm | 100 and 1000 μg/L | Drinking water | 6 weeks |
| [176] |
| C57BL/6NTac | PS 1, 4, and 10 µm | Mixture of 1 µm (1.25 mg/kg), 4 µm (25 mg/kg), and 10 µm (34 mg/kg) | Oral gavage | 28 days |
| [166] |
| C57BL/6 | PE 10–150 μm | 2–20–200 μg/g Food (6, 60, and 600 μg/d) | Food | 5 weeks | In mice treated with 600 μg/day:
| [20] |
| CD-1 | PE 45–53 μm | 100 mg/kg/d | Oral gavage | 30 days |
| [66] |
| ICR | PS 5 μm | 100 and 1000 μg/L | Drinking water | 6 weeks |
| [181] |
| ICR | PE 1–10 µm | 0, 0.002, and 0.2 μg/g/d | Oral gavage | 30 days |
| [182] |
| C57BL/6 | PVC 2 µm | 100 mg/kg | Oral gavage | 60 days |
| [183] |
| ICR | PS 0.5 μm | 10 μg/mL, 50 μg/mL, and 100 μg/mL | Oral gavage | 2 weeks |
| [184] |
| C57BL/6J | PS 1 µm | 80 μg/kg/d | Drinking water | 8 weeks |
| [185] |
| C57BL/6J | PS 5 µm | 18 and 180 μg/kg/d | Drinking water | 90 days |
| [186] |
| KM | PE, PS, PP, PVC, and PET 150–300 µm | 20 mg/mL | Oral gavage | 1 week |
| [187] |
| C57BL/6 | PP 8, 70 µm | 1, 10, and 100 mg/kg/d | Oral gavage | 28 days |
| [188] |
| C57BL/6 J | PS 50, 500, and 5000 nm | 2.5–500 mg/kg | Oral gavage | 28 days |
| [133] |
| C57BL/6 | PS 200 and 800 nm | 109 MPLs | Oral gavage | 4 weeks |
| [189] |
| C57BL/6 | PE 5 μm | 1 and 10 mg/L | Oral administration | 21 days |
| [190] |
| C57BL/6N | PS 100 nm | 5 mg/kg bw/d | Oral gavage | 28 days |
| [191] |
| KM healthy-aged | PS 1 μm | 4.67 × 10−15, 4.67 × 10−12, 4.67 × 10−9, 4.67 × 10−6 mg/kg/d | Oral gavage | 10 days |
| [192] |
| C57BL/6 J | PS 500 nm | 5 mg/kg/d | Oral gavage | 30 days |
| [132] |
| C57BL/6 J | PS 0.5, 5 μm | 0.5 mg/mice in 100 µL sterile deionized water | Oral gavage | 8 weeks |
| [193] |
| C57BL/6 J | PS 0.2, 1, 5 μm | 1 mg/kg/d | Oral gavage | 28 days |
| [194] |
| C57BL/6 J | PS 50 nm | 0.1, 1, 10 mg/L | Drinking water | 32 weeks |
| [132] |
| C57BL/6 | PS 2 μm | 0.5, 2 mg/kg/d | Oral gavage | 8 weeks |
| [195] |
Increase/exacerbation (↑); reduction (↓); polystyrene (PS); polyethylene (PE); polypropylene (PP); polyethylene terephthalate (PET); polyvinyl chloride (PVC); mononuclear leukocytes (MNLs); Intraepithelial lymphocytes (IELs); lamina propria leukocytes (LPLs).
4.2. MNPLs and Inflammatory Bowel Diseases
As reported above, in the intestine, the inflammatory cascade induced by MNPL exposure can disrupt mucosal immunity, alter gut microbiota composition, and impair the function of intestinal immune cells. This disruption may collectively contribute to gastrointestinal diseases, including inflammatory bowel disease (IBDs) such as Crohn’s disease (CD) and ulcerative colitis (UC) and colorectal cancer (CRC) [196].
In many countries, the number of IBD patients is increasing, reaching more than 6.8 million patients diagnosed worldwide [197,198]. IBD incidence in newly industrialized and developing countries is generally higher than in Western developed countries due to more severe environmental pollution conditions [31,32,33,34]. For this reason, IBD patients are considered high-risk individuals in the environmental impact assessment of atmospheric particulate matter [199].
IBDs are characterized by the extensive infiltration of inflammatory cells in the intestinal mucosa, damaged structure of the intestinal barrier, and alteration of the intestinal flora, associated with diarrhea and bloody stools as the main clinical manifestations of the disease [200]. Typically, in IBD patients, a loss of the mucus layer occurs; this phenomenon promotes the attack of epithelial cells by pathogenic microorganisms [201]. Pathogenic bacteria and their product, namely lipopolysaccharide (LPS), activate immune cells, triggering an immune response in the lamina propria [202]. Activated cells, including macrophages, T lymphocytes, and B lymphocytes, secrete pro-inflammatory cytokines such as interleukin (IL)6, IL23, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, which attack intestinal epithelial cells and induce differentiation of immature immune cells in T helper 1 and 17 (Th1 and Th17) cells. Th17 cells also release pro-inflammatory mediators and recruit neutrophils [203], thus contributing to a vicious cycle of inflammation and cell damage, translating into the lamina propria thickening [202]. The regulatory T cells (Treg cells), a subgroup of immunosuppressive CD4+ T cells, produce anti-inflammatory cytokine such as IL10 and IL4 [204], and they are reduced in IBD patients (Figure 3).
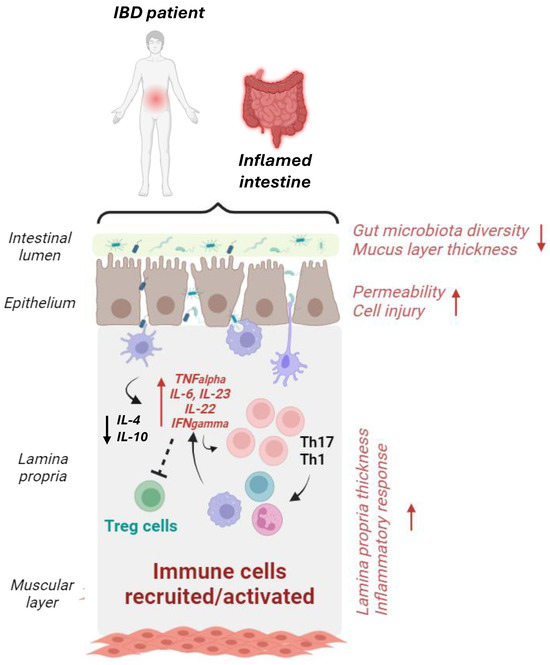
Figure 3.
Graphical schematization of the intestinal microenvironment in IBD patients. The intestine of IBD patients is characterized by dysbiotic microbiota (i.e., lower bacterial diversity, increased pro-inflammatory bacteria, etc.), decreased mucus layer thickness, and increased epithelial permeability, which trigger immune response activation. In this setting, antigens from dysbiotic microbes activate pro-inflammatory macrophages and dendritic cells (DCs), which release pro-inflammatory cytokines (such as TNF-α and IL-6), thus stimulating Th1 and Th17 cells and the subsequent release of other pro-inflammatory mediators and the recruitment of additional immune cells. The generation of anti-inflammatory cytokines IL-10 and IL-4 from DCs and Treg cells is also reduced [24]. Increase/exacerbation (↑); reduction (↓). Created with biorender.com.
Moreover, in IBDs, integrity loss of the enterocyte villus structure, tight junction (TJ) reduction, and oxidative stress have been reported. Thus, the increased cell–cell interspace promotes the crossing of harmful substances, and gut permeability is increased [205,206]. Compared to healthy individuals, in IBD patients, generally, the intestinal microbiota composition has a reduced bacteria diversity but not a significantly different number of bacteria: The abundance of Firmicutes phylum, Bacteroidetes, Lactobacillus, and Bifidobacterium decreased, while Actinobacillus and Proteobacteria were more abundant, thus suggesting that in IBDs, a decrease in the number of beneficial bacteria occurs, and the intestinal microbiota composition may also contribute to the intestinal damage [207] (Figure 3).
