The Impact of Melanoma Imaging Biomarker Cues on Detection Sensitivity and Specificity in Melanoma versus Clinically Atypical Nevi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Background and Recruitment
2.2. Participant Training
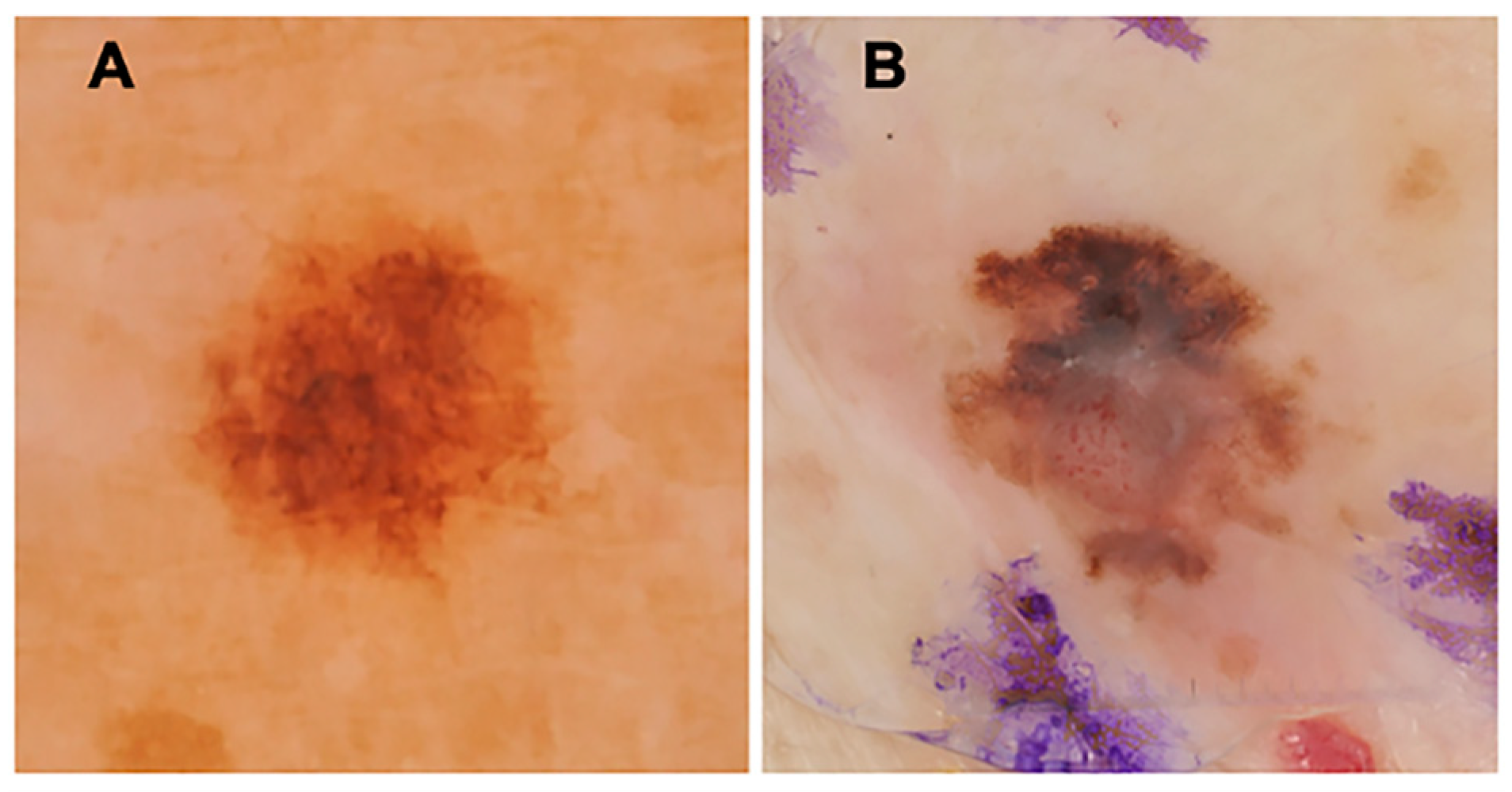
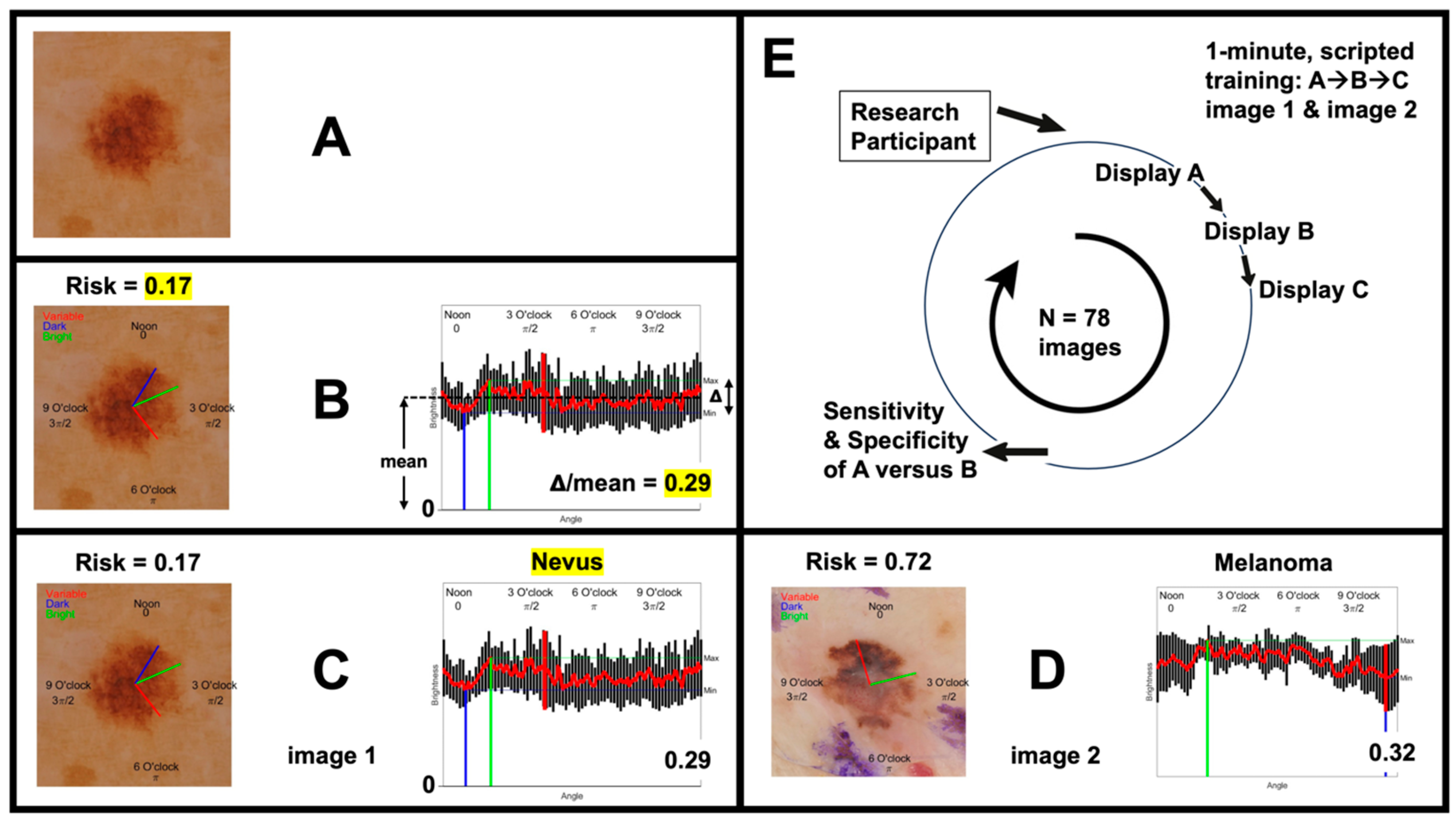
2.3. Dermoscopy Image Acquisition and Presentation
