Proceedings from the First Onco Summit: LATAM Chapter, 19–20 May 2023, Rio de Janeiro, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
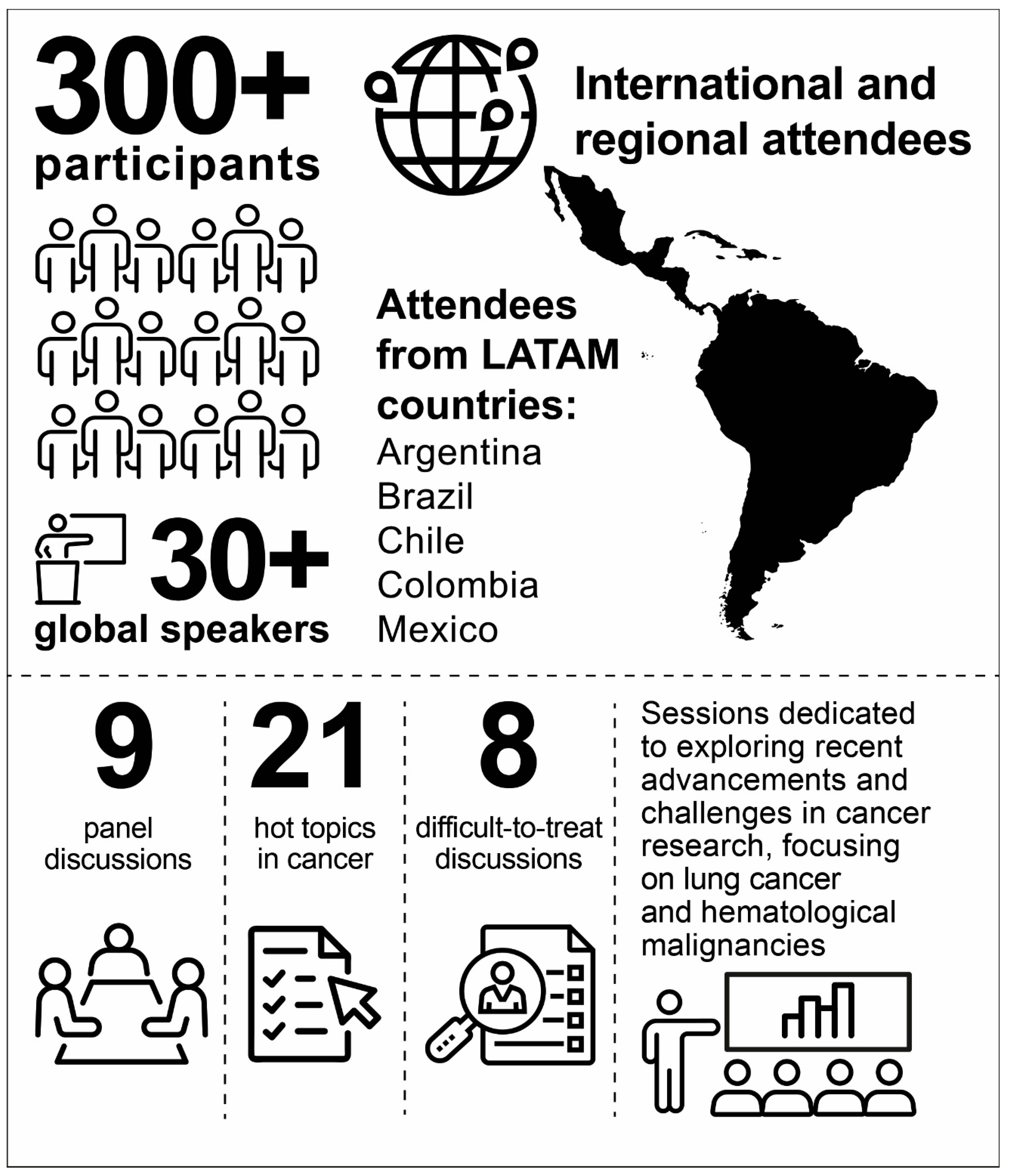
2. Overview of The Onco Summit 2023: The LATAM Chapter
3. Recent Therapeutic Advances in Oncology
4. Common Challenges with the Use of Novel Anti-Cancer Therapies and Potential Solutions
4.1. Lung Cancer
4.1.1. Limited Treatment Options Post-PD-L1 Antibodies in NSCLC
4.1.2. Selecting Targeted Therapy Based on Biomarker Selection
4.1.3. Treatment Resistance to Targeted Therapies
4.1.4. Key Takeaways to Address the Challenges in Lung Cancer
4.2. MM
4.2.1. Making Treatment Decisions in RRMM Solely Based on Clinical Trial Data
4.2.2. Treatment of Patients with RRMM with Prior Exposure to Lenalidomide
4.2.3. Selecting the Type and Duration of Maintenance and Continuous Therapies
4.2.4. Treatment of Elderly Patients
4.2.5. Treatment of Smoldering Myeloma
4.2.6. Key Takeaways to Address the Challenges in MM
4.3. Lymphomas
4.3.1. Improving SCT Rates and Clinical Outcomes with ASCT in RRHL
4.3.2. Treatment of SCT-Ineligible Patients with RRHL
4.3.3. Treatment of T-Cell Lymphoma
4.3.4. Key Takeaways to Address the Challenges in Lymphomas
5. Challenges with Therapeutic Advances in Cancer—Focus on Oncology Clinical Trials and Drug Development Process
5.1. Improving Generalizability and Applicability of Clinical Trial Data in Clinical Practice
5.2. Improve the Drug Development Process in Oncology
5.3. Key Takeaways to Address the Challenges with Clinical Trials and the Drug Development Process
6. Challenges with Cancer Care in LATAM
6.1. Improved and Equitable Access to Modern Diagnostic Techniques and Novel Therapies
6.2. Early Diagnosis and Early Intervention
6.3. Key Takeaways to Address the Challenges with Cancer Care in LATAM
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cardone, C.; Arnold, D. The cancer treatment gap in lower- to middle-income countries. Oncology 2023, 101, 2–4. [Google Scholar] [CrossRef]
- Pramesh, C.S.; Badwe, R.A.; Bhoo-Pathy, N.; Booth, C.M.; Chinnaswamy, G.; Dare, A.J.; de Andrade, V.P.; Hunter, D.J.; Gopal, S.; Gospodarowicz, M.; et al. Priorities for cancer research in low- and middle-income countries: A global perspective. Nat. Med. 2022, 28, 649–657. [Google Scholar] [CrossRef]
- Stefan, D.C.; Tang, S. Addressing cancer care in low- to middle-income countries: A call for sustainable innovations and impactful research. BMC Cancer 2023, 23, 756. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, M.; Laversanne, M.; Barrios, E.; Cancela, M.C.; de Vries, E.; Pardo, C.; Bray, F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg. Health Am. 2022, 13, 100294. [Google Scholar] [CrossRef] [PubMed]
- Cazap, E.; de Almeida, L.M.; Arrossi, S.; García, P.J.; Garmendia, M.L.; Gil, E.; Hassel, T.; Mayorga, R.; Mohar, A.; Murillo, R.; et al. Latin America and the Caribbean code against cancer: Developing evidence-based recommendations to reduce the risk of cancer in Latin America and the Caribbean. J. Glob. Oncol. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Llera, A.S. A fresh perspective on Latin America cancer care: Uncovering hidden messages in unconventional data sources. Lancet Reg. Health Am. 2023, 24, 100559. [Google Scholar] [CrossRef] [PubMed]