Recently, a relationship between MPs in feces and IBDs was described [208]. The study showed that the MP fecal concentration in IBD patients was significantly higher (41.8 items/g dm) than in healthy individuals (28.0 items/g dm). The detected MPs included 15 types, mainly sheets and fibers [208]. Moreover, the concentration of MPs and the activity level of IBDs were positively correlated. These results suggest (i) a relationship between MP exposure and the disease process or that (ii) in IBD patients, the disease process promotes MP retention.
Knowledge about the influence of MNPLs in patients with IBDs is still limited. However, several studies support the idea that MNPL exposure could aid intestinal barrier damage in these patients, thus promoting the severe development of intestinal pathology [35,36].
Recent studies in mouse models further support these suspicions. When mice were administered 100 nm PS-NPs at 1 mg/kg, 5 mg/kg, and 25 mg/kg for 28 consecutive days, inflammation and intestinal injury induced by sodium dextran sulfate (DSS) were exacerbated [209].
The most recent studies about the toxic effect of MNPLs in different animal models of IBDs are reported in Table 4. Compared to healthy controls, mice with IBDs induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) showed an increased accumulation of PS particles in the mucus layer and ulcerated regions as well as increased particle attachment to the colon mucosa [210]. In mice with acute colitis induced by DSS, PS-MPs upregulated the expression of inflammation factors (TNF-α, IL-1β, and IFN-γ) and oxidative stress factors [36]. In mice injected with LPS to cause intestinal inflammation and orally challenged with PS-NPs, LPS-induced duodenal damage and intestinal permeability were increased as well as the ROS levels and the expression of NF-κB [211].
The plastics mainly used by researchers are above 1 μm in size. However, in healthy intestinal mucosa, cell gaps have a range of 2–6 nm; thus, micron-sized plastics hardly penetrate through the tight junctions of healthy epithelial cells. In the damaged intestinal barrier, cell-to-cell gap spaces expand due to epithelial cell atrophy, thus permitting the MPs to cross the barrier. Interestingly, it has been proposed that in the intestinal lamina propria of IBD patients, the MNPLs could be engulfed by macrophages, thus inducing their immuno-activation and promoting the recruitment of more macrophages [212]. Altogether, these results indicate that IBD patients are more susceptible to MNLPs than healthy individuals and suggest the clinical need to pay attention to the toxic effects of MNLP exposure on individuals suffering from IBDs.
Recently, the comparison of MNLP content in feces from healthy volunteers and patients with IBD revealed a positive correlation between the number of fecal MNLPs and the severity of IBDs [208].


Table 4.
Effects of oral exposure to MNPLs on the intestine in murine models of IBDs.
Table 4.
Effects of oral exposure to MNPLs on the intestine in murine models of IBDs.
| Mouse Model | MNPLs | MNPLs Dose | Routes of Exposure | Time of Exposure | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| C57BL/6 and DSS-induced colitis | PS 5 µm | 100 μg/L, ~1.456 × 106 MPLs/L | Drinking water | 42 days |
| [213] |
| C57 and DSS-induced colitis | PS 5 µm | 500 μg/L | Oral gavage | 28 days |
| [214] |
| C57BL/6 J and DSS-induced colitis | PS 5 µm | 7280/72,800 MPLs/day | Oral gavage | 14 days |
| [215] |
| Lewis rats TNBS induced acute colitis | PS 0.1, 1, and 10 µm | 12.5 mg particles/kg bw | Oral administration | 3 days |
| [210] |
| C57-BL/6 mice and DSS-induced colitis | PS 5 µm | 500 μg/L | Drinking water | 28 days |
| [36] |
| C57BL/6 mice and LPS-induced inflammation | PS~102 nm | 5 μg/g | Intraperitoneal injection | 14 days |
| [210] |
Increase/exacerbation (↑); reduction (↓); polystyrene (PS); polyethylene (PE); polypropylene (PP); polyethylene terephthalate (PET); polyvinyl chloride (PVC); mononuclear leukocytes (MNLs); intraepithelial lymphocytes (IELs); lamina propria leukocytes (LPLs); trinitrobenzenesulfonic acid (TNBS); lipopolysaccharide (LPS).
4.3. MNPLs in Colorectal Cancer Development and Progression
CRC is the third most common cancer worldwide, with 1.9 million cases in 2020, and in many countries, it is increasing under 50 years of age [38,39,216]. The reasons for epidemiological change are not entirely understood [217]. Still, it may be partly the result of an environmental influence, as suggested by the increased risk of early-onset CRC in individuals born after 1960 compared to previous generations [46]. This may be explained by several factors, including the increasing trends in obesity, sedentary lifestyles, smoking, the Westernization of diets, the use of antibiotics in early life [38], and the use of contraceptive pills starting from the late 1950s [218]. However, mechanistic links between this new epidemiological trend and these factors have yet to be identified [219].
It is well recognized that early-onset CRC and, in general, sporadic colorectal cancer are likely to be associated with the interaction of microbiota with the gut mucosa [220,221]. The mucosal epithelium is protected by a mucus layer that has an increased thickness from the proximal to the distal of the healthy colon [220] to act as the first line of defense for colonocytes from luminal bacteria [221]. The colon mucus layer consists of an inner sublayer, which expands distally into an outer layer. The inner mucus layer represents a barrier separating colonic bacteria from epithelium [222], while the outer mucus layer provides a nutrient-rich habitat for some colonic bacterial species [223]. It was proposed that some bacteria in this layer are associated with an increased risk of CRC [224].
By reaching the colon with the diet, MNPLs may alter the balance between the microbiota and the mucus layer and influence the colonocytes’ exposure to potentially harmful bacteria, thus promoting the incidence of CRC. To date, studies addressing the carcinogenic potential of MNPLs mainly include in vitro models and/or short-term studies in rodents, and therefore, they do not allow clear conclusions [225,226].
Carcinogenesis can involve genotoxic or non-genotoxic mechanisms [227]. In genotoxic carcinogenesis, direct DNA damage occurs due to the action of mutagens.
The chemical nature of MNPL surfaces enables them to adsorb hydrophobic compounds (including some carcinogens), charged molecules, ions such as toxic metals, and pathogenic bacteria. Thus, the health risks associated with MNLP exposures also include the delivery of MP-associated toxic chemicals to the underlying epithelium [228]. MPs may also release carcinogenic bacterial toxins to the colonic epithelium. For example, the colonic presence of Escherichia coli increases the risk of CRC [229] through the expression of a genotoxin [230]. MNPLs may act as a vehicle for delivering these genotoxic bacteria to the colonic epithelium; this phenomenon seems dependent on losing an intact inner mucus layer [231]. A dysbiosis condition characterized by a dietary-related shift in the abundance of bacterial species degrading colonic mucus may increase the relative abundance of genotoxic bacterial species at the surface of the colonic epithelium. The association of these bacteria with MNPLs may promote bacterial toxin delivery to the colonic epithelium, thus supporting the hypothesis that the long-term carriage of genotoxic bacteria could guide carcinogenesis in an otherwise healthy colon [232].
Non-genotoxic carcinogenesis is a process associated with the occurrence of errors during cell division. MNPLs may contribute to this process by promoting inflammation and/or delivering estrogenic compounds activating cell proliferation signaling and non-genotoxic carcinogenesis due to their interaction with estrogen receptors expressed by some intestinal epithelial cells. This phenomenon gives meaning to the idea that dietary estrogenic compounds and estrogen mimics may act as CRC promoters [233] and that some types of plastics, such as 4-nonyl phenol, are estrogen mimics [234].
It is well recognized that intestinal macrophages are essential players in preserving mucosal homeostasis and epithelial barrier integrity. Once activated, macrophages shift towards an M1 phenotype with a pro-inflammatory phenotype, thus contributing to chronic intestinal inflammation, which is, in turn, associated with an increased risk of carcinogenesis [235].
The exposure of MNPLs to a damaged mucus layer facilitates MNPL translocation through the colonocytes to the lamina propria. Here, MNPLs may be phagocytosed by resident intestinal macrophages [166] and act as a vector to deliver particle-associated toxins and surface-associated bacteria. MNPLs do not necessarily require surface-associated toxins and bacteria to elicit inflammation, as demonstrated by the finding that macrophages ingesting pristine MPs show altered cell surface markers and cytokine gene expression [212]. It has been demonstrated that when macrophages phagocytose MNPLs, a glycolysis process occurs in these cells to provide energy [212]. This supports the idea that the phenotypic plasticity of macrophages may be affected by chronic exposure to ingested MNPLs and suggests that their role in driving inflammation-associated colorectal neoplasia has been underestimated.