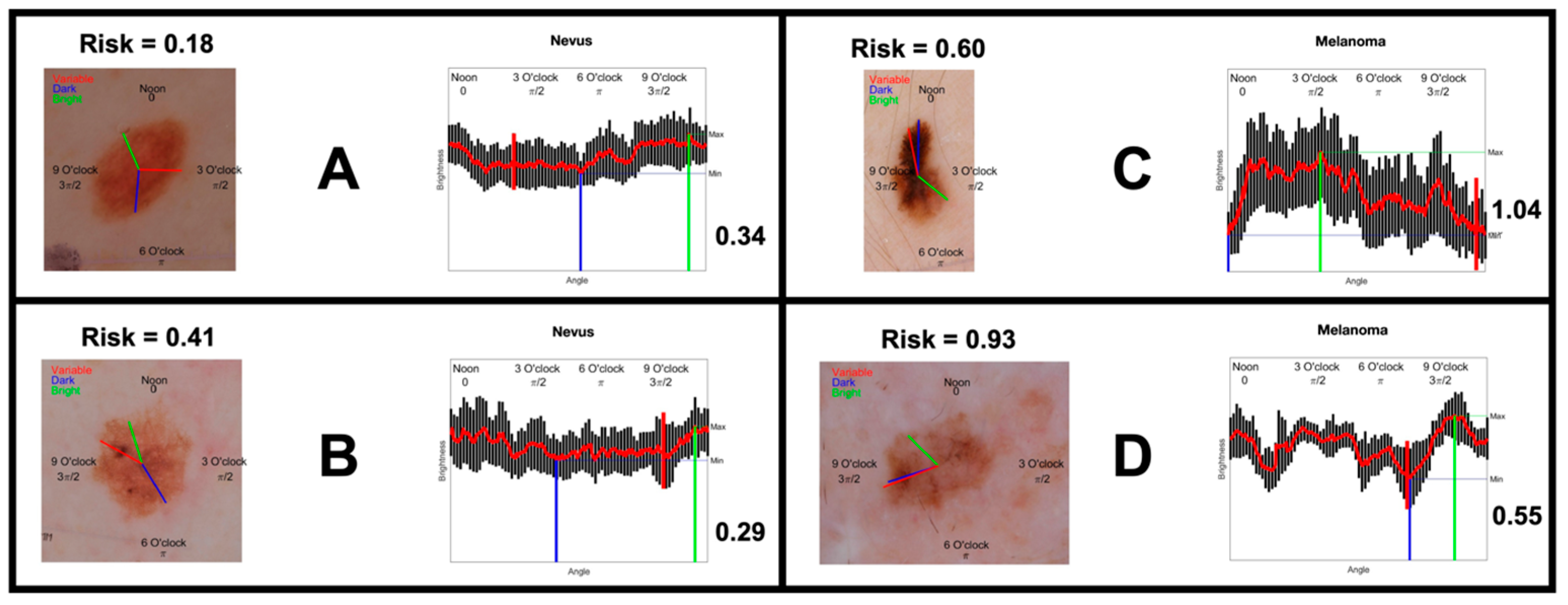
2.4. Outcome Measures
3. Results
3.1. Dermoscopy Image Evaluation Sequence
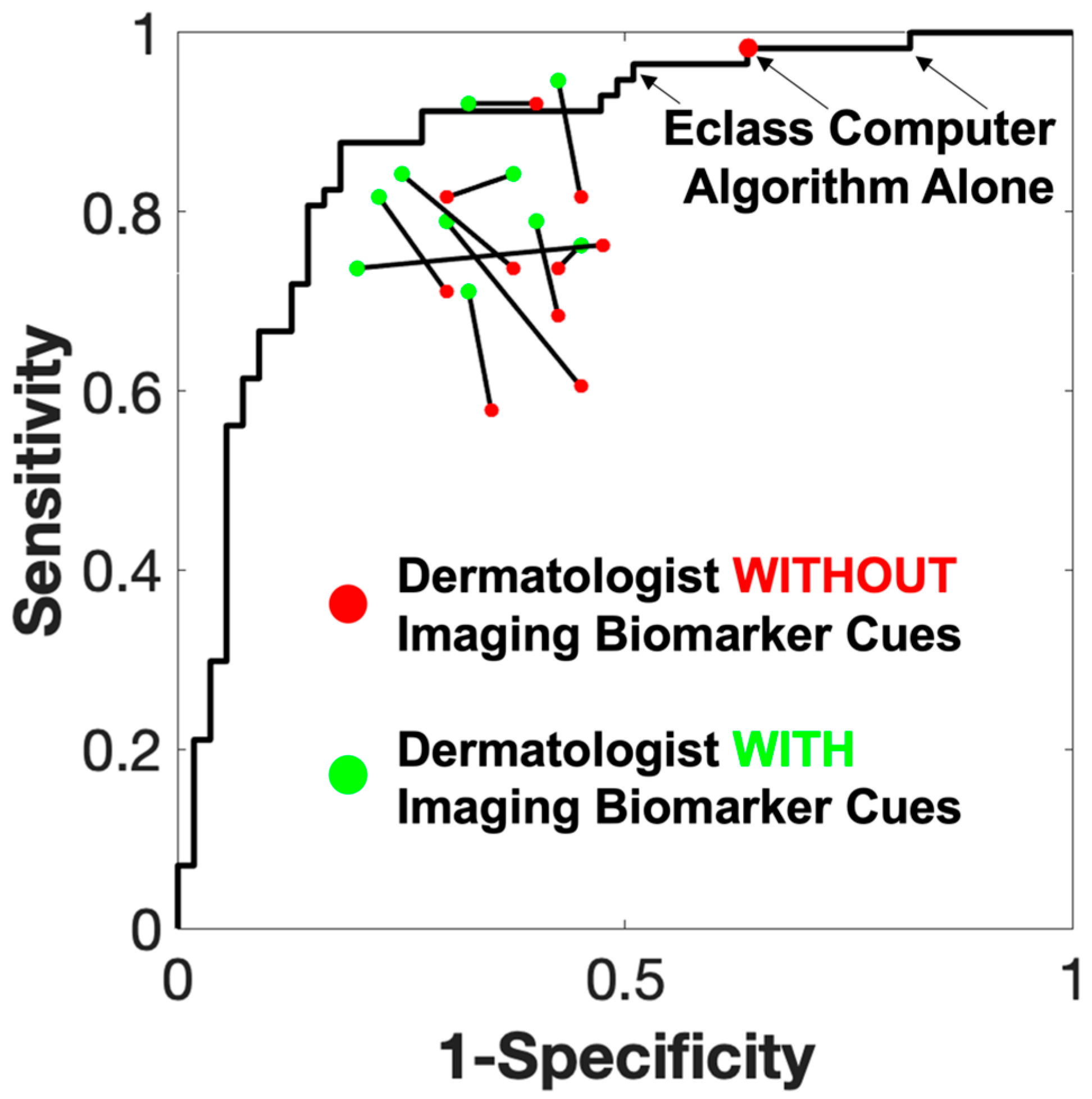
3.2. Participant Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, R.J.; Rigel, D.S.; Kopf, A.W. Early detection of malignant melanoma: The role of physician examination and self-examination of the skin. CA Cancer J. Clin. 1985, 35, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Dermatology Ad Hoc Task Force for the ABCDEs of Melanoma; Tsao, H.; Olazagasti, J.M.; Cordoro, K.M.; Brewer, J.D.; Taylor, S.C.; Bordeaux, J.S.; Chren, M.M.; Sober, A.J.; Tegeler, C.; et al. Early detection of melanoma: Reviewing the ABCDEs. J. Am. Acad. Dermatol. 2015, 72, 717–723. [Google Scholar] [CrossRef]
- Goldsmith, S.M. A unifying approach to the clinical diagnosis of melanoma including “D” for “Dark” in the ABCDE criteria. Dermatol. Pract. Concept. 2014, 4, 75–78. [Google Scholar] [CrossRef]
- Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024.