- Kreidieh, F.Y.; Tawbi, H.A.; Alexaki, A.; Borghaei, H.; Kandalaft, L.E. Novel immunotherapeutics: Perspectives on checkpoints, bispecifics, and vaccines in development. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e391278. [Google Scholar] [CrossRef]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: Current landscape and future perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef]
- Li, X.; Dai, H.; Wang, H.; Han, W. Exploring innate immunity in cancer immunotherapy: Opportunities and challenges. Cell Mol. Immunol. 2021, 18, 1607–1609. [Google Scholar] [CrossRef]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Ghosh, S. Lifting the innate immune barriers to antitumor immunity. J. Immunother. Cancer 2020, 8, e000695. [Google Scholar] [CrossRef]
- Yang, L.; Gu, X.; Yu, J.; Ge, S.; Fan, X. Oncolytic virotherapy: From bench to bedside. Front. Cell Dev. Biol. 2021, 9, 790150. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ignatius Ou, S.H.; Weiss, J.; Ades, S.; Shapiro, G.I.; Socinski, M.A.; Murphy, D.A.; Conte, U.; et al. Crizotinib in Patients With MET-Amplified NSCLC. J. Thorac. Oncol. 2021, 16, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Hasanovic, A.; Suehara, Y.; Lipson, D.; Stephens, P.; Ross, J.; Miller, V.; Ginsberg, M.; Zakowski, M.F.; et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013, 3, 630–635. [Google Scholar] [CrossRef]
- Gautschi, O.; Pauli, C.; Strobel, K.; Hirschmann, A.; Printzen, G.; Aebi, S.; Diebold, J. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J. Thorac. Oncol. 2012, 7, e23–e24. [Google Scholar] [CrossRef]
- Mazières, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef]
- Ou, S.H.; Kwak, E.L.; Siwak-Tapp, C.; Dy, J.; Bergethon, K.; Clark, J.W.; Camidge, D.R.; Solomon, B.J.; Maki, R.G.; Bang, Y.J.; et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J. Thorac. Oncol. 2011, 6, 942–946. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Riudavets, M.; Sullivan, I.; Abdayem, P.; Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021, 6, 100260. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Catalano, M.; Shabani, S.; Venturini, J.; Ottanelli, C.; Voltolini, L.; Roviello, G. Lung cancer immunotherapy: Beyond common immune checkpoints inhibitors. Cancers 2022, 14, 6145. [Google Scholar] [CrossRef]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the use of targeted therapies in non-small cell lung cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Clay, T.D.; Majem, M.; Felip, E.; Doger, B.; Costa, E.C.; Forster, M.; Krebs, M.; Peguero, J.A.; Mueller, C.; Triebel, F. Results from a phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with PD-L1 unselected metastatic non-small cell lung carcinoma. J. Clin. Oncol. 2021, 39, 9046. [Google Scholar] [CrossRef]
- Kluger, H.; Weiss, S.; Olszanski, A.; Schuchter, L.; Linette, G.; Garland, L.; Iannotti, N.; Johnson, M.; Avsar, E.; Srivastava, M.; et al. Abstract CT089: Phase Ib/II of CD40 agonistic antibody APX005M in combination with nivolumab (nivo) in subjects with metastatic melanoma (M) or non-small cell lung cancer (NSCLC). Cancer Res. 2019, 79, CT089. [Google Scholar] [CrossRef]
- Kim, T.W.; Burris, H.A.; de Miguel Luken, M.J.; Pishvaian, M.J.; Bang, Y.J.; Gordon, M.; Awada, A.; Camidge, D.R.; Hodi, F.S.; McArthur, G.A.; et al. First-in-human phase I study of the OX40 agonist MOXR0916 in patients with advanced solid tumors. Clin. Cancer Res. 2022, 28, 3452–3463. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Chiappori, A.A.; Thompson, J.A.; Doi, T.; Hu-Lieskovan, S.; Eskens, F.A.L.M.; Ros, W.; Diab, A.; Spano, J.-P.; Rizvi, N.A.; et al. First-in-human study of an OX40 (ivuxolimab) and 4-1BB (utomilumab) agonistic antibody combination in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e005471. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Champiat, S.; Lai, W.V.; Izumi, H.; Govindan, R.; Boyer, M.; Hummel, H.D.; Borghaei, H.; Johnson, M.L.; Steeghs, N.; et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: An open-label, phase I study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