The generation of ROS species induced by MNPL exposure and the consequent oxidative stress [236] is one of the mechanisms potentially linking MNPL-induced macrophage activation and the resultant inflammation with increased risk of CRC (Figure 4) [237]. ROS generation may be promoted by the increased expression of pro-inflammatory cytokines and by their aberrant glycosylation, which contributes to mucus layer damage [237,238]. Since ROS highly reacts with other molecules, including DNA and proteins, ROS-induced oxidative damage to DNA may occur, potentially promoting oncogenic signaling and thus increasing carcinogenesis risk [239] (Figure 4).
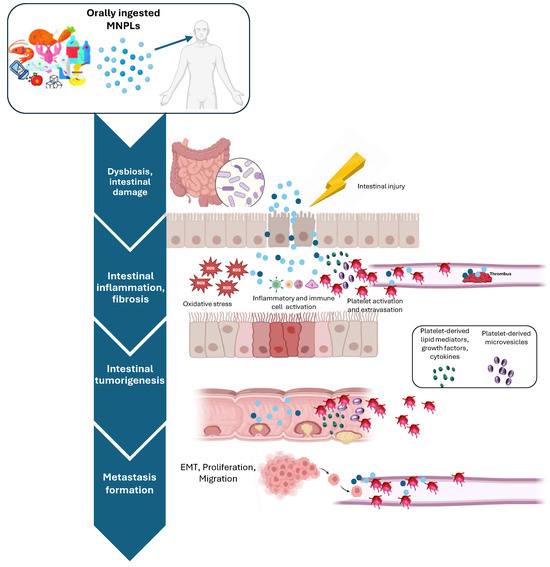
Figure 4.
MNPLs can easily accumulate in the gastrointestinal tract. As a consequence, they can exacerbate damage to the intestinal barrier in high-risk patients (for example, with inflammatory bowel diseases), in the presence of an epithelial injury (as it occurs due to lifestyle and aging), and alter intestinal flora. These effects promote plastic particle passage through the intestinal mucus and epithelial cell layers and trigger oxidative stress, inflammatory response, impaired immune function, inhibition of cell proliferation, and tissue degeneration. Colonic mucus layer disruption may further facilitate MNPL passage in the bloodstream, thus promoting the toxic effects of MNPLs on cardiac functions, microvascular sites, and distant organs. Recently, the possible link between MNPL exposure and increased colorectal cancer (CRC) has been explored, and several potential mechanisms have been proposed. It has been suggested that platelets, beyond hemostasis and thrombosis, once activated in response to tissue damage, can mediate other processes, including immune response, inflammation, fibrosis, cancer, and metastasis formation. This is mainly due to the capacity of activated platelets to extravasate, interact, and activate other cell types such as vascular and immune cells, fibroblasts, and cancer cells through direct contact and/or the release of several soluble factors and extracellular vesicles (EVs). Created with biorender.com.
A recently published study revealed the prolonged presence of MNPLs during cell division and provide preliminary evidence suggesting that plastic particles may promote tumor metastasis [240]. The study analyzed the interaction between PS-MNPLs (0.25 and 1 μm in size) and four human colorectal cancer cell lines, showing that the effects of the interaction depend on concentration, time, particle size, and cell type in both 2D and 3D cell cultures. In particular, they found that MNPLs exhibited high persistence in monolayer and spheroid cultures, accumulating in the non-proliferating parts of multicellular spheroids. Despite their persistence, they did not interrupt cell proliferation or division. However, particles smaller than 1 μm were able to enhance cell migration, potentially promoting metastasis. This observation aligns with recent research exploring the effect of MNPLs on breast cancer metastasis [241]. In this study, different human breast cancer cell lines (MCF-7 and MDA-MB-231) were exposed to irregularly shaped particles, reflecting the shapes most commonly present in the environment. The authors reported that plastics, when used at concentrations that were not cytotoxic (1.6 mg/mL), did not significantly affect breast cancer cells’ morphology and migration ability. However, RNA sequencing and gene expression analysis revealed that the level of cell cycle-related transcripts and genes were changed considerably in MDA-MB-231 cells exposed to MPs, thus suggesting that particles promoted metastatic capacity (Figure 4).
Altogether, these recent studies point out the persistency and bioaccumulation of MNPLs in cancer cell lines [240] and call for more studies on chronic plastic exposure and cancer progression reflecting the vast diversity of MNPLs to which humans can be exposed.
5. Platelet Activation in Intestinal Inflammation and Cancer: The Unexplored Contribution of MNPL Ingestion
5.1. Role of Platelets in Intestinal Inflammation and Cancer
Over the last decade, several pieces of evidence from pre-clinical and clinical studies corroborated the hypothesis that activated platelets participate in intestinal tumorigenesis [43,242,243,244]. Firstly, the analyses of randomized clinical trials (RCTs) revealed that the chronic use of the antiplatelet agent low-dose aspirin for cardiovascular prevention was associated with a significant reduction in the incidence and mortality of CRC but also of other types of cancer [245], thus supporting the idea that the antiplatelet effect of the drug was responsible for aspirin chemopreventive effects. In particular, it was proposed that low-dose aspirin may prevent tumorigenesis mechanisms by inhibiting platelet function [245]. From a mechanical point of view, platelets may participate in intestinal tumorigenesis by promoting intestinal inflammation and its chronic development. This may occur through interacting and activating other cells (such as immune and vascular cells, fibroblasts, and tumor cells) via direct contact and/or releasing different soluble factors and vesicles (Figure 4) [246]. In a setting of alteration of tissue microenvironment, for example, when vascular or intestinal damage occurs, platelets can be activated and in turn release several lipid mediators (such as thromboxane (TX)A2, prostaglandin (PG)E2 and 12S-Hydroxyeicosatetraenoic acid (HETE)), proteins (growth factors, proteases, and cytokines), genetic material, and extracellular vesicles (EVs), all rich in miRNAs. The release of this plethora of mediators triggers the activation of stromal cells (including fibroblasts, inflammatory cells, and endothelial cells), which, in turn, contributes to chronic inflammation and intestinal tumorigenesis [246]. Cyclooxygenase (COX)-2 expression induction, leading to the aberrant biosynthesis of pro-inflammatory and protumorigenic PGE2, is a key event induced by platelet activation. Using a conditional knock-out murine model with the specific deletion of COX-1 in megakaryocytes/platelets highlighted the role of platelet-derived TXA2 in developing intestinal inflammation and fibrosis [247]. Moreover, in ApcMin/+ mice, a model of intestinal tumorigenesis, the specific deletion of COX-1 in megakaryocytes/platelets was associated with a profound reduction in the biosynthesis of platelet TXA2 in vivo, the decrease in the number and size of intestinal polyps, and the restraining of COX-2 expression in intestinal adenomas [248]. In inflamed intestinal tissue, platelets, being able to extravasate, can interact and activate stromal cells. In fact, in platelets co-cultured in vitro with human myofibroblasts, platelet-derived TXA2 promotes the induction of COX-2-dependent PGE2 in myofibroblasts [248].
In addition to their contribution to inflammation and the early stage of tumorigenesis, the role of platelets in cancer cell metastatization is well recognized. Platelets, by interacting with cancer cells in the bloodstream and by the release of lipid mediators (i.e., TXA2 and 12-HETE) and growth factors, including platelet-derived growth factor (PDGF) and transforming growth factor (TGF)β, may induce in cancer cells epithelial–mesenchymal transition (EMT) and the COX-2-signaling pathway, typically associated with a metastatic phenotype [249,250,251].
Platelets can also promote cancer development by releasing EVs, which circulate in plasma [252]. It was recently demonstrated that platelet-derived EVs were characterized by different size distribution and proteomic cargo in CRC patients compared with those from healthy individuals. Interestingly, platelet-derived EVs from CRC patients were able to promote prometastatic and prothrombotic phenotypes in cancer cells [253].