- Guy, G.P., Jr.; Ekwueme, D.U.; Tangka, F.K.; Richardson, L.C. Melanoma treatment costs: A systematic review of the literature, 1990–2011. Am. J. Prev. Med. 2012, 43, 537–545. [Google Scholar] [CrossRef]
- Guerry, D.t.; Synnestvedt, M.; Elder, D.E.; Schultz, D. Lessons from tumor progression: The invasive radial growth phase of melanoma is common, incapable of metastasis, and indolent. J. Investig. Dermatol. 1993, 100, 342S–345S. [Google Scholar] [CrossRef]
- Merlino, G.; Herlyn, M.; Fisher, D.E.; Bastian, B.C.; Flaherty, K.T.; Davies, M.A.; Wargo, J.A.; Curiel-Lewandrowski, C.; Weber, M.J.; Leachman, S.A.; et al. The state of melanoma: Challenges and opportunities. Pigment Cell Melanoma Res. 2016, 29, 404–416. [Google Scholar] [CrossRef]
- Kimball, A.B.; Resneck, J.S., Jr. The US dermatology workforce: A specialty remains in shortage. J. Am. Acad. Dermatol. 2008, 59, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Wilkinson, D.; Hansen, M.; Argenziano, G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J. Am. Acad. Dermatol. 2009, 61, 599–604. [Google Scholar] [CrossRef]
- Salerni, G.; Teran, T.; Puig, S.; Malvehy, J.; Zalaudek, I.; Argenziano, G.; Kittler, H. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: A study on behalf of the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 805–814. [Google Scholar] [CrossRef]
- Engasser, H.C.; Warshaw, E.M. Dermatoscopy use by US dermatologists: A cross-sectional survey. J. Am. Acad. Dermatol. 2010, 63, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Carli, P.; de Giorgi, V.; Chiarugi, A.; Nardini, P.; Weinstock, M.A.; Crocetti, E.; Stante, M.; Giannotti, B. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: A randomized study. J. Am. Acad. Dermatol. 2004, 50, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Carli, P.; De Giorgi, V.; Crocetti, E.; Mannone, F.; Massi, D.; Chiarugi, A.; Giannotti, B. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: A retrospective study 1997–2001. Br. J. Dermatol. 2004, 150, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Wolner, Z.J.; Yelamos, O.; Liopyris, K.; Rogers, T.; Marchetti, M.A.; Marghoob, A.A. Enhancing Skin Cancer Diagnosis with Dermoscopy. Dermatol. Clin. 2017, 35, 417–437. [Google Scholar] [CrossRef]
- Mayer, J. Systematic review of the diagnostic accuracy of dermatoscopy in detecting malignant melanoma. Med. J. Aust. 1997, 167, 206–210. [Google Scholar] [CrossRef]
- Morris, J.B.; Alfonso, S.V.; Hernandez, N.; Fernandez, M.I. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol. Pract. Concept. 2017, 7, 7–16. [Google Scholar] [CrossRef]
- Terushkin, V.; Oliveria, S.A.; Marghoob, A.A.; Halpern, A.C. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: An update. J. Am. Acad. Dermatol. 2010, 62, 794–803. [Google Scholar] [CrossRef]
- Fried, L.J.; Tan, A.; Berry, E.G.; Braun, R.P.; Curiel-Lewandrowski, C.; Curtis, J.; Ferris, L.K.; Hartman, R.I.; Jaimes, N.; Kawaoka, J.C.; et al. Dermoscopy Proficiency Expectations for US Dermatology Resident Physicians: Results of a Modified Delphi Survey of Pigmented Lesion Experts. JAMA Dermatol. 2021, 157, 189–197. [Google Scholar] [CrossRef]