- Gajra, A.; Zalenski, A.; Sannareddy, A.; Jeune-Smith, Y.; Kapinos, K.; Kansagra, A. Barriers to chimeric antigen receptor T-Cell (CAR-T) therapies in clinical practice. Pharm. Med. 2022, 36, 163–171. [Google Scholar] [CrossRef]
- Mikhael, J.; Fowler, J.; Shah, N. Chimeric antigen receptor T-cell therapies: Barriers and solutions to access. JCO Oncol. Pract. 2022, 18, 800–807. [Google Scholar] [CrossRef]
- Daher, M.; Rezvani, K. Outlook for new CAR-based therapies with a focus on CAR NK Cells: What lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov. 2021, 11, 45–58. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Pinto, S.; Pahl, J.; Schottelius, A.; Carter, P.J.; Koch, J. Reimagining antibody-dependent cellular cytotoxicity in cancer: The potential of natural killer cell engagers. Trends Immunol. 2022, 43, 932–946. [Google Scholar] [CrossRef]
- Zhang, D.K.Y.; Adu-Berchie, K.; Iyer, S.; Liu, Y.; Cieri, N.; Brockman, J.M.; Neuberg, D.; Wu, C.J.; Mooney, D.J. Enhancing CAR-T cell functionality in a patient-specific manner. Nat. Commun. 2023, 14, 506. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Oliveres, H.; Caglevic, C.; Passiglia, F.; Taverna, S.; Smits, E.; Rolfo, C. Vaccine and immune cell therapy in non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S1602–S1614. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Qin, C.; Yan, H.; Liu, T.; Hu, H.; Tang, S.; Tang, S.; Zhou, H. Biomarker-targeted therapies in non-small cell lung cancer: Current status and perspectives. Cells 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Aisner, D.L.; Wood, D.E.; Akerley, W.; Bauman, J.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; Dobelbower, M.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J. Natl. Compr. Canc Netw. 2018, 16, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Wu, Y.L.; Planchard, D.; Lu, S.; Sun, H.; Yamamoto, N.; Kim, D.W.; Tan, D.S.W.; Yang, J.C.; Azrif, M.; Mitsudomi, T.; et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 171–210. [Google Scholar] [CrossRef]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef]
- Russo, A.; Franchina, T.; Ricciardi, G.; Battaglia, A.; Picciotto, M.; Adamo, V. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: New insights and future perspectives in this complex clinical scenario. Int. J. Mol. Sci. 2019, 20, 1431. [Google Scholar] [CrossRef]
- Zwierenga, F.; van Veggel, B.; Hendriks, L.E.L.; Hiltermann, T.J.N.; Hiddinga, B.I.; Hijmering Kappelle, L.B.M.; ter Elst, A.; Hashemi, S.M.S.; Dingemans, A.-M.C.; van der Leest, C.; et al. High dose osimertinib in patients with advanced stage EGFR exon 20 mutation-positive NSCLC: Results from the phase 2 multicenter POSITION20 trial. Lung Cancer 2022, 170, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vyse, S.; Huang, P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 5. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, C.; Zhao, H. Immune checkpoint inhibitors combined with tyrosine kinase inhibitors is the treatment option of previously treated advanced non-small cell lung cancer harboring EGFR or ALK genetic aberration. Transl. Lung Cancer Res. 2022, 11, 2164–2166. [Google Scholar] [CrossRef]