Altogether, the clinical and experimental evidence presented here suggests that low-dose aspirin, by irreversibly affecting TXA2 generation and inhibiting platelet activation, may lead to the drug recommendation for primary prevention of cardiovascular disease and cancer. This finding, while promising, is not without its limitations, particularly the risk of bleeding associated with the use of low-dose aspirin [254]. However, the results of ongoing and future clinical investigations, which may lead to the discovery of biomarkers for the identification of individuals with high-risk bleeding or who will benefit from low-dose aspirin treatment, could revolutionize the use of this well-known antiplatelet drug. This precision medicine strategy could significantly impact cardiovascular disease and cancer prevention, potentially changing how to approach these conditions in the future [243]. Moreover, the benefit of other antiplatelet agents, such as antagonists of the P2Y12 receptor for ADP and TXA2 receptor (TP) antagonists, should be verified by performing appropriate RCTs in these settings, further expanding our understanding and potential treatment options.
5.2. Effects of MNLPs on Platelet Function
The knowledge about the effects of MNPLs on platelet function is mainly derived from investigations in the cardiovascular system [109]. Due to its primary function, i.e., to drive blood circulation across the body, the cardiovascular system is highly vulnerable to MNPL toxic effects. Dysfunctional heart rates in different systems have been reported. The internalization of MNPLs by cardiac sarcomeres induced oxidative stress and subsequent apoptosis, thus probably leading to arrhythmic heart functionality [255,256,257]. Moreover, MNPLs can drive vascular toxicity through thrombosis, a considerable risk factor for cardiovascular disease.
The capacity of MNLPs to stimulate platelet aggregation is contingent upon multiple factors, including size, surface charge/modifications, and their concentration [258].
In vivo, animal studies showed that the exposure to unmodified particles did not exert effects on thrombosis, while in contrast, carboxylate–PS particles inhibited thrombus formation at 500 and 100 μg/kg, and amine–PS caused thrombosis at both 50 and 500 μg/kg [259]. Additionally, exposure to MNPLs can alter the equilibrium between pro- and anticoagulant pathways. A study conducted by Oslakovic et al. in 2012 [260] with NPs different in size and modifications explored their effects on thrombin generation and coagulation in human plasma. The aminated NPs (57 and 330 nm diameter) decreased thrombin formation in plasma, with the 57 nm group exhibiting a more substantial effect due to their binding of factors VII and IX. In contrast, carboxylated NPs acted as a surface for activation of the intrinsic pathway of blood coagulation. The ability of amine-modified NPs to bind FVII and FIX could increase the risk of bleeding, whereas the ability of carboxyl-modified NPs to activate the intrinsic pathway of coagulation could instead lead to coagulation activation and a thrombotic state [260].
In addition to the role of MNPLs on thrombin levels and blood coagulation in plasma, it was shown that MNPLs, when intravenously and intratracheally administered, lead to platelet activation and thrombus formation in animal models [259]. Some evidence proposed that P-selectin-increased expression is responsible for MNPL-dependent platelet activation [261]. Moreover, it was reported that, unlike unmodified particles, both the carboxylated and aminated NPs caused platelet aggregation by following different mechanisms [262]. Carboxylated NPs caused aggregation by classical upregulation of adhesion receptors such as glycoprotein Ⅱb/Ⅲa fibrinogen receptor (PAC-1) and P-selectin (CD62P), while aminated NPs appeared to act by perturbation of the platelet membrane via an unexplained mechanism, resulting in the display of anionic phospholipids on the external platelet plasma membrane [262]. Dobrovolskaia et al. [263] also reported that aminated NPs mediated platelet activation by inducing the perturbation of the platelet membrane through interactions with anionic phospholipids [263]. Moreover, the authors suggested that the increased platelet aggregation caused by aminated NPs could be associated with the disruption of membrane integrity and the subsequent release of mediators [263].
Most of the studies assessing the effects of MNPLs on thrombosis are performed using acute exposures at a high concentration rather than a more representative chronic exposure model. Thus, there is a need to establish more accurate animal models for a better estimation of human MNPL exposure. The chronic exposure experiments available are mainly performed in invertebrate aquatic models; however, they are less applicable to human health. Moreover, the limited knowledge in this setting is also due to the challenges associated with the analysis of MNPLs in biological samples. Recently, Wang et al. [264], by using multimodal methods, showed that MNPLs with different particle numbers, sizes, shapes, and densities accumulated in thrombi surgically collected from patients with ischemic stroke and highlighted the potential association between the mass concentration of MNPLs in thrombi and disease severity.
6. Conclusions and Perspectives
MNPLs are ubiquitous in the environment, and human exposure occurs from multiple sources. They may cause adverse effects in different human organ systems. Evidence shows that ingested MNPLs can easily accumulate in the gastrointestinal tract [265,266], thus causing damage to the intestinal barrier and altering intestinal flora. This promotes plastic particle passage through the intestinal mucus and epithelial cell layers and triggers oxidative stress, inflammatory response, impaired immune function, inhibition of cell proliferation, and tissue degeneration (Figure 4). Although numerous experimental studies in animal and cell culture models have evidenced the adverse biological effects of MNPLs on human health, the underlying mechanisms are still unclear.
IBDs are a group of autoimmune diseases characterized by chronic intestinal inflammation resulting from host–microbial interactions in genetically susceptible individuals. IBD patients are considered a high-risk population susceptible to the effects of MNPLs. These effects would be distinct from those on healthy people [24]. Recently, the possible link between MNPL exposure and increased CRC incidence in individuals under 50 was explored [267]. It is plausible that this changing epidemiology of CRC matches the time necessary to see the effects of the rapid increase in MNPLs in the environment [267].
Several possible mechanisms have been proposed to explain the capability of MNPLs to disrupt the colonic mucus layer, thus leading to the loss of its protective effect and a higher risk of CRC development. Recently, other functions of platelets beyond hemostasis and thrombosis have been proposed. These include immune response, inflammation, fibrosis, cancer, and metastasis formation [242]. These effects are due to the capacity of activated platelets to extravasate, interact, and activate different cell types, including vascular and immune cells, fibroblasts, and cancer cells, through direct contact and/or the release of several soluble factors and EVs [243]. It is well known that platelets can be activated in response to intestinal epithelial damage, which is possibly dependent on several risk factors, such as lifestyle and aging.
Altogether, the evidence summarized in this review supports the idea that oral MNPL exposure may potentially contribute to intestinal epithelial damage, thus promoting the chronic development of intestinal inflammation and fibrosis, mainly in high-risk populations such as IBD patients. This phenomenon may contribute to explaining the higher incidence of CRC in individuals under 50, potentially linked to the increase in human exposure to MNPLs. Finally, colonic mucus layer disruption may further facilitate MNPL passage in the bloodstream, thus promoting the toxic effects of MNPLs on cardiac functions, microvascular sites, and distant organs.
It is essential to recognize that challenges and knowledge gaps about the harmful effects of MNPL exposure on human health are still numerous. Many of the mechanisms described in MNPL toxicity have yet to be demonstrated in humans. For instance, the interaction of the mucus layer, MNPLs, and the gut microbiota requires further clarification. Moreover, most animal exposure studies are performed over short study periods, highlighting the need for longer-term studies to be designed. These challenges underscore the complexity and importance of the topic and the need for further research and understanding in this area.
It is crucial to recognize that human MNPL intake is highly variable in terms of composition and particle numbers. Geographical position, dietary intake, and lifestyle are significant factors that can influence MNPL exposure. This variability underscores the urgent need for experimental studies to clarify the distribution and accumulation of MNPLs in the human body. Limited reports have uncovered the accumulation of MNPLs in the environment or organisms, primarily due to inadequate detection technology. Therefore, it is of utmost importance that more accurate analytical methods are developed in the future to characterize MNPLs, especially for their localization in vivo. This is a pressing need in our understanding of the adverse effects of MNPLs on human health, and it requires interdisciplinary collaboration among researchers, policymakers, and stakeholders.
Moreover, proactive measures aimed at curbing plastic pollution are indispensable. These measures should be addressed to improve the technologies currently used or in development to prevent the leakage of plastic pollution or to collect existing plastic pollution. In combination with efforts to reduce the source of plastic waste and subsequent microplastic generation, policymakers should provide incentives for expanding and implementing these technologies. However, technological development cannot be separated from policy, which likewise cannot be separated from individual and industry efforts.