- Jitian Mihulecea, C.R.; Fratila, S.; Rotaru, M. Clinical-dermoscopic similarities between atypical nevi and early stage melanoma. Exp. Ther. Med. 2021, 22, 854. [Google Scholar] [CrossRef]
- Manolakos, D.; Patrick, G.; Geisse, J.K.; Rabinovitz, H.; Buchanan, K.; Hoang, P.; Rodriguez-Diaz, E.; Bigio, I.J.; Cognetta, A.B. Use of an elastic-scattering spectroscopy and artificial intelligence device in the assessment of lesions suggestive of skin cancer: A comparative effectiveness study. JAAD Int. 2024, 14, 52–58. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Masood, A.; Al-Jumaily, A.A. Computer aided diagnostic support system for skin cancer: A review of techniques and algorithms. Int. J. Biomed. Imaging 2013, 2013, 323268. [Google Scholar] [CrossRef]
- Hoorens, I.; Vossaert, K.; Lanssens, S.; Dierckxsens, L.; Argenziano, G.; Brochez, L. Value of Dermoscopy in a Population-Based Screening Sample by Dermatologists. Dermatol. Pract. Concept. 2019, 9, 200–206. [Google Scholar] [CrossRef]
- Vestergaard, M.E.; Macaskill, P.; Holt, P.E.; Menzies, S.W. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, C.; Ambrosio, L.; Cucchi, C.; Meznerics, F.A.; Kiss, N.; Banvolgyi, A.; Rega, F.; Grignaffini, F.; Barbuto, F.; Frezza, F.; et al. Melanoma Detection by Non-Specialists: An Untapped Potential for Triage? Diagnostics 2022, 12, 2821. [Google Scholar] [CrossRef]
- Gareau, D.S.; Browning, J.; Correa Da Rosa, J.; Suarez-Farinas, M.; Lish, S.; Zong, A.M.; Firester, B.; Vrattos, C.; Renert-Yuval, Y.; Gamboa, M.; et al. Deep learning-level melanoma detection by interpretable machine learning and imaging biomarker cues. J. Biomed. Opt. 2020, 25, 112906. [Google Scholar] [CrossRef]
- Gareau, D.S.; Correa da Rosa, J.; Yagerman, S.; Carucci, J.A.; Gulati, N.; Hueto, F.; DeFazio, J.L.; Suarez-Farinas, M.; Marghoob, A.; Krueger, J.G. Digital imaging biomarkers feed machine learning for melanoma screening. Exp. Dermatol. 2017, 26, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.M.; Coakley, B.J.; Chang, D.; Talebi-Liasi, F.; Lish, S.; Lee, S.W.; Zong, A.M.; Moore, I.; Browning, J.; Jacques, S.L.; et al. Hyperspectral imaging in automated digital dermoscopy screening for melanoma. Lasers Surg. Med. 2019, 51, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Chanda, T.; Hauser, K.; Hobelsberger, S.; Bucher, T.C.; Garcia, C.N.; Wies, C.; Kittler, H.; Tschandl, P.; Navarrete-Dechent, C.; Podlipnik, S.; et al. Dermatologist-like explainable AI enhances trust and confidence in diagnosing melanoma. Nat. Commun. 2024, 15, 524. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Dusza, S.W.; Liopyris, K.; Marghoob, A.A.; Halpern, A.C.; Marchetti, M.A. Automated Dermatological Diagnosis: Hype or Reality? J. Investig. Dermatol. 2018, 138, 2277–2279. [Google Scholar] [CrossRef]
- Sun, X.Y.; Feng, Q.X.; Xu, X.; Zhang, J.; Zhu, F.P.; Yang, Y.H.; Zhang, Y.D. Radiologic-Radiomic Machine Learning Models for Differentiation of Benign and Malignant Solid Renal Masses: Comparison With Expert-Level Radiologists. AJR Am. J. Roentgenol. 2020, 214, W44–W54. [Google Scholar] [CrossRef] [PubMed]