- Nelson, T.A.; Wang, N. Targeting lung cancer brain metastases: A narrative review of emerging insights for anaplastic lymphoma kinase (ALK)-positive disease. Transl. Lung Cancer Res. 2023, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for brain metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhu, L.; Sun, Y.; Stebbing, J.; Selvaggi, G.; Zhang, Y.; Yu, Z. Targeting ALK rearrangements in NSCLC: Current state of the art. Front. Oncol. 2022, 12, 863461. [Google Scholar] [CrossRef]
- Ferreira, C.G. Lung cancer in developing countries: Access to molecular testing. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 327–331. [Google Scholar] [CrossRef]
- O’Reilly, D.; Botticella, A.; Barry, S.; Cotter, S.; Donington, J.S.; Le Pechoux, C.; Naidoo, J. Treatment decisions for resectable non-small-cell lung cancer: Balancing less with more? Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389950. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodéré, F.; Jamet, B.; Bezzi, D.; Zamagni, E.; Moreau, P.; Nanni, C. New developments in myeloma treatment and response assessment. J. Nucl. Med. 2023, 64, 1331–1343. [Google Scholar] [CrossRef]
- Dima, D.; Jiang, D.; Singh, D.J.; Hasipek, M.; Shah, H.S.; Ullah, F.; Khouri, J.; Maciejewski, J.P.; Jha, B.K. Multiple myeloma therapy: Emerging trends and challenges. Cancers 2022, 14, 4082. [Google Scholar] [CrossRef]
- Bahlis, N.J. Darwinian evolution and tiding clones in multiple myeloma. Blood 2012, 120, 927–928. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef]
- Brioli, A.; Melchor, L.; Cavo, M.; Morgan, G.J. The impact of intra-clonal heterogeneity on the treatment of multiple myeloma. Br. J. Haematol. 2014, 165, 441–454. [Google Scholar] [CrossRef]
- Richardson, P.G.; San Miguel, J.F.; Moreau, P.; Hajek, R.; Dimopoulos, M.A.; Laubach, J.P.; Palumbo, A.; Luptakova, K.; Romanus, D.; Skacel, T.; et al. Interpreting clinical trial data in multiple myeloma: Translating findings to the real-world setting. Blood Cancer J. 2018, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Richardson, P.; Lonial, S. Treatment options for patients with heavily pretreated relapsed and refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P. Management of multiple myeloma in the relapsed/refractory patient. Hematol. Am. Soc. Hematol. Educ. Program. 2017, 2017, 508–517. [Google Scholar] [CrossRef]
- Martinez-Høyer, S.; Karsan, A. Mechanisms of lenalidomide sensitivity and resistance. Exp. Hematol. 2020, 91, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.J.; Freeman, C.L.; Rosko, A.E. Treatment of older adult or frail patients with multiple myeloma. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 46–54. [Google Scholar] [CrossRef]
- Bishnoi, R.; Xie, Z.; Shah, C.; Bian, J.; Murthy, H.S.; Wingard, J.R.; Farhadfar, N. Real-world experience of carfilzomib-associated cardiovascular adverse events: SEER-Medicare data set analysis. Cancer Med. 2021, 10, 70–78. [Google Scholar] [CrossRef]
- Lonial, S.; Jacobus, S.; Fonseca, R.; Weiss, M.; Kumar, S.; Orlowski, R.Z.; Kaufman, J.L.; Yacoub, A.M.; Buadi, F.K.; O’Brien, T.; et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J. Clin. Oncol. 2020, 38, 1126–1137. [Google Scholar] [CrossRef]
- Derman, B.A.; Belli, A.J.; Battiwalla, M.; Hamadani, M.; Kansagra, A.; Lazarus, H.M.; Wang, C.-K. Reality check: Real-world evidence to support therapeutic development in hematologic malignancies. Blood Rev. 2022, 53, 100913. [Google Scholar] [CrossRef]