Author Contributions
Conceptualization, A.B. and P.B.; writing—original draft preparation, A.B., M.D., C.M., E.A., M.P., and M.G.; writing—review and editing, A.B., M.D., P.C., P.D.C. and P.B.; funding acquisition, P.D.C. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—Next Generation EU—under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 -M4C2, Investment 1.5—Call for Tender No. 3277 of 30 December 2021, Italian Ministry of University, Award No. ECS00000041, Project Title: “Innovation, digitalization, and sustainability for the diffused economy in Central Italy”, Concession Degree No. 1057 of 23 June 2022, adopted by the Italian Ministry of University. CUP: D73C22000840006; Ministero dell’Università e della Ricerca (MUR) (University Scientific Research Funds).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501. [Google Scholar] [CrossRef]
- Kihara, S.; Köper, I.; Mata, J.P.; McGillivray, D.J. Reviewing nanoplastic toxicology: It’s an interface problem. Adv. Colloid. Interface Sci. 2021, 288, 102337. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Science Assessment of Plastic Pollution. 2020. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/pded/plastic-pollution/Science-assessment-plastic-pollution.pdf (accessed on 17 June 2024).
- Parks Canada. Microplastics: More Than a Drop in the Ocean. 2018. Available online: https://parks.canada.ca/nature/science/conservation/plastique-plastic/microplastique-microplastic (accessed on 17 June 2024).
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Woods, M.N.; Stack, M.E.; Fields, D.M.; Shaw, S.D.; Matrai, P.A. Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. Pollut. Bull. 2018, 137, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 19. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Chen, L.; Lv, S.; Liu, S.; Nie, P.; Aguilar, Z.P.; Xu, H. Restraining the TiO2 nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol. Environ. Saf. 2020, 206, 111393. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, C.Y.; Lu, T.H.; Liao, C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Guilloteau, E.; Djouina, M.; Caboche, S.; Waxin, C.; Deboudt, K.; Beury, D.; Hot, D.; Pichavant, M.; Dubuquoy, L.; Launay, D.; et al. Exposure to atmospheric Ag, TiO2, Ti and SiO2 engineered nanoparticles modulates gut inflammatory response and microbiota in mice. Ecotoxicol. Environ. Saf. 2022, 236, 113442. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Xu, H. Effects of microplastic and engineered nanomaterials on inflammatory bowel disease: A review. Chemosphere 2023, 326, 138486. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2021, 14, 1512–1522. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Liu, Y.; Tang, Y.; Xu, X.; Wang, M.; Tao, X.; Xu, H. Pre-Exposure to TiO2-NPs Aggravates Alcohol-Related Liver Injury by Inducing Intestinal Barrier Damage in Mice. Toxicol. Sci. 2021, 185, 28–37. [Google Scholar] [CrossRef]
- Remmelts, M. Effect of Nano-And Microplastics on the Human Immune System and Their Influence on Inflammatory Bowel Disease. Bachelor’s Thesis, University of Groningen, Groningen, The Netherlands, 2021. [Google Scholar]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, P.; Ballerini, P.; Buccella, S.; Ciccarelli, R.; Rathbone, M.P.; Romano, S.; D’Alimonte, I.; Caciagli, F.; Di Iorio, P.; Pokorski, M. Guanosine protects glial cells against 6-hydroxydopamine toxicity. Neurotransm. Interact. Cogn. Funct. 2015, 837, 23–33. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Meyer, B.; Are, C. Current Status and Future Directions in Colorectal Cancer. Indian. J. Surg. Oncol. 2018, 9, 440–441. [Google Scholar] [CrossRef]
- Tenailleau, Q.M.; Lanier, C.; Gower-Rousseau, C.; Cuny, D.; Deram, A.; Occelli, F. Crohn’s disease and environmental contamination: Current challenges and perspectives in exposure evaluation. Environ. Pollut. 2020, 263, 114599. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Wang, J.; Wu, B.; Guo, X. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 2022, 833, 155198. [Google Scholar] [CrossRef]
- Cetin, M.; Demirkaya Miloglu, F.; Kilic Baygutalp, N.; Ceylan, O.; Yildirim, S.; Eser, G.; İnci Gul, H. Higher number of microplastics in tumoral colon tissues from patients with colorectal adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Eng, C.; Jácome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022, 23, e116–e128. [Google Scholar] [CrossRef]
- REACCT Collaborative; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens,, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Genbo Xu, E.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef]
- Ballerini, P.; Contursi, A.; Bruno, A.; Mucci, M.; Tacconelli, S.; Patrignani, P. Inflammation and Cancer: From the Development of Personalized Indicators to Novel Therapeutic Strategies. Front. Pharmacol. 2022, 13, 838079. [Google Scholar] [CrossRef]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and nanoplastics–current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef]
- REACCT Collaborative. Microsatellite instability in young patients with rectal cancer: Molecular findings and treatment response. Br. J. Surg. 2022, 109, 251–255. [Google Scholar] [CrossRef]
- Bibi, A.; Can, A.; Pant, U.; Hardiman, G.; Hill, D.; Elliott, C.; Cao, C. A review on state-of-the-art detection techniques for micro- and nano-plastics with prospective use in point-of-site detection. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 101, pp. 143–196. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.; Barcelo, D. Nano- and Microplastic Analysis: Focus on Their Occurrence in Freshwater Ecosystems and Remediation Technologies. TrAC Trends Anal. Chem. 2019, 113, 409–425. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Dehaut, A.; Hermabessiere, L.; Duflos, G. Current frontiers and recommendations for the study of microplastics in seafood. TrAC Trends Anal. Chem. 2019, 116, 346–359. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Yang, T.; Luo, J.; Nowack, B. Characterization of Nanoplastics, Fibrils, and Microplastics Released during Washing and Abrasion of Polyester Textiles. Environ. Sci. Technol. 2021, 55, 15873–15881. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene nanoplastics alter the cytotoxicity of human pharmaceuticals on marine fish cell lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I. Secondary Microplastics Generation in the Sea Swash Zone with Coarse Bottom Sediments: Laboratory Experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B..; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Rødland, E.S.; Samanipour, S.; Rauert, C.; Okoffo, E.D.; Reid, M.J.; Heier, L.S.; Lind, O.C.; Thomas, K.V.; Meland, S. A novel method for the quantification of tire and polymer-modified bitumen particles in environmental samples by pyrolysis gas chromatography mass spectroscopy. J. Hazard. Mater. 2022, 423, 127092. [Google Scholar] [CrossRef] [PubMed]
- Karak, N. 15—Biopolymers for paints and surface coatings. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 333–368. [Google Scholar]
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Mapping Exercise—Plastic Additives Initiative—ECHA. Available online: https://european-union.europa.eu/index_en (accessed on 9 August 2024).