- Said, D.; Hectors, S.J.; Wilck, E.; Rosen, A.; Stocker, D.; Bane, O.; Beksac, A.T.; Lewis, S.; Badani, K.; Taouli, B. Characterization of solid renal neoplasms using MRI-based quantitative radiomics features. Abdom. Radiol. 2020, 45, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Eloy, C.; Marques, A.; Pinto, J.; Pinheiro, J.; Campelos, S.; Curado, M.; Vale, J.; Polonia, A. Artificial intelligence-assisted cancer diagnosis improves the efficiency of pathologists in prostatic biopsies. Virchows Arch. 2023, 482, 595–604. [Google Scholar] [CrossRef] [PubMed]
| Sensitivity without IBCs | Specificity without IBCs | Sensitivity with IBCs | Specificity with IBCs | Sensitivity Increase | Specificity Increase | d′ without | d′ with | d′ Increase | |
|---|---|---|---|---|---|---|---|---|---|
| Expert Dermoscopist | 71.1 | 70 | 81.6 | 77.5 | 10.5 | 7.5 | 1.08 | 1.66 | 0.57 |
| Dermatopathologist | 81.6 | 55 | 94.7 | 57.5 | 13.1 | 2.5 | 1.03 | 1.81 | 0.78 |
| Expert Dermoscopist | 92.1 | 60 | 92.1 | 67.5 | 0 | 7.5 | 1.67 | 1.87 | 0.2 |
| Mohs Surgeon | 68.4 | 57.5 | 78.9 | 60 | 10.5 | 2.5 | 0.67 | 1.06 | 0.39 |
| Dermatology Resident | 73.7 | 57.5 | 76.3 | 55 | 2.6 | −2.5 | 0.82 | 0.84 | 0.02 |
| Dermatopathologist | 81.6 | 70 | 84.2 | 62.5 | 2.5 | −7.5 | 1.42 | 1.32 | −0.1 |
| Mohs Surgeon | 57.9 | 65 | 71.1 | 67.5 | 13.2 | 2.5 | 0.58 | 1.01 | 0.43 |
| Mohs Surgeon | 60.5 | 55 | 78.9 | 70 | 18.4 | 15 | 0.39 | 1.33 | 0.94 |
| Expert Dermoscopist | 73.7 | 62.5 | 84.2 | 75 | 10.5 | 12.5 | 0.95 | 1.68 | 0.72 |
| Mohs Surgeon | 76.3 | 52.5 | 73.7 | 80 | −2.6 | 27.5 | 0.78 | 1.48 | 0.7 |
| Mean | 73.69 | 60.50 | 81.57 | 67.25 | 7.87 | 6.75 | 0.94 | 1.41 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero, R.; Buchanan, K.L.; Navarrete-Dechent, C.; Marghoob, A.A.; Stein, J.A.; Landy, M.S.; Leachman, S.A.; Linden, K.G.; Garcet, S.; Krueger, J.G.; et al. The Impact of Melanoma Imaging Biomarker Cues on Detection Sensitivity and Specificity in Melanoma versus Clinically Atypical Nevi. Cancers 2024, 16, 3077. https://doi.org/10.3390/cancers16173077
Agüero R, Buchanan KL, Navarrete-Dechent C, Marghoob AA, Stein JA, Landy MS, Leachman SA, Linden KG, Garcet S, Krueger JG, et al. The Impact of Melanoma Imaging Biomarker Cues on Detection Sensitivity and Specificity in Melanoma versus Clinically Atypical Nevi. Cancers. 2024; 16(17):3077. https://doi.org/10.3390/cancers16173077
Chicago/Turabian StyleAgüero, Rosario, Kendall L. Buchanan, Cristián Navarrete-Dechent, Ashfaq A. Marghoob, Jennifer A. Stein, Michael S. Landy, Sancy A. Leachman, Kenneth G. Linden, Sandra Garcet, James G. Krueger, and et al. 2024. "The Impact of Melanoma Imaging Biomarker Cues on Detection Sensitivity and Specificity in Melanoma versus Clinically Atypical Nevi" Cancers 16, no. 17: 3077. https://doi.org/10.3390/cancers16173077
APA StyleAgüero, R., Buchanan, K. L., Navarrete-Dechent, C., Marghoob, A. A., Stein, J. A., Landy, M. S., Leachman, S. A., Linden, K. G., Garcet, S., Krueger, J. G., & Gareau, D. S. (2024). The Impact of Melanoma Imaging Biomarker Cues on Detection Sensitivity and Specificity in Melanoma versus Clinically Atypical Nevi. Cancers, 16(17), 3077. https://doi.org/10.3390/cancers16173077