- Roberts, M.H.; Ferguson, G.T. Real-world evidence: Bridging gaps in evidence to guide payer decisions. PharmacoEconomics-Open 2021, 5, 3–11. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Stewart, A.K.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.; Mihaylov, G.G.; Goranova-Marinova, V.; et al. Carfilzomib-lenalidomide-dexamethasone vs lenalidomide-dexamethasone in relapsed multiple myeloma by previous treatment. Blood Cancer J. 2017, 7, e554. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Lee, H.C.; Ramasamy, K.; Macro, M.; Davies, F.E.; Abonour, R.; van Rhee, F.; Hungria, V.T.; Puig, N.; Ren, K.; Silar, J.; et al. P925: Impact of prior treatment exposure on the effectiveness of ixazomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma patients treated in routine clinical practice (the INSURE study). Hemasphere 2022, 6, 815–816. [Google Scholar] [CrossRef]
- María-Victoria, M.; Tamas, M.; Norbert, G.; Markus, H.; Irwindeep, S.; Ludek, P.; Luísa, V.; Sharon, R.J.; Anne-Marie, S.; Peter, G.; et al. Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1. Haematologica 2017, 102, 1767–1775. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Davies, F.; Rifkin, R.; Costello, C.; Usmani, S.; Abonour, R.; Palumbo, A.; Romanus, D.; Hajek, R.; Terpos, E.; Stull, D.M.; et al. PS1419 Comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in routine care in the US. HemaSphere 2019, 3, 652–653. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Gavriatopoulou, M.; Oriol, A.; Rabin, N.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol. 2022, 23, 65–76. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.V.; Belch, A.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.J.; et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018, 131, 301–310. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Hemasphere 2021, 5, e528. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, O.; Redd, R.A.; Prescott, J.; Tague, K.; Romines, V.; Metivier, A.; Savell, A.; Leblebjian, H.; Distaso, A.; Mo, C.C.; et al. A phase II trial of the combination of ixazomib, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. Blood 2021, 138, 2749. [Google Scholar] [CrossRef]
- Hungria, V.; Gaiolla, R.; Galvez, K.; Remaggi, G.; Schutz, N.; Bittencourt, R.; Maiolino, A.; Quintero, G.; Cugliari, M.S.; Tobias, W.M.; et al. P954: Multiple myeloma in Latin America cancer registry: The MYLACRE study. Hemasphere 2023, 7, e6700375. [Google Scholar] [CrossRef]
- Hungria, V.T.M.; Bittencourt, R.; Martinez, G.; Santos, J.D.A.; Almeida, D.R.d.; Figueiredo, V.L.D.P.; De Farias, D.L.C.; Zanella, K.R.; Muniz, L.B.; Carvalho, P.; et al. A Brazilian real-life experience of multiple myeloma patients: Final results from the Mmybrave multi-center study. Blood 2022, 140, 4352–4353. [Google Scholar] [CrossRef]
- Tietsche de Moraes Hungria, V.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Avendano Flores, O.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of hematologic malignancies in real-world settings: Findings from the Hemato-Oncology Latin America Observational registry study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Evens, A.M.; Hutchings, M.; Diehl, V. Treatment of Hodgkin lymphoma: The past, present, and future. Nat. Clin. Pract. Oncol. 2008, 5, 543–556. [Google Scholar] [CrossRef]