- Gao, D.; Junaid, M.; Chen, X.; Liao, H.; Chen, G.; Wang, J. Interaction of micro(nano)plastics and bisphenols in the environment: A recent perspective on adsorption mechanisms, influencing factors and ecotoxic impacts. TrAC Trends Anal. Chem. 2023, 165, 117132. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xiang, L.; Gu, C.; Redmile-Gordon, M.; Sheng, H.; Wang, Z.; Fu, Y.; Bian, Y.; Jiang, X. Risk Assessment of Agricultural Plastic Films Based on Release Kinetics of Phthalate Acid Esters. Environ. Sci. Technol. 2021, 16, 3676–3685. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Advancements in Raman imaging for nanoplastic analysis: Challenges, algorithms and future Perspectives. Anal. Chim. Acta 2024, 1290, 342069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Eriksen, M.; Liboiron, M.; Kiessling, T.; Charron, L.; Alling, A.; Lebreton, L.; Richards, H.; Roth, B.; Ory, N.C.; Hidalgo-Ruz, V.; et al. Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 2018, 232, 430–439. [Google Scholar] [CrossRef]
- Schönlau, C.; Karlsson, T.M.; Rotander, A.; Nilsson, H.; Engwall, M.; van Bavel, B.; Kärrman, A. Microplastics in sea-surface waters surrounding Sweden sampled by manta trawl and in-situ pump. Mar. Pollut. Bull. 2020, 153, 111019. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.; Taladriz-Blanco, P.; Lehner, R.; Lubskyy, A.; Ortuso, R.D.; Rothen-Rutishauser, B.; Petri-Fink, A. The micro-, submicron-, and nanoplastic hunt: A review of detection methods for plastic particles. Chemosphere 2022, 293, 133514. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Delshab, H.; Soltani, N.; Sorooshian, A. Investigation of microrubbers, microplastics and heavy metals in street dust: A study in Bushehr city, Iran. Environ. Earth Sci. 2017, 76, 798. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and nanoplastics analysis: Options, imaging, advancements and challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. Int. 2017, 24, 20360–20371. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An overview of analytical methods for detecting microplastics in the atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Peñalver, R.; Costa-Gómez, I.; Arroyo-Manzanares, N.; Moreno, J.M.; López-García, I.; Moreno-Grau, S.; Córdoba, M.H. Assessing the level of airborne polystyrene microplastics using thermogravimetry-mass spectrometry: Results for an agricultural area. Sci. Total Environ. 2021, 787, 147656. [Google Scholar] [CrossRef]
- Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. Int. J. Environ. Res. Public Health 2022, 19, 12252. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. Int. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Overview on the occurrence of microplastics in air and implications from the use of face masks during the COVID-19 pandemic. Sci. Total Environ. 2021, 800, 149555. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, M.; Lee, Y.; Park, J.; Lee, H.; Song, Y.C.; Ro, C.U. Novel Single-Particle Analytical Technique for Inhalable Airborne Microplastic Particles by the Combined Use of Fluorescence Microscopy, Raman Microspectrometry, and SEM/EDX. Anal. Chem. 2023, 95, 8552–8559. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Dümichen, E.; Barthel, A.K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef]
- Niu, S.; Liu, R.; Zhao, Q.; Gagan, S.; Dodero, A.; Ying, Q.; Ma, X.; Cheng, Z.; China, S.; Canagaratna, M. Quantifying the Chemical Composition and Real-Time Mass Loading of Nanoplastic Particles in the Atmosphere Using Aerosol Mass Spectrometry. Environ. Sci. Technol. 2024, 58, 3363–3374. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 15, 202–209. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process Impacts. 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Dini, L.; Panzarini, E.; Mariano, S.; Passeri, D.; Reggente, M.; Rossi, M.; Vergallo, C. Microscopies at the Nanoscale for Nano-Scale Drug Delivery Systems. Curr. Drug Targets 2015, 16, 1512–1530. [Google Scholar] [CrossRef] [PubMed]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tu, C.; Li, R.; Wu, D.; Yang, J.; Xia, Y.; Peijnenburg, W.J.G.M.; Luo, Y. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eco Environ. Health 2023, 2, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Dorsch, A.; Förschner, F.; Ravandeh, M.; da Silva Brito, W.A.; Saadati, F.; Delcea, M.; Wende, K.; Bekeschus, S. Nanoplastic Size and Surface Chemistry Dictate Decoration by Human Saliva Proteins. ACS Appl. Mater. Interfaces 2024, 16, 25977–25993. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Techol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, S.; Bittar, T.B.; Stubbins, A.; Li, D. Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts. J. Hazard. Mater. 2020, 383, 121065. [Google Scholar] [CrossRef]
- Hüffer, T.; Praetorius, A.; Wagner, S.; von der Kammer, F.; Hofmann, T. Microplastic Exposure Assessment in Aquatic Environments: Learning from Similarities and Differences to Engineered Nanoparticles. Environ. Sci. Technol. 2017, 51, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jia, Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, W.; Choi, D.; Heo, J.; Han, U.; Jung, S.Y.; Park, H.H.; Hong, S.-T.; Park, J.H.; Hong, J. Potential threats of nanoplastic accumulation in human induced pluripotent stem cells. Chem. Eng. J. 2022, 427, 131841. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Zhou, C.S.; Wu, J.W.; Liu, B.F.; Ma, W.L.; Yang, S.S.; Cao, G.L. Micro nanoplastics promote the risk of antibiotic resistance gene propagation in biological phosphorus removal system. J. Hazard. Mater. 2022, 431, 128547. [Google Scholar] [CrossRef]
- Poma, A.M.G.; Morciano, P.; Aloisi, M. Beyond genetics: Can micro and nanoplastics induce epigenetic and gene-expression modifications? Front. Epigenet. Epigenom. 2023, 1, 1241583. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Garcia, M.M.; Romero, A.S.; Merkley, S.D.; Meyer-Hagen, J.L.; Forbes, C.; Hayek, E.E.; Sciezka, D.P.; Templeton, R.; Gonzalez-Estrella, J.; Jin, Y.; et al. In Vivo Tissue Distribution of Microplastics and Systemic Metabolomic Alterations After Gastrointestinal Exposure. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Junaid, M.; Liu, S.; Yue, Q.; Wei, M.; Wang, J. Trophic transfer and interfacial impacts of micro(nano)plastics and per-and polyfluoroalkyl substances in the environment. J. Hazard. Mater. 2024, 465, 133243. [Google Scholar] [CrossRef]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarfl, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 287. [Google Scholar] [CrossRef]
- Huang, B.; Tan, Z.; Bohinc, K.; Zhang, S. Interaction between nanoparticles and charged phospholipid membranes. Phys. Chem. Chem. Phys. 2018, 20, 29249–29263. [Google Scholar] [CrossRef]
- Kihara, S.; Ashenden, A.; Kaur, M.; Glasson, J.; Ghosh, S.; van der Heijden, N.; Brooks, A.E.S.; Mata, J.P.; Holt, S.; Domigan, L.J.; et al. Cellular interactions with polystyrene nanoplastics-The role of particle size and protein corona. Biointerphases 2021, 16, 041001. [Google Scholar] [CrossRef]
- Milillo, C.; Aruffo, E.; Di Carlo, P.; Patruno, A.; Gatta, M.; Bruno, A.; Dovizio, M.; Marinelli, L.; Dimmito, M.P.; Di Giacomo, V.; et al. Polystyrene nanoplastics mediate oxidative stress, senescence, and apoptosis in a human alveolar epithelial cell line. Front. Public Health 2024, 12, 1385387. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef]
- Pulvirenti, E.; Ferrante, M.; Barbera, N.; Favara, C.; Aquilia, E.; Palella, M.; Cristaldi, A.; Conti, G.O.; Fiore, M. Effects of Nano and Microplastics on the Inflammatory Process: In Vitro and In Vivo Studies Systematic Review. Front. Biosci. 2022, 27, 287. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Xiong, F.; Xu, K.; Huang, J.; Liu, J.; Wang, D.; Pu, Y. Polystyrene micro- and nanoplastics induce gastric toxicity through ROS mediated oxidative stress and P62/Keap1/Nrf2 pathway. Sci. Total Environ. 2024, 912, 169228. [Google Scholar] [CrossRef]
- Li, L.; Lv, X.; He, J.; Zhang, L.; Li, B.; Zhang, X.; Liu, S.; Zhang, Y. Chronic exposure to polystyrene nanoplastics induces intestinal mechanical and immune barrier dysfunction in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115749. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Jung, B.K.; Han, S.W.; Park, S.H.; Bae, J.S.; Choi, J.; Ryu, K.Y. Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. Neurotoxicology 2020, 81, 189–196. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- German Federal Institute for Risk Assessment (BfR), Department of Food Safety, Unit Effect-based Analytics and Toxicogenomics Unit and Nanotoxicology Junior Research Group, Berlin, Germany; Shopova, S.; Sieg, H.; Braeuning, A. Risk assessment and toxicological research on micro- and nanoplastics after oral exposure via food products. EFSA J. 2020, 18, e181102. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.E.; Smyth, S.H.; McCullough, M.T.; Morris, J.F.; Moyes, S.M. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012, 46, 185–252. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Smith, J.R.H.; Etherington, G.; Shutt, A.L.; Youngman, M.J. A study of aerosol deposition and clearance from the human nasal passage. Ann. Occup. Hyg. 2002, 46, 309–313. [Google Scholar] [CrossRef]
- Asgharian, B.; Hofmann, W.; Miller, F.J. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J. Aerosol Sci. 2001, 32, 817–832. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. Int. 2013, 20, 4339–4355. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Helsdingen, R.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology 2015, 9, 886–894. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Hendriksen, P.J.; Woutersen, R.A.; van der Zande, M.; Undas, A.K.; Helsdingen, R.; van den Berg, H.H.; Rietjens, I.M.; Bouwmeester, H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanopart. Res. 2015, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Sarlo, K.; Blackburn, K.L.; Clark, E.D.; Grothaus, J.; Chaney, J.; Neu, S.; Flood, J.; Abbott, D.; Bohne, C.; Casey, K.; et al. Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology 2009, 263, 117–126. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hirota, K.; Tomoda, K.; Ito, F.; Inagawa, H.; Kochi, C.; Soma, G.; Makino, K.; Terada, H. Phagocytic activity of alveolar macrophages toward polystyrene latex microspheres and PLGA microspheres loaded with anti-tuberculosis agent. Colloids Surf. B Biointerfaces. 2007, 60, 221–228. [Google Scholar] [CrossRef]
- Tomazic-Jezic, V.J.; Merritt, K.; Umbreit, T.H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001, 55, 523–529. [Google Scholar] [CrossRef]
- Volkheimer, G. Passage of particles through the wall of the gastrointestinal tract. Environ. Health Perspect. 1974, 9, 215–225. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumores in Nude Mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D. Enterocyte-like differentiation and polarization of the human colon Carcinoma Cell Line Caco-2 in culture. Biol. Cell. 1983, 47, 323–330. [Google Scholar]
- Hilgers, A.R.; Conradi, R.A.; Burton, P.S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceut. Res. 1990, 7, 902–910. [Google Scholar] [CrossRef]
- Buhrke, T.; Lengler, I.; Lampen, A. Analysis of proteomic changes induced upon cellular differentiation of the human intestinal cell line Caco-2. Dev. Growth Differ. 2011, 53, 411–426. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Guo, J.; Gu, A.Z.; Li, D.; Chen, J. Toxicity Assessment of Nano-ZnO Exposure on the Human Intestinal Microbiome, Metabolic Functions, and Resistome Using an In Vitro Colon Simulator. Environ. Sci. Techol. 2021, 55, 6884–6896. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Hu, X.; Liu, C.; Shi, J.; Wang, H.; Zhan, L.; Song, H. Inhibition of Epithelial-Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro(nano)plastics and Their Potential Impact on Human Gut Health: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, X.; Wang, Y.; Su, J.; Li, G.; Wang, X.; Yang, Y.; Zhang, Y.; Li, B.; Zhang, G.; et al. Biological interactions of polystyrene nanoplastics: Their cytotoxic and immunotoxic effects on the hepatic and enteric systems. Ecotoxicol. Environ. Saf. 2023, 264, 115447. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous oral exposure to micro- and nanoplastics induced gut microbiota dysbiosis, intestinal barrier and immune dysfunction in adult mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Makarova, O.; Fokichev, N. Harmful Effects of the Microplastic Pollution on Animal Health: A Literature Review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef]
- Almeida, A.S.; de Souza, C.B. The Impact of Microplastic on Human Health. Curr. Biotechnol. 2021, 10, 158–167. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo Hasselerharm, P.E.; Mohamed Nor, N.H.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. Microplastic in bottled natural mineral water–literature review and considerations on exposure and risk assessment. Food Addit. Contam. Part A 2018, 35, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Techol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Jin, Y.J.; Seo, S.; Lee, J.H.; Hwang, D.Y. Inflammatory response in the mid colon of ICR mice treated with polystyrene microplastics for two weeks. Lab. Anim. Res. 2021, 37, 31. [Google Scholar] [CrossRef] [PubMed]
- Rawle, D.J.; Dumenil, T.; Tang, B.; Bishop, C.R.; Yan, K.; Le, T.T.; Suhrbier, A. Microplastic consumption induces inflammatory signatures in the colon and prolongs a viral arthritis. Sci. Total Environ. 2022, 809, 152212. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, S.; Chen, Y.; Yuan, H.; Xu, H. Polystyrene microplastics exacerbated liver injury from cyclophosphamide in mice: Insight into gut microbiota. Sci. Total Environ. 2022, 840, 156668. [Google Scholar] [CrossRef]
- Xie, L.; Chen, T.; Liu, J.; Hou, Y.; Tan, Q.; Zhang, X.; Li, Z.; Farooq, T.H.; Yan, W.; Li, Y. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice. Ecotoxicol. Environ. Saf. 2022, 246, 114194. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lin, W.Y.; Cheng, T.J. Microbiota-mediated metabolic perturbations in the gut and brain of mice after microplastic exposure. Chemosphere 2024, 350, 141026. [Google Scholar] [CrossRef]
- Xu, R.; Cao, J.W.; Lv, H.L.; Geng, Y.; Guo, M.Y. Polyethylene microplastics induced gut microbiota dysbiosis leading to liver injury via the TLR2/NF-κB/NLRP3 pathway in mice. Sci. Total Environ. 2024, 917, 170518. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Y.; Wei, Y.; Guo, Y.; Wang, Y.; Song, P.; Yu, J. Gut microbiota and liver metabolomics reveal the potential mechanism of Lactobacillus rhamnosus GG modulating the liver toxicity caused by polystyrene microplastics in mice. Environ. Sci. Pollut. Res. Int. 2024, 31, 6527–6542. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Li, P.; Zhu, X.; Chen, D.; Ommati, M.M.; Wang, H.; Han, L.; Xu, S.; Sun, P. Hepatotoxic of polystyrene microplastics in aged mice: Focus on the role of gastrointestinal transformation and AMPK/FoxO pathway. Sci. Total Environ. 2024, 917, 170471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene microplastics induce size-dependent multi-organ damage in mice: Insights into gut microbiota and fecal metabolites. J. Hazard. Mater. 2024, 461, 132503. [Google Scholar] [CrossRef]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene microplastic-induced oxidative stress triggers intestinal barrier dysfunction via the NF-κB/NLRP3/IL-1β/MCLK pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, D.; Zhan, J.; Liu, X.; Li, P.; Ma, X.; Hou, H.; Wang, P. Polystyrene microplastics induce kidney injury via gut barrier dysfunction and C5a/C5aR pathway activation. Environ. Pollut. 2024, 342, 122909. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Meng, Z.; Fu, B.; Yang, Z.; Xu, Y.; Huang, H.; Bai, Y.; Fang, X.; Shen, S.; Yang, J.; Yong, J.; et al. Polydopamine-coated thalidomide nanocrystals promote DSS-induced murine colitis recovery through Macrophage M2 polarization together with the synergistic anti-inflammatory and anti-angiogenic effects. Int. J. Pharm. 2023, 630, 122376. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Duan, R.; Wu, Y.; Wang, M.; Wu, J.; Wang, X.; Wang, Z.; Hu, Y.; Duan, L. Association between short-term exposure to fine particulate pollution and outpatient visits for ulcerative colitis in Beijing, China: A time-series study. Ecotoxicol. Environ. Saf. 2021, 214, 112116. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Curtis, V.F.; Campbell, E.L. The inflammatory tissue microenvironment in IBD. Inflamm. Bowel Dis. 2013, 19, 2238–2244. [Google Scholar] [CrossRef]
- Strugala, V.; Dettmar, P.W.; Pearson, J.P. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int. J. Clin. Pract. 2008, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic. Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Ogino, T.; Nishimura, J.; Barman, S.; Kayama, H.; Uematsu, S.; Okuzaki, D.; Osawa, H.; Haraguchi, N.; Uemura, M.; Hata, T.; et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 2013, 145, 1380–1391.e1. [Google Scholar] [CrossRef]