- Sureda, A.; André, M.; Borchmann, P.; da Silva, M.G.; Gisselbrecht, C.; Vassilakopoulos, T.P.; Zinzani, P.L.; Walewski, J. Improving outcomes after autologous transplantation in relapsed/refractory Hodgkin lymphoma: A European expert perspective. BMC Cancer 2020, 20, 1088. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Müller, H.; Gillessen, S.; Yang, X.; Koeppel, L.; Pilz, V.; Marinello, P.; Kaskel, P.; Raut, M.; Fuchs, M.; et al. Clinical outcomes of relapsed and refractory Hodgkin lymphoma patients after contemporary first-line treatment: A German Hodgkin Study Group analysis. Leukemia 2022, 36, 772–780. [Google Scholar] [CrossRef]
- Kaloyannidis, P.; Hertzberg, M.; Webb, K.; Zomas, A.; Schrover, R.; Hurst, M.; Jacob, I.; Nikoglou, T.; Connors, J.M. Brentuximab vedotin for the treatment of patients with relapsed or refractory Hodgkin lymphoma after autologous stem cell transplantation. Br. J. Haematol. 2020, 188, 540–549. [Google Scholar] [CrossRef]
- Takiar, R.; Karimi, Y. Novel salvage therapy options for initial treatment of relapsed/refractory classical Hodgkin’s lymphoma: So many options, how to choose? Cancers 2022, 14, 3526. [Google Scholar] [CrossRef]
- Ma, H.; Abdul-Hay, M. T-cell lymphomas, a challenging disease: Types, treatments, and future. Int. J. Clin. Oncol. 2017, 22, 18–51. [Google Scholar] [CrossRef]
- Dippel, E.; Assaf, C.; Becker, J.C.; von Bergwelt-Baildon, M.; Bernreiter, S.; Cozzio, A.; Eich, H.T.; Elsayad, K.; Follmann, M.; Grabbe, S.; et al. S2k-Guidelines—Cutaneous lymphomas (ICD10 C82–C86): Update 2021. J. Dtsch. Dermatol. Ges. 2022, 20, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017, 92, 1085–1102. [Google Scholar] [CrossRef]
- Maranzano, M.; Mead, M. The role of transplantation in Hodgkin lymphoma. Front. Oncol. 2023, 12, 1054314. [Google Scholar] [CrossRef]
- Tomassetti, S.; Herrera, A.F. Update on the role of brentuximab vedotin in classical Hodgkin lymphoma. Ther. Adv. Hematol. 2018, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.P.; Chatzidimitriou, C.; Asimakopoulos, J.V.; Arapaki, M.; Tzoras, E.; Angelopoulou, M.K.; Konstantopoulos, K. Immunotherapy in Hodgkin lymphoma: Present status and future strategies. Cancers 2019, 11, 1071. [Google Scholar] [CrossRef]
- Sureda-Balari, A.; Terol, M.J.; Domingo-Domènech, E.; Rodriguez, A.P.G.; Mohedo, F.H.; Céspedes, J.N.; De La Cruz Vicente, F.; Muñoz, C.M.; Díaz, M.E.A.; Córdoba, R.; et al. T098: Brentuximab vedotin plus ESHAP (BRESHAP) versus ESHAP in patients with relapsed or refractory classical Hodgkin’s lymphoma. Interim results of the BRESELIBET prospective clinical trial. Hemasphere 2022, 6, 45. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Walewski, J.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Viviani, S.; et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 2018, 132, 2639–2642. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Shah, G.; Schöder, H.; Ganesan, N.; Drill, E.; Hancock, H.; Davey, T.; Perez, L.; Ryu, S.; Sohail, S.; et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J. Clin. Oncol. 2021, 39, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Chen, Y.B.; Redd, R.A.; Joyce, R.M.; Bsat, J.; Jeter, E.; Merryman, R.W.; Coleman, K.C.; Dahi, P.B.; Nieto, Y.; et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood 2019, 134, 22–29. [Google Scholar] [CrossRef]
- De Filippi, R.; Marcacci, G.; Derenzini, E.; Musso, M.; Donnarumma, D.; Morelli, E.; Patti, C.; Maraglino, A.M.E.; Scalone, R.; Simeone, L.; et al. Anti-PD1 consolidation in patients with Hodgkin lymphoma at high risk of relapse after autologous stem cell transplantation: A multicenter real-life study. Cancers 2022, 14, 5846. [Google Scholar] [CrossRef]
- Mohty, R.; Dulery, R.; Bazarbachi, A.H.; Savani, M.; Hamed, R.A.; Bazarbachi, A.; Mohty, M. Latest advances in the management of classical Hodgkin lymphoma: The era of novel therapies. Blood Cancer J. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadidi, S.A.; Lee, H.J. Checkpoint inhibition therapy in transplant-ineligible relapsed or refractory classic Hodgkin lymphoma. JCO Oncol. Pract. 2021, 17, 64–71. [Google Scholar] [CrossRef]
- Randall, M.P.; Spinner, M.A. Optimizing treatment for relapsed/refractory classic Hodgkin lymphoma in the era of immunotherapy. Cancers 2023, 15, 4509. [Google Scholar] [CrossRef] [PubMed]
- Walewski, J.; Hellmann, A.; Siritanaratkul, N.; Ozsan, G.H.; Ozcan, M.; Chuncharunee, S.; Goh, A.S.; Jurczak, W.; Koren, J.; Paszkiewicz-Kozik, E.; et al. Prospective study of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma patients who are not suitable for stem cell transplant or multi-agent chemotherapy. Br. J. Haematol. 2018, 183, 400–410. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Zagadailov, E.A.; Corman, S.L.; Chirikov, V.; Johnson, C.; Macahilig, C.; Seal, B.; Dalal, M.R.; Illidge, T. Brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma who are Ineligible for autologous stem cell transplant: A Germany and United Kingdom retrospective study. Eur. J. Haematol. 2017, 99, 553–558. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A.; et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862. [Google Scholar] [CrossRef]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries- a systematic review. Int. J. Equity Health 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H.; Werutsky, G.; Martinez-Mesa, J. The global conduct of cancer clinical trials: Challenges and opportunities. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e132–e139. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Carson, L.M.; Berry, S.; Moraes, F.Y. Challenges of globalization of cancer drug trials—Recruitment in LMICs, approval in HICs. Lancet Reg. Health Am. 2021, 7, 100157. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Kaushik, N. Challenges in oncology studies: Review from a global perspective. Eur. J. Clin. Oncol. 2019, 1, 3. [Google Scholar]
- Arrowsmith, J.; Miller, P. Trial watch: Phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; de Lima Lopes, G.; Yusof, M.M.; Rubagumya, F.; Rutkowski, P.; Sengar, M. Barriers in access to oncology drugs—A global crisis. Nat. Rev. Clin. Oncol. 2023, 20, 7–15. [Google Scholar] [CrossRef]
- Paolo, T.; Dario, T.; Stefania, M.; Emanuela, F.; Giulia, V.; Paolo, D.A.; Bruno Achutti, D.; Giuseppe, C. Opportunities and challenges of implementing pharmacogenomics in cancer drug development. Cancer Drug Resist. 2019, 2, 43–52. [Google Scholar] [CrossRef]
- Tan, S.C.; Poh, W.T.; Yong, A.C.H.; Chua, E.W.; Ooi, J.; Mahmud, R.; Thiagarajan, M.; Stanslas, J. Challenges and strategies for improving access to cancer drugs in Malaysia: Summary of opinions expressed at the 2nd MACR International Scientific Conference 2022. Cancer Manag. Res. 2023, 15, 851–862. [Google Scholar] [CrossRef]
- Timothy, S. Weighing Two Drivers for Pharmaceutical Stocks. Available online: https://www.fisherinvestments.com/en-us/marketminder/weighing-two-drivers-for-pharmaceutical-stocks (accessed on 25 November 2023).