- Makita, S.; Kanai, T.; Oshima, S.; Uraushihara, K.; Totsuka, T.; Sawada, T.; Nakamura, T.; Koganei, K.; Fukushima, T.; Watanabe, M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J. Immunol. 2004, 173, 3119–3130. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Yao, J.; Wei, C.; Wang, J.Y.; Zhang, R.; Li, Y.X.; Wang, L.S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Techol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Ma, J.; Wan, Y.; Song, L.; Wang, L.; Wang, H.; Li, Y.; Huang, D. Polystyrene nanobeads exacerbate chronic colitis in mice involving in oxidative stress and hepatic lipid metabolism. Part. Fibre Toxicol. 2023, 20, 49. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef]
- Merkley, S.D.; Moss, H.C.; Goodfellow, S.M.; Ling, C.L.; Meyer-Hagen, J.L.; Weaver, J.; Campen, M.J.; Castillo, E.F. Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol. Toxicol. 2022, 38, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, R.; Li, Z.; Liu, C.; Chen, Y.; Yu, Q. Microplastics perturb colonic epithelial homeostasis associated with intestinal overproliferation, exacerbating the severity of colitis. Environ. Res. 2023, 217, 114861. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci. Total Environ. 2022, 844, 156884. [Google Scholar] [CrossRef]
- Chlittleborough, T.J.; Gutlic, I.; Pearson, J.F.; Watson, A.; Bhatti, L.A.; Buchwald, P.; Potter, J.D.; Wakeman, C. Increasing Incidence of Young-Onset Colorectal Carcinoma A 3-Country Population Analysis. Dis. Colon. Rectum 2020, 63, 903–910. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Speizer, F.E.; Willett, W.C. A prospective study of family history and the risk of colorectal cancer. N. Engl. J. Med. 1994, 331, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.V.; Dollin, J. Half a century of the oral contraceptive pill: Historical review and view to the future. Can. Fam. Physician 2012, 58, e757–e760. [Google Scholar] [PubMed]
- Murphy, C.C.; Singal, A.G. Establishing a research agenda for early-onset colorectal cancer. PLoS Med. 2018, 15, e1002577. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Coleman, O.I.; Haller, D. Microbe-Mucus Interface in the Pathogenesis of Colorectal Cancer. Cancers 2021, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Korpela, K. Diet, Microbiota, and Metabolic Health: Trade-Off Between Saccharolytic and Proteolytic Fermentation. Annu. Rev. Food Sci. Technol. 2018, 9, 65–84. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef]
- Domenech, J.; Annangi, B.; Marcos, R.; Hernandez, A.; Catalan, J. Insights into the potential carcinogenicity of micro- and nano-plastics. Mutat. Res. Rev. Mutat. Res. 2023, 791, 108453. [Google Scholar] [CrossRef]
- Shaw, I.; Jones, H. Shaw and Jones reply: The multifactorial nature of carcinogenesis. Trends Pharmacol. Sci. 1994, 15, 323. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Iyadorai, T.; Mariappan, V.; Vellasamy, K.M.; Wanyiri, J.W.; Roslani, A.C.; Lee, G.K.; Sears, C.; Vadivelu, J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 2020, 15, e0228217. [Google Scholar] [CrossRef] [PubMed]
- Nougayrede, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Reuter, C.; Alzheimer, M.; Walles, H.; Oelschlaeger, T.A. An adherent mucus layer attenuates the genotoxic effect of colibactin. Cell. Microbiol. 2018, 20, e12812. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shaw, I.C.; Ye, H.; Shaw, I.C. Dietary isoflavone-induced, estrogen receptor-β-mediated proliferation of Caco-2 cells is modulated by gallic acid. Food Chem. Toxicol. 2020, 145, 111743. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Shaw, I. Does the oestrogen receptor encourage oestrogenicity in environmental pollutants? The case of 4-nonylphenol. SAR QSAR Environ. Res. 2011, 22, 329–350. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell. Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.J. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Enss, M.L.; Cornberg, M.; Wagner, S.; Gebert, A.; Henrichs, M.; Eisenblatter, R.; Beil, W.; Kownatzki, R. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm. Res. 2000, 49, 162–169. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; Del Favero, G.; et al. Microplastics role in cell migration and distribution during cancer cell division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, S.; Kim, O.H.; Kim, C.H.; Kim, J.; Kim, J.W.; Hong, S.; Lee, H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Tacconelli, S.; Contursi, A.; Ballerini, P.; Patrignani, P. Cyclooxygenases and platelet functions. Adv. Pharmacol. 2023, 97, 133–165. [Google Scholar] [CrossRef] [PubMed]
- Dovizio, M.; Ballerini, P.; Fullone, R.; Tacconelli, S.; Contursi, A.; Patrignani, P. Multifaceted Functions of Platelets in Cancer: From Tumorigenesis to Liquid Biopsy Tool and Drug Delivery System. Int. J. Mol. Sci. 2020, 21, 9585. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dovizio, M.; Tacconelli, S.; Contursi, A.; Ballerini, P.; Patrignani, P. Antithrombotic Agents and Cancer. Cancers 2018, 10, 253. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Contursi, A.; Sacco, A.; Grande, R.; Dovizio, M.; Patrignani, P. Platelets as crucial partners for tumor metastasis: From mechanistic aspects to pharmacological targeting. Cell Mol. Life Sci. 2017, 74, 3491–3507. [Google Scholar] [CrossRef]
- Sacco, A.; Bruno, A.; Contursi, A.; Dovizio, M.; Tacconelli, S.; Ricciotti, E.; Guillem-Llobat, P.; Salvatore, T.; Di Francesco, L.; Fullone, R.; et al. Platelet-Specific Deletion of Cyclooxygenase-1 Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. J. Pharmacol. Exp. Ther. 2019, 370, 416–426. [Google Scholar] [CrossRef]
- Bruno, A.; Contursi, A.; Tacconelli, S.; Sacco, A.; Hofling, U.; Mucci, M.; Lamolinara, A.; Del Pizzo, F.; Ballerini, P.; Di Gregorio, P.; et al. The specific deletion of cyclooxygenase-1 in megakaryocytes/platelets reduces intestinal polyposis in ApcMin/+ mice. Pharmacol. Res. 2022, 185, 106506. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Dovizio, M.; Maier, T.J.; Alberti, S.; Di Francesco, L.; Marcantoni, E.; Münch, G.; John, C.M.; Suess, B.; Sgambato, A.; Steinhilber, D.; et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol. Pharmacol. 2013, 84, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Llobat, P.; Dovizio, M.; Bruno, A.; Ricciotti, E.; Cufino, V.; Sacco, A.; Grande, R.; Alberti, S.; Arena, V.; Cirillo, M.; et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016, 7, 32462–32477. [Google Scholar] [CrossRef]
- Dovizio, M.; Bruno, A.; Contursi, A.; Grande, R.; Patrignani, P. Platelets and extracellular vesicles in cancer: Diagnostic and therapeutic implications. Cancer Metastasis Rev. 2018, 37, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Contursi, A.; Fullone, R.; Szklanna-Koszalinska, P.; Marcone, S.; Lanuti, P.; Taus, F.; Meneguzzi, A.; Turri, G.; Dovizio, M.; Bruno, A.; et al. Tumor-Educated Platelet Extracellular Vesicles: Proteomic Profiling and Crosstalk with Colorectal Cancer Cells. Cancers 2023, 15, 350. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; et al. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 1577–1584. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schürch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef]
- Li, H.; Kilgallen, A.B.; Münzel, T.; Wolf, E.; Lecour, S.; Schulz, R.; Daiber, A.; Van Laake, L.W. Influence of mental stress and environmental toxins on circadian clocks: Implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2020, 177, 5393–5412. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Dinsdale, D.; Smith, T.; Xu, H.; Vermylen, J.; Nemery, B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 2002, 166, 998–1004. [Google Scholar] [CrossRef]
- Oslakovic, C.; Cedervall, T.; Linse, S.; Dahlbäck, B. Polystyrene nanoparticles affecting blood coagulation. Nanomedicine 2012, 8, 981–986. [Google Scholar] [CrossRef]
- Bihari, P.; Holzer, M.; Praetner, M.; Fent, J.; Lerchenberger, M.; Reichel, C.A.; Rehberg, M.; Lakatos, S.; Krombach, F. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology 2010, 269, 148–154. [Google Scholar] [CrossRef] [PubMed]
- McGuinnes, C.; Duffin, R.; Brown, S.L.; Mills, N.; Megson, I.L.; Macnee, W.; Johnston, S.; Lu, S.L.; Tran, L.; Li, R.; et al. Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol. Sci. 2011, 119, 359–368. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).