- Senior, M. Fresh from the biotech pipeline: Fewer approvals, but biologics gain share. Nat. Biotechnol. 2023, 41, 174–182. [Google Scholar] [CrossRef]
- Chodankar, D. Introduction to real-world evidence studies. Perspect. Clin. Res. 2021, 12, 171–174. [Google Scholar] [CrossRef]
- Dickson, D.; Johnson, J.; Bergan, R.; Owens, R.; Subbiah, V.; Kurzrock, R. The master observational trial: A new class of master protocol to advance precision medicine. Cell 2020, 180, 9–14. [Google Scholar] [CrossRef]
- NHS. Publishing Your Research Findings. Available online: https://www.hra.nhs.uk/planning-and-improving-research/research-planning/publishing-your-research-findings/ (accessed on 25 November 2023).
- Schuhmacher, A.; Gassmann, O.; Bieniok, D.; Hinder, M.; Hartl, D. Open innovation: A paradigm shift in pharma R&D? Drug Discov. Today 2022, 27, 2395–2405. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Atanasov, A.G.; Sheridan, H.; Klager, E.; Eibensteiner, F.; Völkl-Kernsock, S.; Kletecka-Pulker, M.; Willschke, H.; Schaden, E. Open innovation in medical and pharmaceutical research: A literature landscape analysis. Front. Pharmacol. 2021, 11, 587526. [Google Scholar] [CrossRef] [PubMed]
- Ali, C.-A.; Andrea, O. Precision oncology in Latin America: Current situation, challenges and perspectives. Ecancermedicalscience 2019, 13, 920. [Google Scholar] [CrossRef]
- Barrios, C.H.; Werutsky, G.; Mohar, A.; Ferrigno, A.S.; Müller, B.G.; Bychkovsky, B.L.; Castro, E.C.J.; Uribe, C.J.; Villarreal-Garza, C.; Soto-Perez-de-Celis, E.; et al. Cancer control in Latin America and the Caribbean: Recent advances and opportunities to move forward. Lancet Oncol. 2021, 22, e474–e487. [Google Scholar] [CrossRef] [PubMed]
- Riano, I.; Velazquez, A.I.; Viola, L.; Abuali, I.; Jimenez, K.; Abioye, O.; Florez, N. State of cancer control in South America: Challenges and advancement strategies. Hematol. Oncol. Clin. N. Am. 2024, 38, 55–76. [Google Scholar] [CrossRef]
- de Moraes Hungria, V.T.; Martínez-Baños, D.M.; Peñafiel, C.R.; Miguel, C.E.; Vela-Ojeda, J.; Remaggi, G.; Duarte, F.B.; Cao, C.; Cugliari, M.S.; Santos, T.; et al. Multiple myeloma treatment patterns and clinical outcomes in the Latin America Haemato-Oncology (HOLA) Observational Study, 2008–2016. Br. J. Haematol. 2020, 188, 383–393. [Google Scholar] [CrossRef]

| Challenges | Potential Solutions |
|---|---|
| Clinical trials | |
|
|
|
|
| Drug development process | |
|
|
| Challenges | Potential Solutions |
|---|---|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hungria, V.; Sureda, A.; Campelo, G.R.; Salvino, M.A.; Ramasamy, K. Proceedings from the First Onco Summit: LATAM Chapter, 19–20 May 2023, Rio de Janeiro, Brazil. Cancers 2024, 16, 3063. https://doi.org/10.3390/cancers16173063
Hungria V, Sureda A, Campelo GR, Salvino MA, Ramasamy K. Proceedings from the First Onco Summit: LATAM Chapter, 19–20 May 2023, Rio de Janeiro, Brazil. Cancers. 2024; 16(17):3063. https://doi.org/10.3390/cancers16173063
Chicago/Turabian StyleHungria, Vania, Anna Sureda, Garcia Rosario Campelo, Marco Aurélio Salvino, and Karthik Ramasamy. 2024. "Proceedings from the First Onco Summit: LATAM Chapter, 19–20 May 2023, Rio de Janeiro, Brazil" Cancers 16, no. 17: 3063. https://doi.org/10.3390/cancers16173063
APA StyleHungria, V., Sureda, A., Campelo, G. R., Salvino, M. A., & Ramasamy, K. (2024). Proceedings from the First Onco Summit: LATAM Chapter, 19–20 May 2023, Rio de Janeiro, Brazil. Cancers, 16(17), 3063. https://doi.org/10.3390/cancers16173063





